Caenorhabditis Sieve: A Low-tech Instrument and Methodology for Sorting Small Multicellular Organisms
Summary
The current protocol includes a methodology for the sorting and cleaning of age-matched populations of Caenorhabditis elegans. It uses a simple, inexpensive, and efficient custom-made tool to obtain a large experimental population of nematodes for research.
Abstract
Caenorhabditis elegans (C. elegans) is a well-established model organism used across a range of basic and biomedical research. Within the nematode research community, there is a need for an affordable and effective way to maintain large, age-matched populations of C. elegans. Here, we present a methodology for mechanically sorting and cleaning C. elegans. Our aim is to provide a cost-effective, efficient, fast, and simple process to obtain animals of uniform sizes and life stages for their use in experiments. This tool, the Caenorhabditis Sieve, uses a custom-built lid system that threads onto common conical lab tubes and sorts C. elegans based on body size. We also demonstrate that the Caenorhabditis Sieve effectively transfers animals from one culture plate to another allowing for a rapid sorting, synchronizing, and cleaning without impacting markers of health, including motility and stress-inducible gene reporters. This accessible and innovative tool is a fast, efficient, and non-stressful option for maintaining C. elegans populations.
Introduction
The nematode worm, Caenorhabditis elegans, is a premier model organism. In addition to the straightforward and controlled nature of their cultivation in the laboratory, their entire genome is sequenced1 and the developmental fate of each cell is known2. Due to these features, C. elegans is a widely used model organism for genetic studies. However, along with these beneficial characteristics come some challenges for researchers. Due to their rapid generation time, C. elegans populations can quickly run out of food and/or become mixed populations with multiple generations and developmental stages present at once. Thus, experiments performed on solid nematode growth media (NGM) require researchers to physically move animals to fresh plates before the bacterial food source depletes and new larvae develop. This can be tedious as a frequent transferring of the animals is required to prevent the experimental populations from becoming mixed with offspring generations. Still, some experiments require both large numbers of animals and extended time points (e.g., DNA or RNA extraction in adulthood). This compounds the challenges of accurately maintaining a synchronized population and transferring large numbers of animals.
Current methods of transferring C. elegans cultured on NGM are picking or washing the animals from plate to plate; chemically treating the animals (e.g., with the DNA replication inhibitor fluorodeoxyuridine or FUDR); or using flow cytometry to sort the animals in multi-well plates. Picking involves the use of a hand tool, made with either a thin platinum wire or an eyelash, to manually transfer individual or multiple animals3,4. This method is accurate but requires both skill and time and is a limitation for studies involving large numbers of animals. Picking may also be physically damaging and stressful to the animals by potentially subjecting individuals to unnatural and inconsistent amounts of disturbance and force. Washing involves rinsing a culture dish with a buffer solution and transferring the solution with the animals via glass Pasteur pipette to a new culture plate. This method is rapid and efficient but is not accurate as multiple generations and developmental stages of animals are transferred in bulk. Chemical treatments, such as FUDR, can be dissolved in the culturing media to prevent the production of offspring through blocking any DNA replication, and thus, the gamete production and egg development. While effective, this method must be applied after developmental maturation as to not disrupt the normal developmental processes, and this means that there is still a requirement to transfer the animals prior to its administration3. This method also influences multiple cellular signaling pathways, resulting in noticeable effects on the animals as they age (e.g., a lifespan extension or an altered proteostasis) depending on the strain of C. elegans used5,6,7,8,9,10. Flow cytometry methods automatically sort and transfer individual C. elegans from one multi-well plate to another11. While this method is very effective and efficient, flow cytometry equipment is prohibitively expensive and inaccessible to many researchers. An alternative to transferring animals is to use mutant models that are temperature sensitive, such as fer-15 and fem-1, which become sterile with temperature adjustment12. While using mutant animals is useful in some situations, these specific strains grow slower than wild-type animals and they rely on an altered genome, serving as poor representatives for aging or healthy worms. In addition, the reliance on a temperature shift to induce sterility also results in the absence of a static environment, and temperature changes have been readily shown to influence gene expressions13,14,15. Research groups have previously published techniques describing the use of a mesh to filter C. elegans by size16. However, we were unable to find previous work testing for any changes in the overall health outcomes that may be associated with the use of such filters.
There is, thus, a need within the C. elegans research community for an affordable, efficient, rapid, and accurate method for transferring large numbers of animals between culture plates. We have developed an improved, accessible piece of equipment (named the Caenorhabditis Sieve) and an associated protocol for its manufacture and operation that meets the needs of the C. elegans research community. Herein, we share the design of the Caenorhabditis Sieve and methods for its use, and we demonstrate that its use does not impact the common health or any stress markers when compared to standard manual picking and a treatment with the commonly-used, fertility-restricting chemical FUDR.
Protocol
1. Caenorhabditis Sieve Construction and Use
- Construction protocol
- Acquire 2 lids from 50 mL conical tubes (Figure 1A).
- Remove the center area inside the inner lip of the lids (when viewed from the bottom, Figure 1B) using a Bunsen burner and a hot metal probe or a soldering iron or stepped drill bit.
NOTE: Using heat to cut the plastic lid is preferable to a blade because there is less risk of injury. - Clean and sand the cut edges and top the surface with a curved file or a rotary grinding tool (e.g., Dremel). See Figure 1C.
- Cut the circle of the monofilament mesh to the appropriate diameter (Figure 1D). For this, trace a lid onto a monofilament nylon mesh sheet and cut just inside the line drawn.
- Cut grooves/slashes into the top surface of the lids to enhance the adhesion of the two lids when glue is applied to the plastic (Figure 1E).
- Clean the lids with ethanol and let them dry.
- Apply cyanoacrylate glue to the top surface of both lids, keeping to the outer edge.
NOTE: A little glue goes a long way. - Lay the monofilament mesh, according to Table 1, on one glued lid (Figure 1F). Place the second lid inverted on top of the mesh; both lids must have their tops together. Press firmly together (Figure 1G). Ensure that the mesh is taut.
NOTE: As a safety measure, use tweezers to place the mesh on the lids. - Once the initial layer of glue has dried, apply a ring of cyanoacrylate glue around the outer gap between the lids. Be generous as this adds integrity and prevents any peeling apart or leaking.
- Label the filter with the mesh pore size of the monofilament mesh.
NOTE: Here, we use two mesh pore sizes—20 µm and 50 µm.
- Use protocol
- Pre-wet the sieve.
- Pipette a saline solution, such as M9 [5 g of NaCl, 6 g of Na2HPO4, 3 g of KH2PO4, 1 L of ultrapure H2O, and 1 mL of MgSO4 (1 M)]17, through the center of the sieve until a droplet forms, condenses, and drips off the center of the filter bottom (Figure 2A). Optionally, apply a lint-free wipe (e.g., Kimwipe) to the bottom to shape or spread a moisture droplet on the mesh.
- Place the sieve over a 50 mL conical tube. Label the tube as 'Waste tube' (Figure 2B).
- Wash a population of C. elegans off an agar plate.
- Wash the plate with the M9 buffer and place the worm-containing medium on the topside of the monofilament mesh 1 mL at a time (Figure 2C). Make sure to operate in the center of the mesh. Wash all worms from the plate.
NOTE: Typically, 3 mL of M9 for a 60 mm plate is sufficient. - To minimize the number of worms lost in pipetting, use a glass Pasteur pipette to move the worms off the plate and onto the mesh center (repeat as needed).
- Rinse the filter with the M9 buffer from the top. Again, make sure to operate in the center of the mesh and rinse all worms in that area. Wash as many times as necessary to ensure that all the bacteria and smaller worms have passed through the mesh.
- Wash the plate with the M9 buffer and place the worm-containing medium on the topside of the monofilament mesh 1 mL at a time (Figure 2C). Make sure to operate in the center of the mesh. Wash all worms from the plate.
- Harvest the size-matched animals from the sieve.
- Attach a new 50 mL conical tube to the top of the sieve, with the adult worms from step 1.2.2.3 facing the inside of the new collection tube (Figure 2D).
- Remove the first tube (waste tube) and quickly flip over the sieve and new tube to keep the droplet from migrating (Figure 2E).
NOTE: If sterility is not required, use a lint-free wipe (e.g., Kimwipe) to wick fluid from bottom of the mesh prior to flipping. - Rinse the mesh with M9 into the new 50 mL tube from the new topside (Figure 2F). Again, operate in the mesh center and maintain a droplet on the underside of the mesh.
- Allow the worms to settle or gently spin them down (e.g., <16 x g) for approximately 1 min (Figure 2G).
- Aspirate the buffer solution from the pellet of worms, ideally down to >0.5 mL, or remove as much fluid as possible without disturbing the pellet.
- Pipette the worms using a glass Pasteur pipette onto an NGM plate and let them dry. Space multiple droplets out on a new plate so they dry faster (Figure 2H).
- Clean the sieve.
- Rinse the sieve gently and thoroughly with ethanol and reverse-osmosis water. Let it dry.
- Store it in a clean container for indefinite future use.
- Discard it when the mesh develops a "sag" appearance.
- Pre-wet the sieve.
2. Validation of Caenorhabditis Sieve Sorting Method
- General maintenance
- For all experiments, culture worms on a standard Nematode Growth Media17 (1 L of NGM consists of 2.5 g of peptone, 17 g of agar, 3 g of NaCl, 975 mL of double-distilled water, 1 mL of 5 mg/mL cholesterol, 1 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, 25 mL of 1 M KHPO4, and 0.5 mL of 100 mg/mL streptomycin) at 25 °C.
- Experimental treatment administration
- Compare the three treatment groups: pick, fluorodeoxyuridine (FUDR), and Caenorhabditis Sieve.
- For the FUDR treatment group, add 100 mg/mL FUDR to the NGM media to a final concentration of 100 μM to prevent any progeny production, and transfer the worms every other day to a fresh NGM plate to avoid food depletion.
- For the pick group, select and transfer the worms manually using a platinum loop.
- For the Caenorhabditis Sieve treatment group, follow step 1.2 and pipette the worms onto an NGM plate.
- Compare the three treatment groups: pick, fluorodeoxyuridine (FUDR), and Caenorhabditis Sieve.
- Caenorhabditis Sieve percentage yield
- To quantify how effective the sieve is at sorting C. elegans, grow age-synchronous N2 animals to day 1 of adulthood at 25 °C (i.e., 48 h after egg laying) and then transfer them by picking them to fresh NGM plates (totaling N = 50 or N = 100 animals per treatment group).
- After 24 h of recovery, transfer the population to new NGM plates with the Caenorhabditis Sieve following the above protocol (see step 1.2) and count the number of successfully transferred animals.
- Calculate the percentage yield as the ratio of the number of animals transferred in comparison to the starting number at the beginning of the transfer multiplied by 100 (%).
- Healthspan assays
NOTE: Score healthspan parameters of the motility class, the pharyngeal pump rate, and both the anterior and the posterior gentle touch response on days 2, 4, 6, and 8 of adulthood for age-synchronized N2 animals maintained at 25 °C.- Motility tracking
- Assign motility scores based on a class-based system (classes A, B, and C) following the methods of Herndon et al.18. Compare the effects of the three experimental groups using an ordinal logistic statistics model in statistical analysis software.
NOTE: Class A individuals move spontaneously in a normal, sinusoidal pattern. Class B individuals move in markedly non-sinusoidal movements and may require prodding to encourage movement. Class C individuals move their head and/or tail in response to prodding but are unable to move across the agar.
- Assign motility scores based on a class-based system (classes A, B, and C) following the methods of Herndon et al.18. Compare the effects of the three experimental groups using an ordinal logistic statistics model in statistical analysis software.
- Pharyngeal pump rate
- Count the grinder movement of the animal’s terminal pharyngeal bulb visually under a stereomicroscope at a 600X final magnification for 1 min.
- Conduct a statistical analysis with a one-way ANOVA with α = 0.05 and Bonferroni post-tests with α = 0.05.
- Touch response
- Record a gentle touch response and compare between the three treatment groups. Perform the assays based on the methods described by Calixto et al.19.
- Record the anterior and posterior touch response by gently stroking an eyelash pick perpendicularly across the tail or the head (5x each, alternating head and tail) of the animals.
- Score any movement in the opposite direction of the stroke as 1 point on a scale of 0 to 5 for both the anterior and the posterior response.
- Conduct statistical analysis with a one-way ANOVA with α = 0.05 and Bonferroni post-tests with α = 0.05.
- Motility tracking
- Fecundity assay
- To determine the use of the Caenorhabditis Sieve's impact on reproduction, grow age-synchronous N2 animals to day 2 of adulthood at 25 °C.
- 60 h after egg lay, transfer animals to new NGM plates with either a platinum pick or the Caenorhabditis Sieve and give them 4 h to recover (dry the sieved plates for 20–30 min).
- After recovery, individually plate the animals via an eyelash pick to NGM plates, give them 24 h to lay eggs, and remove the animals. Allow the progeny on each plate to develop under normal conditions at 25 °C for another 24 h.
NOTE: An eyelash pick is a human eyelash secured to the end of a Pasteur pipette with nail polish and sterilized with ethanol. - Count the number of viable F1 generation individuals. Perform a statistical analysis using a T-test with α = 0.05.
Note: Viable offspring are considered to be eggs that successfully hatch and begin their development through the regular larval cycles.
- Fluorescent reporter stress response assays
- Perform three commonly used fluorescent reporter assays to detect potential markers of stress: a DAF-16::GFP translocation into the nuclei of cells in a strain [TJ356- zIs356 (pDAF-16::DAF-16-GFP;rol-6)]20; an hsp-16.2 expression [TJ375- gpIs1 (hsp-16.2p::GFP)]21; and a sod-3 expression [CF1553-muIs84([pAD76]sod-3p::GFP+rol-6[su1006])]22.
- For each assay, culture age-synchronous animals from the three treatment groups at 20 °C and examine them at day 3 of adulthood: use a negative control (on a daily basis, transfer a group of animals manually with a platinum pick), a positive control (on a daily basis, transfer a group of animals manually with a platinum pick plus an established stressor), and the Caenorhabditis Sieve (pass the animals through the sieve and allow them to recover for 30 min on NGM just before imaging).
- In the DAF-16::GFP assay, heat shock the positive control animals at 37 °C for 30 min before imaging20. For the hsp-16.2 assay, heat shock the positive control animals for 90 min, 20 h before imaging21. For the sod-3 assay, treat the positive control animals with 100 mM paraquat for 4 h before imaging22.
- Harvest the worms immediately with an eyelash pick and mount them to a coverslip with 1 μL of a 36% poloxamer 407 surfactant solution to immobilize the worms.
- Sandwich the mounted worms with another coverslip. Mount the two coverslips to a standard glass microscopy slide and image the worms using an 8X magnification on an inverted fluorescent microscope (for an overall magnification of 80X) and a constant exposure with a FITC filter.
- To detect any differences between the three groups in the DAF-16::GFP assay, categorize the animals based on the location of the DAF-16::GFP reporter (nuclear if the reporter translocated to nuclei, cytosolic if the reporter located in cytosol, and intermediate if the reporter located in both nuclei and cytosol).
- Compare the results using an ordinal logistics statistical model in statistical analysis software. For the hsp-16.2 and the sod-3 expression assays, use a one-way ANOVA with α = 0.05 and Tukey's post hoc tests with α = 0.05 to compare the total fluorescence of the head region.
Representative Results
The Caenorhabditis Sieve consists of 2 screw caps, securing an area of woven nylon monofilament mesh smaller than the body diameter of the desired developmental age, used to extract live populations of organisms using a simple washing technique. It attaches to standard conical tubes and uses the mesh screen to mechanically sort animals by body diameter, leaving the desired animals in the tube ready for further maintenance and experimentation (e.g., transfer or genetic harvest). A gentle manual washing with the Caenorhabditis Sieve is quick, approximately 5 min per 60–100 mm plate, and the organisms are easily recovered from the mesh.
Percentage yield of animals following Caenorhabditis Sieve use
To establish the percentage yield of the Caenorhabditis Sieve, devices were tested with both 20 μm and 50 μm pore-size meshes on adult animals. A mean yield of a >90% successful animal transfer was achieved for both mesh sizes tested (Table 1).
Monofilament mesh with different size gaps may be used to separate animals of different life stages. Mesh with 20 μm gaps is appropriate for washing away developing embryos and larval stages smaller than the fourth larval stage, retaining the latter (L4; with an average body diameter of 32 μm) and any animals in later life stages, while mesh with 50 μm gaps will allow all other life stages aside from adults (with an average body diameter of 70 μm) to be washed away (Table 2).
Caenorhabditis Sieve use does not impact healthspan metrics
Motility: In C. elegans the normal sinusoidal movement (i.e., motility) declines with age18 and is a marker of overall health. To determine if the Caenorhabditis Sieve influenced motility, motility scores were compared for pick, FUDR, and Caenorhabditis Sieve treatment groups on days 2, 4, 6, and 8 of adulthood. All the animals across every group (n = 10/group) exhibited normal and spontaneous movement patterns (Class A) at multiple ages throughout adulthood (days 2, 4, 6, and 8 of adulthood; p > 0.05 for multiple comparisons on all days, Figure 3).
Pharyngeal pump rate: The ability of C. elegans' pharyngeal muscles to pump declines with age and is another biomarker of healthspan23. To determine if the Caenorhabditis Sieve influenced the animals' pharyngeal pump rate, pick, FUDR, and Caenorhabditis Sieve treatment groups were compared on days 2, 4, 6, and 8 of adulthood (n = 8 to 10 per group). There was a significant difference between the animals that underwent the picking and FUDR methods on days 6 (p <0.001) and 8 (p <0.001). Also, there was a significant difference between the sieve and FUDR groups on days 6 (p <0.001) and day 8 of adulthood (p <0.001). However, there was no statistically significant difference between the pick and the Caenorhabditis Sieve groups for any day (p >0.05, Figure 4), indicating that the sieve does not impact this measure of healthspan.
Gentle touch response: Response to mechanical stimulus is a physiological marker to assess aging or general health24,25; thus, the impact of different transfer methods on both the anterior and the posterior gentle touch responses were tested. There was no statistically significant difference between the pick, FUDR, and Caenorhabditis Sieve treatment groups (n = 8/group), either anteriorly or posteriorly, for any day of testing (p >0.4 for all comparisons; Figure 5A and 5B).
Fecundity: To establish whether or not the Caenorhabditis Sieve influenced the amount of viable progeny produced by C. elegans, the individual offspring produced in a 24 h period during day 3 of adulthood was counted and compared (n = 20 to 22 per group). The use of the Caenorhabditis Sieve did not significantly impact the number of progeny produced when compared to a pick treatment group (p = 0.61, Figure 6).
Molecular reporter assays
DAF-16 nuclear translocation: In C. elegans, the activation of the transcription factor DAF-16 is associated with increased stress resistance26. The nuclear localization of DAF-16 was examined in a transgenic nematode strain TJ356, which expresses DAF-16 fused to a green fluorescent protein (DAF-16::GFP)20. Under normal growth conditions, DAF-16::GFP is localized primarily in the cytosol, but under various stressors (e.g., heat stress), it is rapidly translocated into the nucleus20. To test the impact of sorting with the Caenorhabditis Sieve on DAF-16 translocation, DAF-16::GFP localizations were compared in age-matched day-5 adults in a positive control group (heat stress), a negative control group (manual transfer via pick), and a Caenorhabditis Sieve treatment groups (n = 10/group). The transfer with the Caenorhabditis Sieve did not affect the nuclear translocation of DAF-16::GFP, and showed a similar phenotype to the negative control animals (p >0.05, Figure 7).
hsp-16.2 reporter: Small heat shock proteins like HSP-16.2 are biomarkers of a stress response, and they are highly expressed during an exposure to heat shock or oxidative stress agents21,27. The TJ375 strain has a GFP reporter gene fused with an hsp-16.2 promoter that is not active under normal conditions21. However, after an exposure to a heat shock, HSP-16.2 protein expression is induced, and the animals display high levels of GFP expression21. To test the involvement of the Caenorhabditis Sieve on an HSP-16.2-mediated stress response, the fluorescence density in the pharynx region (n = 10 animals/group) of age-matched animals was compared in day-5 adults between a positive control group (heat stress), a negative control group (picking), and a Caenorhabditis Sieve treatment group. The transfer with the Caenorhabditis Sieve did not significantly induce the expression of HSP-16.2::GFP (hsp-16.2::gfp) when compared to the negative control (p >0.05, Figure 8).
sod-3 reporter: In C. elegans, the anti-oxidant gene that codes for superoxide dismutase 3 (SOD-3) is up-regulated during oxidative stress28. The C. elegans strain CF1553 expresses green fluorescent protein (GFP)-labeled SOD-3 promoter, whose expression is induced by oxidative stressors, such as paraquat5. To test the involvement of the Caenorhabditis Sieve sorting on the antioxidant response in C. elegans, the fluorescence density in the head region of age-matched day-5 adults was compared between a positive control population (100 μM paraquat treatment), a negative control population (manual picking), and a Caenorhabditis Sieve-transferred population. The transfer with the Caenorhabditis Sieve did not significantly induce the expression of sod-3::gfp when compared to the negative control (p >0.05, Figure 9).
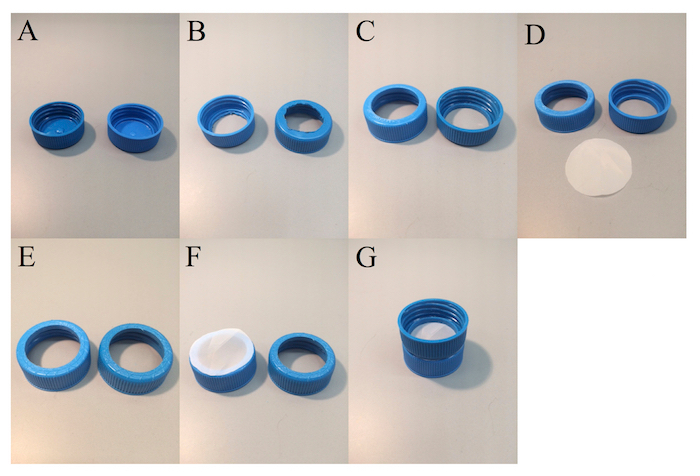
Figure 1: Caenorhabditis Sieve construction. The progression of the 'do-it-yourself' manufacture of the tool is shown. These panels show (A) two 50 mL conical tube caps (B) whose centers have been removed, (C) cut edges smoothed, and (D – F) who are fitted with monofilament mesh corresponding to the desired life stage of C. elegans (see Protocol for details). (G) The completed sieve is also shown. Please click here to view a larger version of this figure.
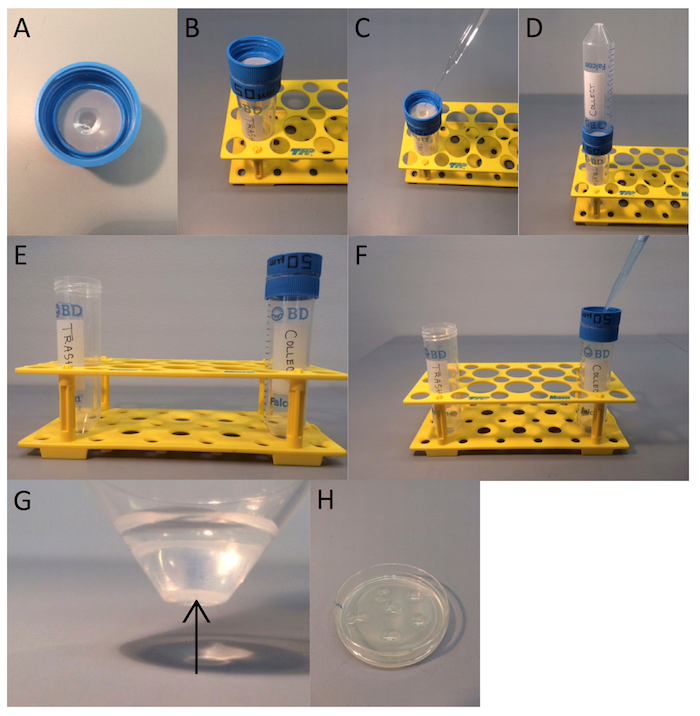
Figure 2: Step-by-step image representation of Caenorhabditis Sieve use. (A) The sieve is pre-wet with a drop of M9 solution, and (B) fit on top of a 50 mL conical tube. (C) 1 mL of M9 solution with worms is pipetted on the topside of the sieve, (D) a 50 mL conical tube is placed on top of the sieve with the worms facing the inside of the tube, and (E) the sieve with a newly attached upper tube is quickly flipped over. (G) The sieve is rinsed with M9 carrying the desired animals into the new 50 mL tube and the worms are allowed to settle by gravity to the bottom of the tube. (H) The worms are pipetted and placed as droplets on a fresh NGM plate. Please click here to view a larger version of this figure.
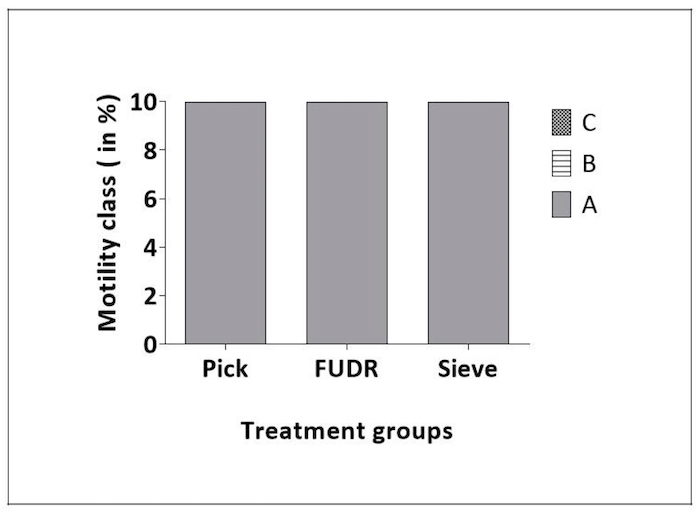
Figure 3: Caenorhabditis Sieve did not impact motility throughout lifespan. This figure shows the motility class distribution of animals at days 2, 4, 6, and 8 of adulthood for pick, FUDR, and Caenorhabditis Sieve treatment groups. Class A animals moved normally and spontaneously, class B animals moved abnormally and may have required prodding, and class C animals were unable to move. There was no difference between Caenorhabditis Sieve, pick and FUDR groups (p >0.05). Three replicates (n = 10 animals per treatment plate) were conducted and analyzed with Ordinal logistic model. class B animals. Please click here to view a larger version of this figure.
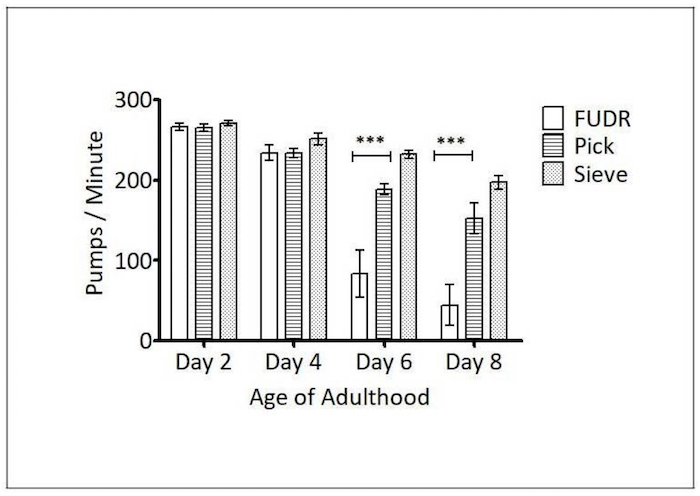
Figure 4: Caenorhabditis Sieve use did not impact pharyngeal pumping throughout the lifespan. This figure shows the pharyngeal pump rates of pick, FUDR, and Caenorhabditis Sieve treatment groups, compared on days 2, 4, 6, and 8 of adulthood. The asterisks denote a significance between the pick, sieve, and FUDR treatment groups for the days specified (*** p <0.05). There was no difference between the Caenorhabditis Sieve and the pick group (p > 0.05). Two replicates (N = 10 animals per treatment plate) were conducted and analyzed with a one-way ANOVA and a Bonferroni post-test. The bars represent the mean ± the standard error of the mean. Please click here to view a larger version of this figure.
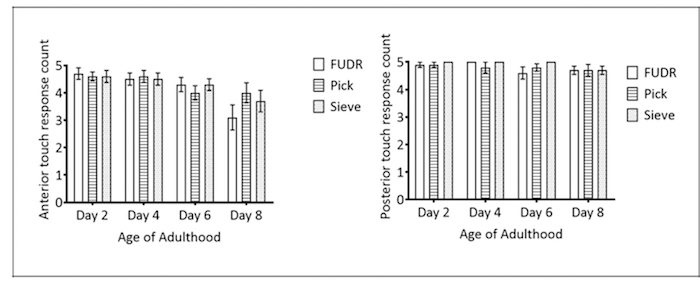
Figure 5: Caenorhabditis Sieve use did not impact anterior touch response throughout lifespan. These panels show (A) the anterior and (B) the posterior touch response count of pick, FUDR, and Caenorhabditis Sieve treatment groups compared on days 2, 4, 6, and 8 of adulthood. Two replicates were conducted with N = 10 for each treatment group and compared with a one-way ANOVA and a Bonferroni post-test (p = 0.4 and p = 0.9 for anterior and posterior, respectively). The bars represent the mean ± the standard error of the mean. Please click here to view a larger version of this figure.
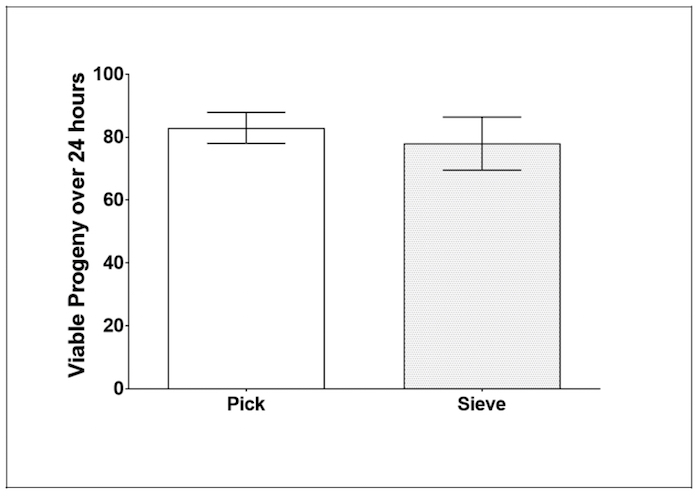
Figure 6: Caenorhabditis Sieve did not impact the amount of viable progeny on day 3 of adulthood. This figure shows the viable progeny of day-3 adults after a 24 h egg-laying period, spanning day 3 of adulthood for parent animals. The bars represent the mean ± the standard error of the mean. N = 20 – 22 animals from at least two separate biological replicates per treatment group. The treatment groups are compared with a t-test, (p >0.05). Please click here to view a larger version of this figure.
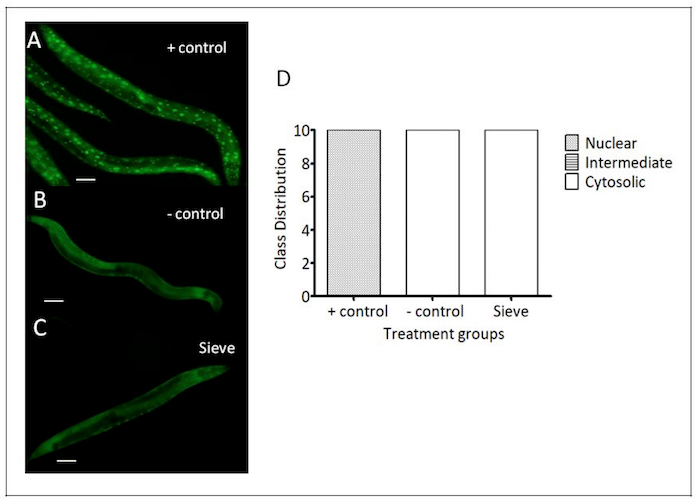
Figure 7: Caenorhabditis Sieve did not affect nuclear translocation of DAF-16::GFP. These panels show representative images of the DAF-16 translocation of (A) a heat shock group (the positive control), (B) a pick group (the negative control), and (C) a Caenorhabditis Sieve treatment group. (D) The animals in the positive control group displayed an activation of the DAF-16 nuclear translocation. The Caenorhabditis Sieve did not induce a nuclear translocation and displayed cytosolic fusion protein similar to the animals in the negative control group. N = 10 animals per treatment group from at least three separate biological replicates. The treatment groups were compared with a one-way ANOVA and a Tukey's post hoc test. Scale bar = 100 μm. Please click here to view a larger version of this figure.
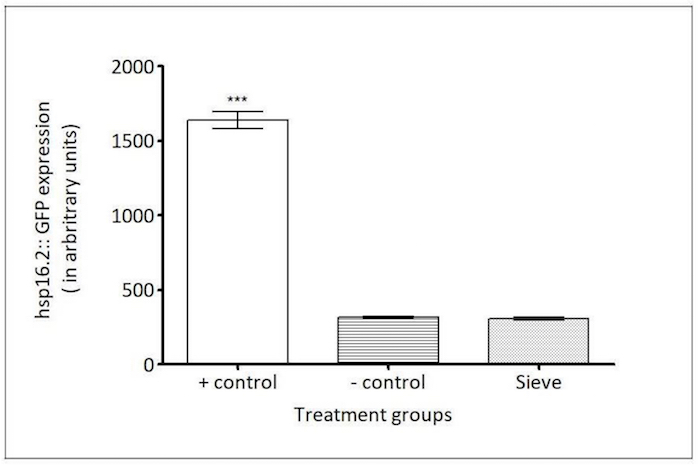
Figure 8: Caenorhabditis Sieve did not affect the expression of hsp16.2::gfp. The HSP-16.2 expressions (in arbitrary fluorescence units) of a heat shock group (the positive control), a pick group (the negative control), and a Caenorhabditis Sieve treatment group are compared. The asterisks denote a high expression of hsp16.2::gfp for the positive control group which was significantly different from the other treatment groups (*** p <0.05). The Caenorhabditis Sieve did not affect the hsp16.2::gfp expression and displayed fluorescence intensities similar to animals in the negative control group (p >0.05). Three replicates were conducted with N = 10 for each treatment group and compared with a one-way ANOVA and a Tukey's post hoc test. Please click here to view a larger version of this figure.
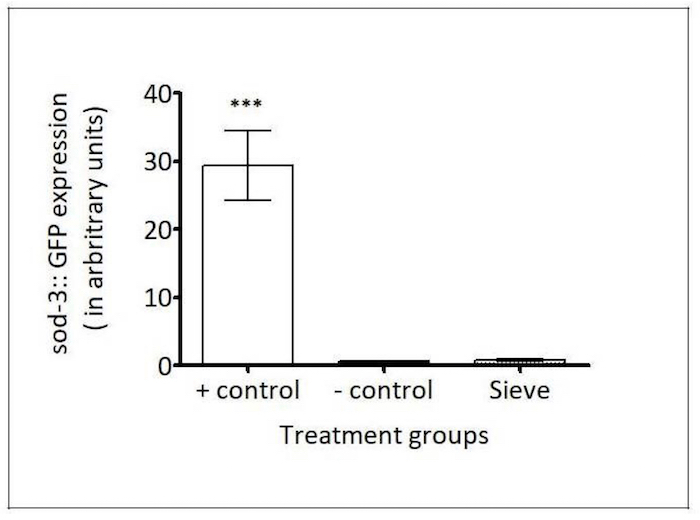
Figure 9: Caenorhabditis Sieve did not affect the expression of sod-3::gfp. The SOD-3 expressions (in arbitrary fluorescence units) of a 100 μM paraquat group (the positive control), a pick group (the negative control), and a Caenorhabditis Sieve treatment group are shown. The asterisks denote a high expression of sod-3::gfp for the positive control group which was significantly different from the other groups (*** p <0.05). The Caenorhabditis Sieve did not affect the sod-3::gfp expression and displayed fluorescence intensities similar to the animals in the negative control group (p >0.05). Three replicates were conducted with N = 10 for each treatment group and compared with a one-way ANOVA and a Tukey's post hoc test. Please click here to view a larger version of this figure.
| Mesh size | N = 50 | N = 100 |
| 20 μm | 95.33% | |
| 50 μm | 99.00% | 93.33% |
Table 1: Percentage yield of mesh sizes. This table shows the results of a 20 μm device tested with N = 50 adults for 3 replicates and a 50 μm device tested with N = 50 adults for 2 replicates and with N = 100 for 3 replicates.
| Mesh Size | Developmental Stage | Body Diameter |
| 20 μm | Larval Stage 4 | ~35 μm |
| 50 μm | Day 1 Adult | ~70 μm |
Table 2: Mean body diameter. This table displays the mean body diameter acquired by averaging 3 measurements equally spread across each worm for 15 animals at each measured life stage.
Discussion
Herein, we introduced the design and use of the accessible, effective Caenorhabditis Sieve as a tool for sorting and maintaining C. elegans. This tool has several advantages to manually picking individual animals, washing populations, chemical treatments (e.g., FUDR), and more expensive methods of segregating animals. First, the Caenorhabditis Sieve efficiently and quickly (less than 20 min) sorts progeny from large mixed populations of animals (Table 2). Also, the use of the tool has no detectable toxic effects on the animals' healthspan (Figure 3, Figure 4, Figure 5, Figure 6), does not induce well-described genetic stress reporters (Figure 7, Figure 8, Figure 9), and reduces the amount of foreign chemical that could influence the applied treatment to the culture plates or any molecular assay thereafter. Its ease of use is a significant advantage; a researcher at any stage in their education can be trained to use it (Figure 1 and Figure 2; Protocol).
The materials required for the fabrication and use of the sieve (e.g., pipettes, buffers) are readily available in standard research laboratories; thus, if broken, the Caenorhabditis Sieve is not expensive to replace or repair. The self-constructed nature of the Caenorhabditis Sieve allows it to be a versatile tool: it can be constructed with mesh sizes appropriate for different C. elegans developmental stages and phenotypes. The Caenorhabditis Sieve may also be used in assays conducted in other nematode species or when isolating small organisms, provided the appropriate mesh size is used upon manufacture.
In addition to the benefits this tool provides for C. elegans population maintenance, the Sieve may be used to clean animals before their use in other experimental applications. For example, with the development of microfluidics to conduct C. elegans research comes the issue of chip use and cleaning. Depending on the amount of bacteria attached to the animals, special precautions need to be taken so as not to clog the microfluidic chip, rendering it unusable29. Often when C. elegans are transferred to a microfluidic chip, residue from the bacterial lawn is brought with them. This residue can and does clog the microfluidic channels, making the chip malfunction, which then requires cleaning or replacement. The device construct in this protocol offers a method for not only harvesting synchronized animals for microfluidics, but also for cleaning the animals prior to their insertion into a microfluidic device. By removing the debris and bacterial residue, taken up when collecting animals, the microfluidic channels are less prone to malfunction, thus increasing the operational life of individual chips and the subsequent throughput of research being conducted with them.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Heather Currey for her initial contribution to the study design, and Dr. Swarup Mitra for his critical review of the manuscript. We would also like to thank Dr. Michael B. Harris for comments, refinements and assistance in producing the demonstration of this methodology. The strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). The research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Numbers UL1GM118991, TL4GM118992, or RL5GM118990 and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number 5P20GM103395-15. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. UA is an AA/EO employer and educational institution and prohibits illegal discrimination against any individual: www.alaska.edu/titleIXcompliance/nondiscrimination.
Materials
| Safety glasses | Uline | S-21076 | |
| Protective heat resistant glove | Grainger | Item # 3AT17 Mfr. Model # 3AT17 Catalog Page # 1703 | |
| 50 mL conical tube | Falcon | 14-432-22 | |
| Synthetic Nylon mesh | Dynamic Aqua-Supply Ltd |
NTX20 and NTX50 | |
| Cyanoacrylate glue | Scotch Super Glue Liquid | SAD114 | |
| Pliers | Vampliers | VMPVT-001-8 | |
| Dremmel tool with circular file | Lowe's | Item # 525945 Model # 100-LG | |
| FUDR | Sigma | F0503 | |
| M9 chemicals ( NaCl, Na2HPO4, KH2PO4, MgSO4) | Sigma | S7653, RES20908-A7, 1551139, M7506 | |
| NGM plate chemicals (Bactopeptone, Agar, KH2PO4, K2HPO4, CaCl2,Cholesterol, Streptomycin) | BD Biosciences (bactopeptone) , Lab express (agar), Sigma ( rest) | BD bioscience 211677, Lab Express 1001, Sigma 1551139, 1551128, C1016, C8667, S6501 | |
| Pluronic F-127 | Sigma | P2443 | |
| Paraquat dichloride hydrate | Sigma | 36541 | |
| Inverted fluorescence microscope | Olympus | FSX100 |
Referências
- C elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 282 (5396), 2012-2018 (1998).
- Herman, M. A. Hermaphrodite cell-fate specification. WormBook. , 1-16 (2006).
- Stiernagle, T. Maintenance of C. elegans. WormBook. , 1-11 (2006).
- Chalfie, M., Hart, A. C., Rankin, C. H., Goodman, M. B. Assaying mechanosensation. WormBook. , (2014).
- Van Raamsdonk, J. M., Hekimi, S. Deletion of the Mitochondrial Superoxide Dismutase sod-2 Extends Lifespan in Caenorhabditis elegans. PLoS Genetics. 5 (2), e1000361 (2009).
- Van Raamsdonk, J. M., Hekimi, S. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mechanisms of Ageing Development. 132 (10), 519-521 (2011).
- Gandhi, S., Santelli, J., Mitchell, D. H., Stiles, J. W., Sanadi, D. R. A simple method for maintaining large, aging populations of Caenorhabditis elegans. Mechanisms of Ageing Development. 12 (2), 137-150 (1980).
- Aitlhadj, L., Stürzenbaum, S. R. The use of FUdR can cause prolonged longevity in mutant nematodes. Mechanisms of Ageing and Development. 131 (5), 364-365 (2010).
- Davies, S. K., Leroi, A. M., Bundy, J. G. Fluorodeoxyuridine affects the identification of metabolic responses to daf-2 status in Caenorhabditis elegans. Mechanisms of Ageing Development. 133 (1), 46-49 (2012).
- Feldman, N., Kosolapov, L., Ben-Zvi, A. Fluorodeoxyuridine improves Caenorhabditis elegans proteostasis independent of reproduction onset. PLoS One. 9 (1), e85964 (2014).
- Pulak, R. Techniques for analysis, sorting, and dispensing of C. elegans on the COPAS flow-sorting system. Methods Molecular Biology. 351, 275-286 (2006).
- Argon, Y., Ward, S. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genética. 96 (2), 413-433 (1980).
- Lee, S. J., Kenyon, C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Current Biology. 19 (9), 715-722 (2009).
- Klass, M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mechanisms of Ageing Development. 6 (6), 413-429 (1977).
- Zhang, B., et al. Environmental Temperature Differentially Modulates C. elegans Longevity through a Thermosensitive TRP Channel. Cell Reports. 11 (9), 1414-1424 (2015).
- Michaelson, L. C. C. elegans: A Practical Approach. Ian A. Hope (ed.). Oxford University Press, Oxford. 1999. Pp. 281. ISBN 0 19 963738 5. Heredity. 85 (1), 97-100 (2000).
- Brenner, S. The genetics of Caenorhabditis elegans. Genética. 77 (1), 71-94 (1974).
- Herndon, L. A., et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 419 (6909), 808-814 (2002).
- Calixto, A., Chelur, D., Topalidou, I., Chen, X., Chalfie, M. Enhanced neuronal RNAi in C. elegans using SID-1. Nature Methods. 7 (7), 554-559 (2010).
- Henderson, S. T., Johnson, T. E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Current Biology. 11 (24), 1975-1980 (2001).
- Rea, S. L., Wu, D., Cypser, J. R., Vaupel, J. W., Johnson, T. E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nature Genetics. 37 (8), 894-898 (2005).
- Libina, N., Berman, J. R., Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 115 (4), 489-502 (2003).
- Keith, S. A., Amrit, F. R., Ratnappan, R., Ghazi, A. The C. elegans healthspan and stress-resistance assay toolkit. Methods. 68 (3), 476-486 (2014).
- Scerbak, C., Vayndorf, E. M., Hernandez, A., McGill, C., Taylor, B. E. Mechanosensory neuron aging: Differential trajectories with lifespan-extending alaskan berry and fungal treatments in Caenorhabditis elegans. Frontiers in Aging Neuroscience. 8, 173 (2016).
- Vayndorf, E. M., et al. Morphological remodeling of C. elegans neurons during aging is modified by compromised protein homeostasis. npj Aging and Mechanisms of Disease. 2, 16001 (2016).
- Murakami, S., Johnson, T. E. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genética. 143 (3), 1207-1218 (1996).
- Abbas, S., Wink, M. Green Tea Extract Induces the Resistance of Caenorhabditis elegans against Oxidative Stress. Antioxidants (Basel). 3 (1), 129-143 (2014).
- Yanase, S., Hartman, P. S., Ito, A., Ishii, N. Oxidative stress pretreatment increases the X-radiation resistance of the nematode Caenorhabditis elegans. Mutation Research. 426 (1), 31-39 (1999).
- Chung, K., Crane, M. M., Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nature Methods. 5 (7), 637-643 (2008).

