Studying Protein Import into Chloroplasts Using Protoplasts
Summary
Here we describe a protocol to express proteins into protoplasts by using PEG-mediated transformation method. The method provides easy expression of proteins of interest, and efficient investigation of protein localization and the import process for various experimental conditions in vivo.
Abstract
The chloroplast is an essential organelle that is responsible for various cellular processes in plants, such as photosynthesis and the production of many secondary metabolites and lipids. Chloroplasts require a large number of proteins for these various physiological processes. Over 95% of chloroplast proteins are nucleus-encoded and imported into chloroplasts from the cytosol after translation on cytosolic ribosomes. Thus, the proper import or targeting of these nucleus-encoded chloroplast proteins to chloroplasts is essential for the proper functioning of chloroplasts as well as the plant cell. Nucleus-encoded chloroplast proteins contain signal sequences for specific targeting to chloroplasts. Molecular machinery localized to the chloroplast or cytosol recognize these signals and carry out the import process. To investigate the mechanisms of protein import or targeting to chloroplasts in vivo, we developed a rapid, efficient protoplast-based method to analyze protein import into chloroplasts of Arabidopsis. In this method, we use protoplasts isolated from leaf tissues of Arabidopsis. Here, we provide a detailed protocol for using protoplasts to investigate the mechanism by which proteins are imported into chloroplasts.
Introduction
The chloroplast is one of the most important organelles in plants. One of the main functions of chloroplasts is to carry out photosynthesis1. Chloroplasts also carry out many other biochemical reactions for the production of fatty acids, amino acids, nucleotides, and numerous secondary metabolites1,2. For all of these reactions, chloroplasts require a large number of different types of proteins. However, the chloroplast genome contains only approximately 100 genes3,4. Therefore, chloroplasts must import the majority of their proteins from the cytosol. In fact, most chloroplast proteins were shown to be imported from the cytosol after translation4,5,6. Plant cells require specific mechanisms to import proteins from the cytosol to chloroplasts. However, although these protein import mechanisms have been investigated for the past several decades, we still do not fully understand them at the molecular level. Here, we provide a detailed method for preparing protoplasts and exogenously expressing genes in protoplasts. This method could be valuable for elucidating the molecular mechanisms underlying protein import into chloroplasts in detail.
Protein import can be studied using many different approaches. One of these methods involves the use of an in vitro protein import system7,8. Using this approach, in vitro-translated protein precursors are incubated with purified chloroplasts in vitro, and protein import is analyzed by SDS-PAGE followed by western blot analysis. The advantage of this approach is that each step of protein import into chloroplasts can be studied in detail. Thus, this method has been widely used to define the components of the protein import molecular machinery and to dissect sequence information for transit peptides. More recently, another approach involving the use of protoplasts from leaf tissues was developed and it has become widely used to study protein import into chloroplasts9,10. The advantage of this approach is that protoplasts provide a cellular environment that is closer to that of intact cells than the in vitro system. Thus, the protoplast system allows us to address many additional aspects of this process, such as the associated cytosolic events and how the specificity of targeting signals is determined. Here, we present a detailed protocol for the use of protoplasts to study protein import into chloroplasts.
Protocol
1. Growth of Arabidopsis Plants
- Prepare 1 L Gamborg B5 (B5) medium by adding 3.2 g of B5 medium including vitamins, 20 g of sucrose, 0.5 g of 2-(N-morpholino) ethane sulfonic acid (MES) to approximately 800 mL of deionized water, and adjust the pH to 5.7 with potassium hydroxide (KOH). Add more deionized water bring the total volume to 1 L. Add 8 g of phytoagar and autoclave for 15 min at 121 °C.
- Allow the medium to cool down to 55 °C and pour 20 – 25 mL of the B5 medium into a Petri dish (9 cm in diameter, 1.5 cm in height) at a clean bench. Dry plates for 2 – 3 days, pack them with clean wrap, and keep them in a refrigerator until use.
- Sterilize Arabidopsis thaliana seeds with 1 mL of 70% (v/v) ethanol in a centrifuge tube (e-tube) by continuously shaking for 2 – 3 min. Remove the supernatant, add 1 mL of 1% (v/v) sodium hypochlorite solution, and shake continuously for 2 – 3 min. Prepare 100 – 150 seeds per Petri dish.
- Remove the supernatants and wash the seeds with 1 mL of distilled water. Repeat this step 4x. Place the sterilized seeds at 4 °C for 3 days to synchronize seed germination.
- Sow 100 – 150 seeds onto B5 plates using a micropipette and seal the plate with surgical tape.
- Grow plants in a growth room with a 16 h/8 h light/dark cycle at 100 µmol·m-2·s-1 light intensity, 70% relative humidity and 20 °C temperature for 2 weeks.
2. Preparation of the Plasmid
NOTE: High-purity plasmids should be used for transformation; the use of a commercial plasmid purification kit is recommended.
- Purify the plasmid (RbcS-nt:GFP) using a DNA isolation kit. To concentrate DNA, perform ethanol precipitation in a 1.5 mL tube and dissolve the DNA pellet in 100 µL of distilled water. Determine the concentration of the plasmid using a spectrophotometer at 260 nm. Dilute the plasmid to a final concentration of ~ 2 µg/µL. Keep the plasmid at -20 °C until use.
3. Isolation of Protoplasts
- Prepare the following solutions.
- Prepare the enzyme solution to contain 0.25% (w/v) macerozyme, 1.0% (w/v) cellulase, 400 mM D-mannitol, 8 mM calcium chloride (CaCl2), 0.1% (w/v) bovine serum albumin (BSA), and 5 mM MES. Adjust the pH to 5.6 with KOH.
- Filter the enzyme solution using a cellulose acetate filter unit with a 0.45 μm pore size. Aliquot 25 mL of the enzyme solution into 50 mL conical tubes and keep at -20 °C. Thaw the enzyme solution at room temperature (RT) and mix well just before use.
- Prepare a 21% (w/v) sucrose solution by dissolving 21 g of sucrose in 100 mL of deionized water and autoclave the solution.
- Prepare W5 solution to contain 154 mM sodium chloride (NaCl), 125 mM CaCl2, 5 mM potassium chloride (KCl), 5 mM glucose, and 1.5 mM MES. Adjust the pH to 5.6 with KOH and autoclave the solution.
- Prepare MaMg solution to contain 400 mM D-mannitol, 15 mM magnesium chloride (MgCl2), and 5 mM MES. Adjust the pH to 5.6 with KOH and autoclave the solution.
- Prepare 1 M D-mannitol and 1 M calcium nitrate stock solutions to make the PEG solution. Autoclave these solutions.
- Prepare the enzyme solution to contain 0.25% (w/v) macerozyme, 1.0% (w/v) cellulase, 400 mM D-mannitol, 8 mM calcium chloride (CaCl2), 0.1% (w/v) bovine serum albumin (BSA), and 5 mM MES. Adjust the pH to 5.6 with KOH.
- Harvest intact leaf tissues from 2 weeks-old plants from ~ 1 – 2 B5 plates using a surgical knife and place the harvested leaves into a 50 mL conical tube containing 25 mL of enzyme solution. Place the conical tube containing the enzyme solution and leaf tissues horizontally on a rotary shaker with gentle agitation in the dark. It takes 8 – 12 h for full digestion of leaf tissues.
- At 8 – 12 h after incubation, pour the enzyme solution (containing protoplasts released from leaf tissues) into a fresh Petri dish through a mesh with 140 µm pore size. Carefully layer the protoplast-containing enzyme solution on top of 15 mL of 21% (w/v) sucrose solution and centrifuge it at 98 x g for 10 min with the lowest acceleration and deceleration settings in a swinging-bucket rotor.
- Using a Pasteur pipette, carefully transfer the protoplasts from the upper-most layer (enzyme solution) and at the interface between the enzyme solution and sucrose solution to a 50 mL conical tube containing 30 mL of W5 solution. Centrifuge this tube at 51 x g for 6 min. At this stage, protoplasts will be in the pellet at the bottom of the conical tube.
- Discard the supernatant carefully using a pipette without disturbing the protoplasts pellet. Add 25 mL of W5 solution, and gently resuspend the protoplasts. Incubate the protoplast solution in a 4 °C refrigerator for at least 1 h for stabilization.
4. Protoplast Transformation using Polyethylene Glycol
- Prepare a 40% PEG solution by adding 4 g of PEG 8000, 4 mL of 1 M mannitol solution, 1 mL of 1 M Ca(NO3)2, and 1.8 mL of distilled water into a 50 mL conical tube and mix well. Dissolve PEG by heating in a microwave oven for 20 – 30 s. Place the 40% PEG solution at RT for cooling down.
NOTE: The 40% PEG solution should be prepared freshly every time. If protoplasts are not pelleted completely during the 4 °C incubation in the refrigerator, centrifuge the material at 46 x g for 2 min. - Remove the supernatant carefully but completely and add MaMg solution to the protoplast pellet to yield the concentration of 5 x 106/mL.
NOTE: The number of protoplast can be determined by a hemocytometer viewed under a microscope. - Place 10 µg of the plasmid DNA each empty 13 mL round-bottom tube and add 300 µL of protoplast solution using a pipette.
NOTE: The end of the pipette tip should be cut off. Whenever protoplasts are sampled, the protoplast-containing solution should be resuspended right before pipetting so that the same number of protoplasts is added to each tube. - Mix the plasmid DNA with protoplasts by gently rotating the tubes and immediately add 300 µL of 40% PEG solution using a pipette. Mix gently but completely by rotating tubes and incubate for 30 min at room temperature; tilt the tube almost horizontally and rotate it several times.
- Add 1 mL of W5 solution and mix gently but completely by rotating the tube by hand in a similar manner. Incubate the sample for 10 min at room temperature.
- Repeat this step two times more using 1.5 mL and 2 mL of W5 solution, respectively. Incubate for 30 min after the final addition of W5 solution.
- Centrifuge at 46 x g for 4 min. Discard the supernatant and add 2 mL of W5 solution. Mix gently but completely. Incubate at 22 °C in a dark chamber.
5. Analysis of the Protein Import into Chloroplasts
NOTE: After PEG-mediated transformation of protoplasts, incubation time ranges from 8 to 24 h.
- Fluorescence Microscopy
- Place 10 µL of the protoplast solution on a slide glass using a pipette with a trimmed tip and carefully cover with a coverslip to avoid damaging the protoplasts.
- Place the slide on the stage of a fluorescence microscope equipped with excitation/emission filter sets for observing green fluorescent protein (GFP) and chlorophyll auto-fluorescence.
- Capture images with a cooled charge-coupled device (CCD) camera and process images using an image-editing software to produce pseudo-color images.
- Total Protein Extraction and Immunoblotting
- Prepare denaturation buffer containing 2.5% (w/v) sodium dodecylsulfate (SDS), and 2% (v/v) 2-mercaptoethanol.
NOTE: Protein import into chloroplasts can be quantified by measuring the degree of transit peptide processing via immunoblot analysis using anti-GFP antibody. - Transfer protoplasts into a centrifuge tube and centrifuge at 46 x g for 4 min.
- Remove the supernatant and add 80 µL of denaturation buffer. Vigorously vortex for 5 s and add 5x SDS sample buffer (250 mM Tris-Cl (pH 6.8), 0.5 M DTT, 10% (w/v) SDS, 0.05% (w/v) Bromophenol blue, and 50% (v/v) glycerol). Mix well and boil for 10 min.
- Subject this protein sample to standard SDS-PAGE and immunoblotting with anti-GFP antibody.
- Prepare denaturation buffer containing 2.5% (w/v) sodium dodecylsulfate (SDS), and 2% (v/v) 2-mercaptoethanol.
Representative Results
The import of proteins into chloroplasts can be examined using two approaches: fluorescence microscopy and immunoblot analysis after SDS-PAGE-mediated separation. Here, we used RbcS-nt:GFP, a fusion construct encoding the 79 N-terminal amino acid residues of RbcS containing the transit peptide fused to GFP. When proteins are imported into chloroplasts, green fluorescence signals from the target protein RbcS-nt:GFP should merge with the red fluorescent signals from chlorophyll auto-fluorescence, as examined by fluorescence microscopy (Figure 1). The close overlap of the two signals indicates protein import into chloroplasts. Often the GFP signals are spread throughout the chloroplasts or are concentrated at the center of chloroplasts, with weakly diffuse signals throughout the chloroplasts, depending on the individual protein. The import of proteins can be confirmed by immunoblot analysis using GFP antibody. Total proteins are prepared from protoplasts and separated by SDS-PAGE followed by Western blot analysis (Figure 2). In most cases, two protein bands should be observed in the immunoblot if a protein was properly imported into chloroplasts: the upper band corresponds to the full-length precursor and the lower band to the processed form after import into chloroplasts. The amount of the processed form of the protein should increase in a time-dependent manner. Such results would imply that the protein RbcS-nt:GFP is imported into chloroplasts in Arabidopsis protoplasts. Moreover, the ratio of the processed form to the total amount of expressed proteins (the processed form plus precursor) can be used as a measure of import efficiency. If necessary, the chloroplasts can be purified from gently lysed protoplasts, and proteins from the chloroplast fraction can be analyzed by western blotting to further confirm the import of proteins into chloroplasts.
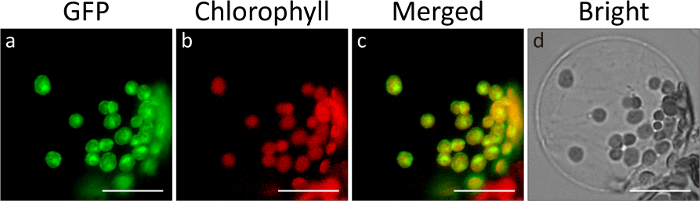
Figure 1. In vivo localization of GFP fused to the RbcS transit peptide to chloroplasts.
Images were taken 18 h after transformation under a fluorescence microscope. Green (a), red (b), merged (c), and bright (d) labels indicate GFP image, chlorophyll image, a merged image of the two signals, and bright field image, respectively. Scale bar = 20 µm. Please click here to view a larger version of this figure.
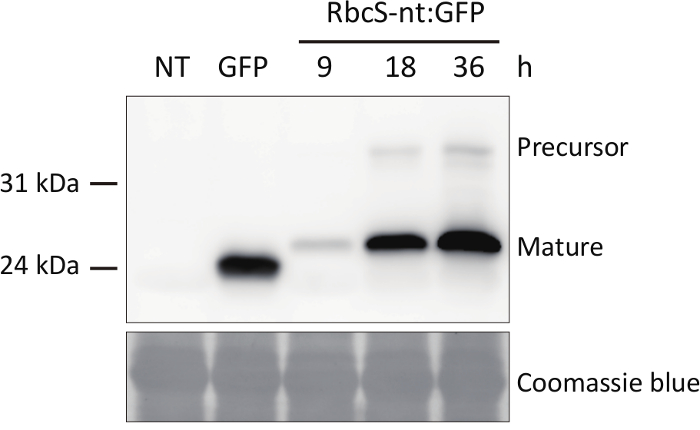
Figure 2. Western blot analysis of RbcS-nt:GFP to investigate protein import into chloroplasts.
Total protein extracts were prepared from protoplasts and separated by 10% (w/v) SDS-PAGE, followed by western blot analysis using anti-GFP antibody. Please click here to view a larger version of this figure.
Discussion
We provided a detailed protocol for the use of protoplasts of Arabidopsis to study protein import into chloroplasts. This method is powerful for investigating the protein import process. This simple, versatile technique is useful for examining the targeting of the intended cargo proteins to chloroplasts. Using this method, protoplasts are prepared from leaf tissues of Arabidopsis11,12 which can be obtained from plants at many different growth stages ranging from very early to fully mature leaves. However, care must be taken when growing plants used for protoplast preparation. One should use very healthy plants, as protoplasts prepared from healthy plants can withstand the many steps involved in PEG-mediated transformation. Another important precaution is to use fresh solutions. Slight changes in the concentrations of the solutions can greatly damage the protoplasts, since they are fragile and very sensitive to osmotic pressure.
We have been using protoplasts to study protein import into chloroplasts9,13,14. Based on these studies, we were able to dissect the sequence motifs in various transit peptides. In addition, we used protoplasts to identify the targeting signals (the positively charged region flanking the C-terminus of the transmembrane domain) of proteins targeted to the outer envelope membrane of the chloroplast15. Similarly, we used protoplasts to investigate protein import into the mitochondria10. Again, we were able to identify many critical sequence motifs in the presequences of mitochondrial proteins. In addition, the outer membrane proteins of the mitochondria also contain a positively charged region flanking the transmembrane domain as their targeting signal15. These targeting signals share a high degree of similarity in amino acid composition. Indeed, chloroplast proteins were mistargeted to mitochondria during in vitro import experiments16. However, chloroplast and mitochondrial proteins are specifically imported into their target organelles in vivo. Thus, protoplasts can be used to elucidate the mechanisms underlying how targeting specificity is determined between chloroplasts and mitochondria.
Protoplasts represent an ideal system for analyzing the import of proteins into chloroplasts in vivo. However, one caveat is that protoplasts may be under strong stress conditions such as wounding stress. Thus, we cannot rule out the possibility that such stress may affect the import process. Thus, in certain cases, the results should be interpreted with caution when protoplasts are used for protein import experiments.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was carried out with the supports of Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ010953012018), Rural Development Administration, and the National Research Foundation (Korea) grant funded by the Ministry of Science and ICT (No. 2016R1E1A1A02922014), Republic of Korea.
Materials
| GAMBORG B5 MEDIUM INCLUDING VITAMINS | Duchefa Biochemie | G0210.0050 | |
| SUCROSE | Duchefa Biochemie | S0809.5000 | |
| MES MONOHYDRATE | Duchefa Biochemie | M1503.0250 | |
| Agar, powder | JUNSEI | 24440S1201 | |
| Micropore Surgical tape | 3M | 1530-0 | |
| Surgical blade stainless No.10 | FEATHER | Unavailable | |
| Conical Tube, 50ml | SPL LIFE SCIENCES | 50050 | |
| Macerozyme R-10 | YAKULT PHARMACEUTICAL IND. | Unavailable | |
| Cellulase ONOZUKA R-10 | YAKULT PHARMACEUTICAL IND. | Unavailable | |
| ALBUMIN, BOVINE (BSA) | VWR | 0332-100G | |
| D-Mannitol | SIGMA | M1902-1KG | |
| CALCIUM CHLORIDE, DIHYDRATE | MP BIOMEDICALS | 0219463505-5KG | |
| Twister | VISION SCIENTIFIC | VS-96TW | |
| Screen cup for CD-1 | SIGMA | S1145 | |
| Screens for CD-1 | SIGMA | S3895 | |
| Petri Dish | SPL LIFE SCIENCES | 10090 | |
| Pasteur pipette | HILGENBERG | 3150102 | |
| LABORATORY CENTRIFUGE / BENCH-TOP | VISION SCIENTIFIC | VS-5500N | |
| Sodium chloride | JUNSEI | 19015S0350 | |
| Potassium chloride | SIGMA | P3911-1KG | |
| D-GLUCOSE, ANHYDROUS | BIO BASIC | GB0219 | |
| Potassium Hydroxide | DUKSAN | 40 | |
| Calcium nitrate tetrahydrate | SIGMA | C2786-500G | |
| Poly(ethylene glycol) | SIGMA | P2139-2KG | |
| Magnesium chloride hexahydrate | SIGMA | M2393-500G | |
| Tube 13ml, 100x16mm, PP | SARSTEDT | 55.515 | |
| Microscope slides | MARIENFELD | 1000412 | |
| Microscope Cover Glasses | MARIENFELD | 101030 | |
| Counting Chamber | MARIENFELD | 650030 | |
| Axioplan 2 Imaging Microscope | Carl Zeiss | Unavailable | |
| Micro tube 1.5ml | SARSTEDT | 72.690.001 | |
| 2-Mercaptoethanol | SIGMA | M3148-250ML | |
| Sodium Dodecyl Sulfate (SDS), Proteomics Grade | VWR | M107-500G | |
| TRIS, Ultra Pure Grade | VWR | 0497-5KG | |
| DTT (DL-Dithiothreitol), Biotechnology Grade | VWR | 0281-25G | |
| Bromophenol blue sodium salt ACS | VWR | 0312-50G | |
| Glycerol | JUNSEI | 27210S0350 | |
| Living Colors A.v. Monoclonal Antibody (JL-8) | TAKARA | 632381 |
Referências
- Jarvis, P., Lopez-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nature Reviews Molecular Cell Biology. 14 (12), 787-802 (2013).
- Neuhaus, H. E., Emes, M. J. Nonphotosynthetic Metabolism in Plastids. Annual Review of Plant Physiology and Plant Molecular Biology. 51, 111-140 (2000).
- Rolland, N., et al. The biosynthetic capacities of the plastids and integration between cytoplasmic and chloroplast processes. Annual Review of Genetics. 46, 233-264 (2012).
- Jarvis, P. Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytologist. 179 (2), 257-285 (2008).
- Li, H. M., Chiu, C. C. Protein Transport into Chloroplasts. Annual Review of Plant Biology. 61, 157-180 (2010).
- Keegstra, K., Cline, K. Protein import and routing systems of chloroplasts. Plant Cell. 11 (4), 557-570 (1999).
- Gasser, S. M., Daum, G., Schatz, G. Import of proteins into mitochondria. Energy-dependent uptake of precursors by isolated mitochondria. Journal of Biological Chemistry. 257 (21), 13034-13041 (1982).
- Smeekens, S., Bauerle, C., Hageman, J., Keegstra, K., Weisbeek, P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 46 (3), 365-375 (1986).
- Lee, D. W., et al. Arabidopsis nuclear-encoded plastid transit peptides contain multiple sequence subgroups with distinctive chloroplast-targeting sequence motifs. Plant Cell. 20 (6), 1603-1622 (2008).
- Lee, S., et al. Mitochondrial targeting of the Arabidopsis F1-ATPase gamma-subunit via multiple compensatory and synergistic presequence motifs. Plant Cell. 24 (12), 5037-5057 (2012).
- Jin, J. B., et al. A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell. 13 (7), 1511-1526 (2001).
- Lee, K. H., Kim, D. H., Lee, S. W., Kim, Z. H., Hwang, I. In vivo import experiments in protoplasts reveal the importance of the overall context but not specific amino acid residues of the transit peptide during import into chloroplasts. Molecules and Cells. 14 (3), 388-397 (2002).
- Lee, D. W., Lee, S., Oh, Y. J., Hwang, I. Multiple sequence motifs in the rubisco small subunit transit peptide independently contribute to Toc159-dependent import of proteins into chloroplasts. Plant Physiology. 151 (1), 129-141 (2009).
- Lee, D. W., Woo, S., Geem, K. R., Hwang, I. Sequence motifs in transit peptides act as independent functional units and can be transferred to new sequence contexts. Plant Physiology. 169 (1), 471-484 (2015).
- Lee, J., et al. Both the hydrophobicity and a positively charged region flanking the C-terminal region of the transmembrane domain of signal-anchored proteins play critical roles in determining their targeting specificity to the endoplasmic reticulum or endosymbiotic organelles in Arabidopsis cells. Plant Cell. 23 (4), 1588-1607 (2011).
- Cleary, S. P., et al. Isolated plant mitochondria import chloroplast precursor proteins in vitro with the same efficiency as chloroplasts. Journal of Biological Chemistry. 277 (7), 5562-5569 (2002).

