Construction of a Low-cost Mobile Incubator for Field and Laboratory Use
Summary
This paper describes a method for building an adaptable, low-cost and transportable incubator for microbial testing of drinking water. Our design is based on widely available materials and can operate under a range of field conditions, while still offering the advantages of higher-end laboratory-based models.
Abstract
Incubators are essential for a range of culture-based microbial methods, such as membrane filtration followed by cultivation for assessing drinking water quality. However, commercially available incubators are often costly, difficult to transport, not flexible in terms of volume, and/or poorly adapted to local field conditions where access to electricity is unreliable. The purpose of this study was to develop an adaptable, low-cost and transportable incubator that can be constructed using readily available components. The electronic core of the incubator was first developed. These components were then tested under a range of ambient temperature conditions (3.5 °C – 39 °C) using three types of incubator shells (polystyrene foam box, hard cooler box, and cardboard box covered with a survival blanket). The electronic core showed comparable performance to a standard laboratory incubator in terms of the time required to reach the set temperature, inner temperature stability and spatial dispersion, power consumption, and microbial growth. The incubator set-ups were also effective at moderate and low ambient temperatures (between 3.5 °C and 27 °C), and at high temperatures (39 °C) when the incubator set temperature was higher. This incubator prototype is low-cost (< 300 USD) and adaptable to a variety of materials and volumes. Its demountable structure makes it easy to transport. It can be used in both established laboratories with grid power or in remote settings powered by solar energy or a car battery. It is particularly useful as an equipment option for field laboratories in areas with limited access to resources for water quality monitoring.
Introduction
Culture-based methods for the detection of microbial contaminants are the state-of-the-art for water quality analysis in both industrialized and developing countries1,2. Microorganisms exist in many environments and require different temperature conditions for optimal growth. Therefore, creating a temperature-stable incubation environment is a precondition for the reliable detection of microbial contaminants of concern in drinking water. According to the World Health Organization, Escherichia coli (E. coli) (or alternatively, thermotolerant coliforms (TTC)) are the most suitable indicators of fecal contamination in drinking water3. Detection of these organisms consists of, for example, filtering a 100 mL water sample through a membrane followed by incubation of the membrane on selective media at 35 – 37 °C (E. coli) or 44 – 45 °C (TTC)3.
Field-based applications of culture-based methods have become increasingly relevant in recent years. Under Sustainable Development Goal 6, Target 6.1, governments have committed to regularly report bacteriological quality of drinking water at the national level4. In addition to such public health surveillance efforts, operational monitoring of water infrastructure is regularly undertaken at the local or regional level5. These surveillance and monitoring campaigns are often in remote locations where the required laboratory infrastructure is inadequate or unavailable.6 Similarly, culture-based methods are widely used in medical diagnosis and microbiological research where local clinics and research institutions may be challenged by limited resources and insecure power supplies7.
In the above contexts, conventional incubators are often inadequate or unavailable. As an alternative, field incubators have been specifically developed for use outside the laboratory, e.g., the Aquatest project8, University of Bristol, United Kingdom; DelAgua9, Marlborough, United Kingdom; or Aquagenx10, University of North Carolina, United States. However, these devices are relatively small in volume, thus limiting the number of samples that can be simultaneously processed. Field incubators on the market are also not designed to operate under very low (< 20 °C) or very high (> 40 °C) ambient temperature conditions, making their use in desert or alpine environments difficult. Further alternative solutions include yogurt-making appliances11, body belts, and phase-change incubators12. However, such unconventional incubators may function unreliably or be burdensome to operate11.
There is thus a need for an incubator that offers the advantages of laboratory-based models (ease of use, larger volume, and temperature precision) while remaining suitable for field applications (low-cost, easily transported and maintained, robustness to a range of ambient temperatures, energy efficient, and resilient to intermittent power supplies) (Table 1). The purpose of this protocol is to detail the fabrication process of a low-cost incubator designed to optimize the advantages of both conventional and field-based models using widely available material.
| Characteristic | Laboratory-based | Field | Optimized |
| User friendly design |  |
 |
 |
| Large capacity |  |
 |
 |
| Robust to wide range of ambient temperatures |  |
 |
 |
| Maintains constant temperature |  |
 |
 |
| Low cost |  |
 |
 |
| Easily transported |  |
 |
 |
| Energy efficient |  |
 |
 |
| Resilient to intermittent power supply |  |
 |
 |
Table 1: Characteristics of commercially available incubators (laboratory-based and field) and the optimized approach.
The following assembly protocol specifies the required materials and steps for building the incubator. It is structured in four steps: first, assembly of the heating unit; second, assembly of the control unit; third, assembly of the incubator electrical core; and fourth, assembly of the incubator. This protocol explains the construction of the electronic core of the incubator, that can work with a variety of incubator shells. See the Table of Materials for a full list of all components used in the Protocol and their technical specifications. The protocol below presents a functional example of the field incubator, but flexible use of different components is possible as long as they fulfill the electrical requirements. Using different components might influence the performances of the incubator. It is advised that the construction and wiring of electrical components be done by a person skilled in the electrical field.
Protocol
1. Heating Unit
- Gather the following components (Figure 1):
 Support plate (280 x 250 mm) with required anchorage holes
Support plate (280 x 250 mm) with required anchorage holes
 Axial fan (60 x 60 x 25 mm); 2x
Axial fan (60 x 60 x 25 mm); 2x
 Spacer (length 20 mm, internal diameter 4.25 mm (M4)); 4x
Spacer (length 20 mm, internal diameter 4.25 mm (M4)); 4x
 Luster terminal with three pins
Luster terminal with three pins
 Screw nut (M4); 4x and (M3); 1x
Screw nut (M4); 4x and (M3); 1x
 Washer (M4); 8x and (M3); 1x
Washer (M4); 8x and (M3); 1x
 Screw (M4); 4x and (M3); 1x
Screw (M4); 4x and (M3); 1x
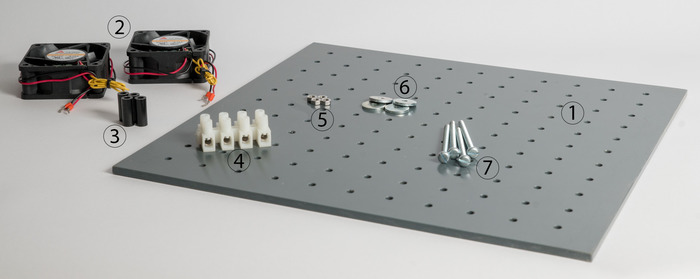
Figure 1: Individual components of the heating unit.  Support plate,
Support plate,  axial fans,
axial fans,  spacers,
spacers,  luster terminal,
luster terminal,  screw nuts,
screw nuts,  washers and
washers and  screws. Please click here to view a larger version of this figure.
screws. Please click here to view a larger version of this figure.
- Drill the required holes (Figure 2) into the support plate
 to secure the axial fans
to secure the axial fans  as well as the luster terminal
as well as the luster terminal  (Figure 1).
(Figure 1).
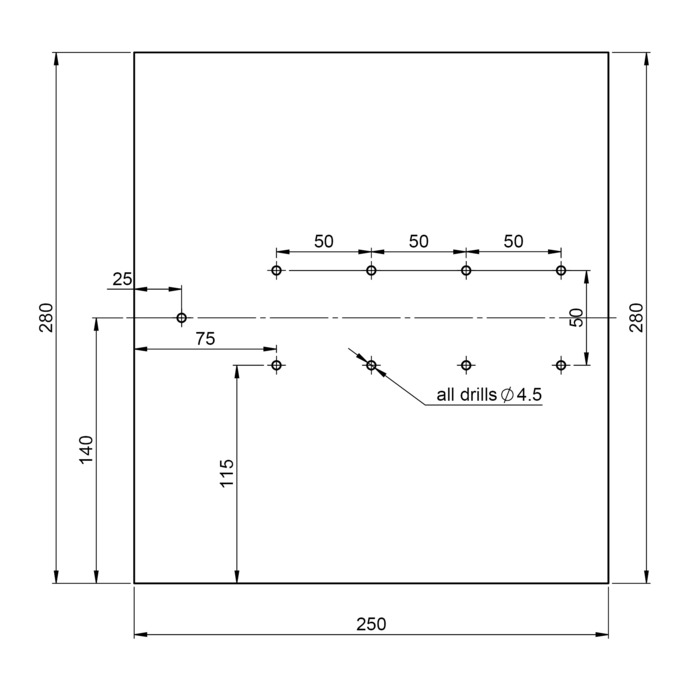
Figure 2: Schematic diagram of support plate. Indications to drill the anchorage holes into the support plate to fix the axial fans as well as the luster terminal. Distances are given in millimeters. Please click here to view a larger version of this figure.
- Anchor the axial fans
 in the center on the support plate
in the center on the support plate  as shown in Figure 3 with two M4 screws, screw nuts, and washers
as shown in Figure 3 with two M4 screws, screw nuts, and washers  ,
,  ,
,  per fan. Use the spacers
per fan. Use the spacers  to leave a distance between the fans and the support plate (Figure 3).
to leave a distance between the fans and the support plate (Figure 3). - Anchor the luster terminal
 to the support plate
to the support plate  using M3 screw, screw nut, and washer. Secure the cable fans. (Figure 3).
using M3 screw, screw nut, and washer. Secure the cable fans. (Figure 3). - Connect the fan cables with the luster terminal. Connect the positive cables of each fan together and the negative cables of each fan together (Figure 3). The speed sensor is not required.
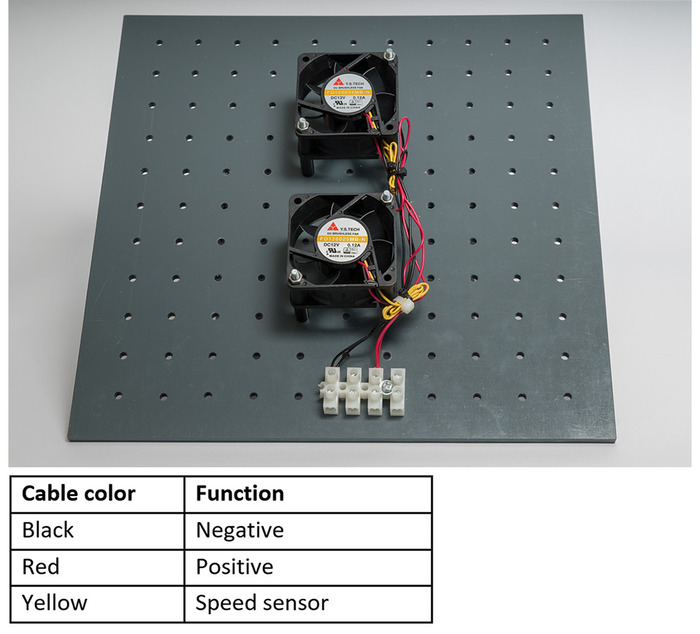
Figure 3: Axial fans fixed on support plate. Please click here to view a larger version of this figure.
NOTE: The cable colors mentioned correspond to the ones used in the figures. The cable colors might change depending on the material used.
2. Control Unit (Power Supply)
- Gather the following components:
 Universal enclosure (here 200 x 120 x 60 mm, but dimensions will depend on the size of the DC/DC converter and the PID temperature controller)
Universal enclosure (here 200 x 120 x 60 mm, but dimensions will depend on the size of the DC/DC converter and the PID temperature controller)
 On/off-Switch
On/off-Switch
 DC/DC converter, input voltage range 9 – 36V, output voltage 12V
DC/DC converter, input voltage range 9 – 36V, output voltage 12V
 PID temperature controller, 12 – 35 V/DC operating voltage
PID temperature controller, 12 – 35 V/DC operating voltage
 Cable gland, M12 x 15 mm, clamping range 2 – 7.5 mm (or according to the cable used)
Cable gland, M12 x 15 mm, clamping range 2 – 7.5 mm (or according to the cable used)
 Temperature sensor Pt100
Temperature sensor Pt100
 AC power supply
AC power supply
NOTE: The incubator can be connected to the mains power supply or to a battery. In the case of the mains operation, the AC power supply is required and if the unit is exclusively connected to mains, the DC/DC converter is not mandatory. In the case of battery operation, the DC/DC converter is highly recommended, and a two-wire cable is required instead of the AC power supply. This protocol presents the version with the DC/DC converter and the AC power supply. An electrical diagram of the incubator electrical core is detailed in the supplementary material (Figure S1). - Mill the openings for the PID temperature controller, on/off switch, and cable glands into the enclosure with a drill and jigsaw, or an equivalent tool (Figure 4).
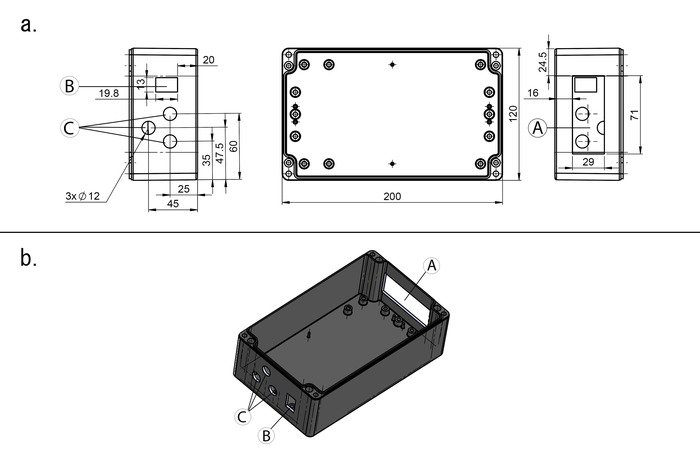
Figure 4: Schematic diagram of the universal enclosure. (a) Indications to place the temperature controller  , on/off switch
, on/off switch 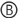 and the cable glands
and the cable glands  into the universal enclosure; distances are given in millimeters. (b) 3D view of the universal enclosure. Please click here to view a larger version of this figure.
into the universal enclosure; distances are given in millimeters. (b) 3D view of the universal enclosure. Please click here to view a larger version of this figure.
- Connect the DC/DC Converter to the on/off switch: connect the positive cable of the AC power adapter to the on/off switch and the negative cable of the AC power adapter to the "– Vin" of the DC/DC converter (Figure 5).
- Use a cable to connect the on/off switch to the "+ Vin" of the DC/DC converter (Figure 5).
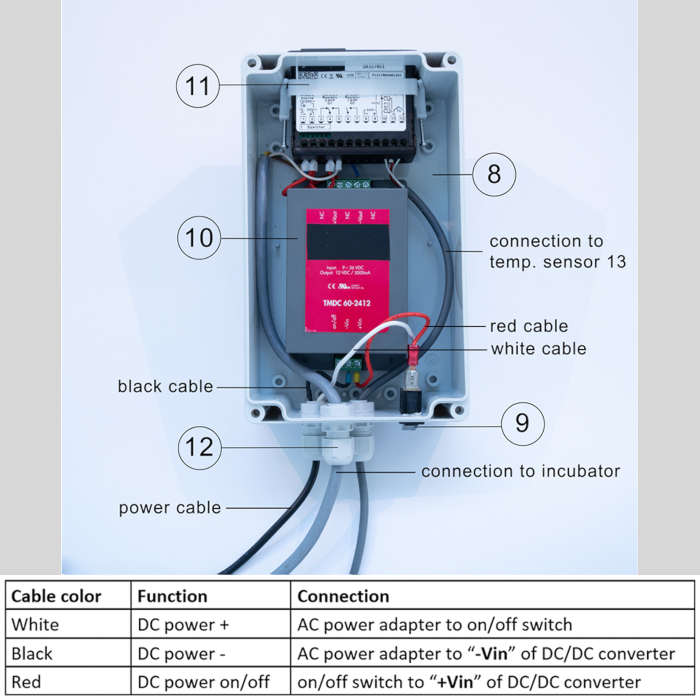
Figure 5: Mounted control unit. Universal enclosure  with DC/DC converter
with DC/DC converter  connected to PID temperature controller
connected to PID temperature controller  and on/off switch
and on/off switch  . Please click here to view a larger version of this figure.
. Please click here to view a larger version of this figure.
- Connect the cables from the heating unit to the PID temperature controller as follows (Figure 6):
- Connect the terminal "1" of the PID temperature controller to the “DC –“ wire from the heating unit connection and to the "– Vout" terminal of the DC/DC converter.
- Connect the “DC +” wire going to the heating unit to the terminal "4" of the PID temperature controller as well as to the terminal "2" of the PID temperature controller (see point 3.2).
- Connect the terminal "2" of the PID temperature controller to the "+ Vout" terminal of the DC/DC converter.
- Connect the terminal “5” of the PID temperature controller to the “command” wire going to the heating unit. (see point 3.2).
- Connect the temperature sensor to the terminals "10", "11" and "12".
NOTE: The red cable of the temperature sensor must be connected to terminal “11” of the PID temperature controller.
- Anchor the DC/DC converter with Velcro tape at the bottom of the enclosure, and close the universal enclosure.
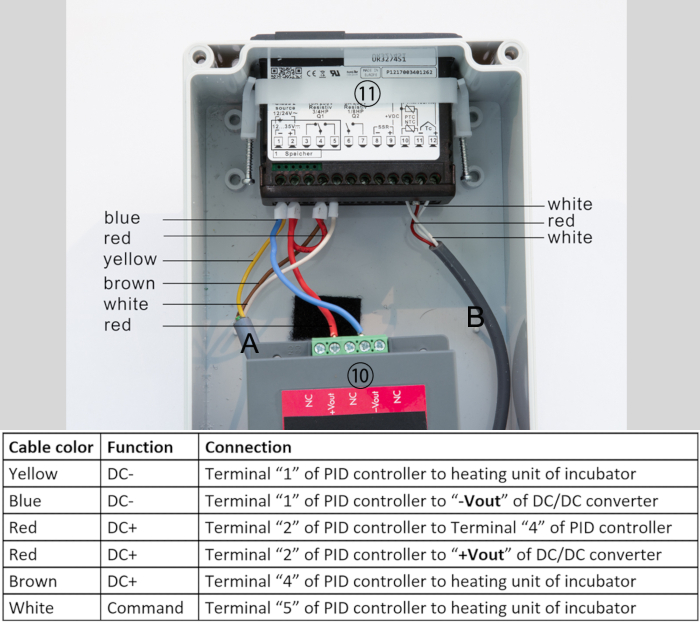
Figure 6: Cable connection of DC/DC converter with PID temperature controller. DC/DC converter  , PID temperature controller
, PID temperature controller  , connection to Incubator (cable A) and connection to temperature sensor (cable B). Please click here to view a larger version of this figure.
, connection to Incubator (cable A) and connection to temperature sensor (cable B). Please click here to view a larger version of this figure.
NOTE: The functions of the PID temperature controller terminals used are given in Table 2.
| PID temperature controller terminal | Function |
| Terminal "1" | Supply input + |
| Terminal "2" | Supply input – |
| Terminal "4" | Control Output Common Contact |
| Terminal "5" | Control Output Normally Open Contact |
Table 2: Functions corresponding to the PID temperature controller terminals.
3. Assembly of the Incubator Electrical Core
- Gather the following components:
Heating unit from section 1
Control unit from section 2
 Heating foils, self-adhesive, 100 x 200 mm, 12 V/20 W, 2x
Heating foils, self-adhesive, 100 x 200 mm, 12 V/20 W, 2x - Link the connection cables from the control unit to the heating unit as follows (Figure 7):
- Connect the “DC-” wire from the control unit with one conductor of each of the heating foils and the negative wire of each fan.
- Connect the “DC+” wire coming from the control unit with the positive cable of each fan.
- Connect the “command” wire from the control unit to the remaining two conductors of the heating foils.
Figure 7: Cable connection of heating foils  with PID temperature controller. Please click here to view a larger version of this figure.
with PID temperature controller. Please click here to view a larger version of this figure.
NOTE: The completed field incubator electrical core of the incubator is shown in Figure 8.
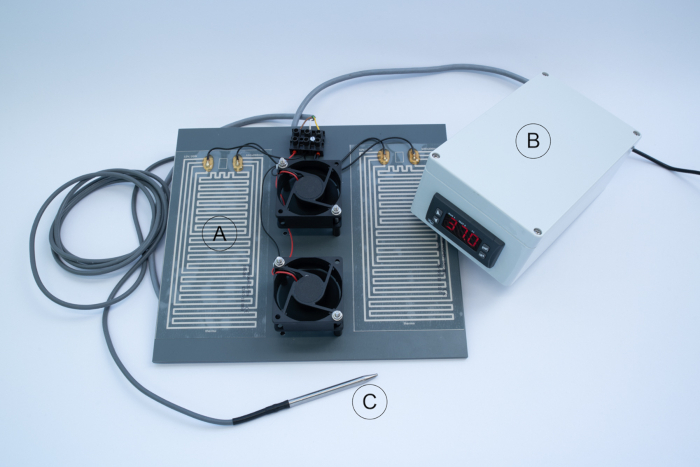
Figure 8: Complete field incubator electrical core. Heating unit  , control unit
, control unit 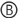 and temperature probe
and temperature probe  . Please click here to view a larger version of this figure.
. Please click here to view a larger version of this figure.
4. Assembly of the Incubator
- Gather the following components:
Incubator electrical core
Incubator shell (here a polystyrene foam box, but can be any type of box made of insulating material)
Support rack (here a metal rack, but can be another material) - Place the incubator components together as follows (Figure 9):
- Place the incubator shell on its side, so that the opening of the incubator (door) is located on a side.
- Place the support plate with the heating unit at the bottom of the incubator shell.
- Place the support rack on top of the heating unit, leaving a space of a minimum of 10 cm between the heating unit and the support rack.
- Place the temperature probe on the support rack and secure it in the incubator.
- Drill holes in the door of the incubator to allow for the entry of the cables (Figure 9).
- Connect the incubator to the power source.
- Turn the incubator on and adjust the settings of the PID temperature controller (see Table S1 in the supplementary material for detailed settings).
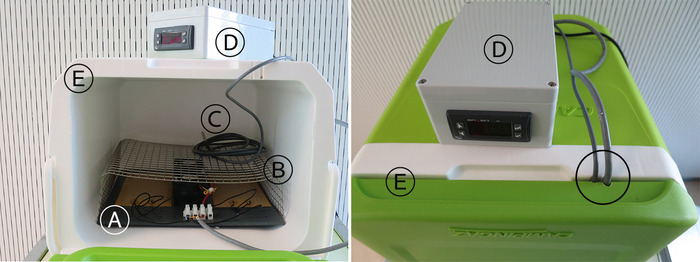
Figure 9: Complete field incubator. Open (left) and closed (right). Heating unit  , support rack
, support rack 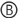 , temperature probe
, temperature probe  , control unit
, control unit  , incubator shell
, incubator shell  and holes for cables in the incubator shell (circled area). Please click here to view a larger version of this figure.
and holes for cables in the incubator shell (circled area). Please click here to view a larger version of this figure.
NOTE: The shell of the incubator can be a box of any type of material. It is recommended to use an insulating material, and that the box closes tightly to avoid dissipation of the heat. The support rack should contain large holes to avoid the accumulation of heat in the rack, and the material can be metal or other (e.g. plastic).
Representative Results
The reliability of a robust field incubator lies in its ability to reach and maintain a set temperature under various conditions. To monitor the performance of the various incubator set-ups, the following measurements were made: time needed to reach the set temperature, effect of opening the door for one minute, power consumption over 24 hours of operation, inner temperature stability over 24 hours of operation, and observation of E. coli growth. The temperature inside the incubator was measured every minute with 4 temperature logging devices placed in different positions in the structure (support rack, wall, top, inside a growth plate). The set temperature was considered to be attained when all measurements were within plus or minus 2 °C, which is the acceptable range for the incubation of E. coli.13
The electronic core was tested with three types of shells, using materials that are typically found in many countries: a polystyrene foam box (78 liters), a hard plastic cooler box (30 liters), and a cardboard box covered with a survival blanket (46 liters) (Figure 10). To cover a range of ambient conditions that can be experienced in the field, these incubator set-ups were tested at three ambient temperatures: ambient (about 27 °C), cold (about 3.5 °C and 7.5 °C) and hot (about 39 °C). Performance measures were tested setting the inner temperature at 37 °C and 44.5 °C.
The time to reach the set temperature in the incubators was influenced by the ambient temperature and the material of the incubator shell. At an ambient temperature of about 27 °C, the three incubators set-ups reached the set temperatures (37 °C and 44.5 °C) in a similar time (Figure 11a and Figure 12a) and comparable with the performance of a standard incubator (Table 3). In cold environments (3.5 °C and 7.5 °C), the incubators with thicker shells, i.e., the polystyrene foam and cooler box, reached the target set temperatures (37 °C and 44.5 °C) in a similar time; about four times longer than under an ambient temperature of 27 °C. With its lower insulation, the cardboard box with survival blanket never fully reached the set temperatures under cold ambient temperature conditions (Figure 11b and Figure 12b). In a warm environment (39 °C), the three incubator set-ups reached the target temperature of 44.5 °C in under 10 minutes (Figure 12c). However, when the set temperature was of 37 °C, i.e., lower than the ambient temperature, none of the incubators could lower the temperature, resulting in overheating for all three incubator set ups (Figure 11c).
The ambient temperature and the type of incubator shell influenced the impact of opening the door of the incubator for one minute. The heat loss was greater in the cold environment, and the time to recover the inner set temperatures was longer, with the exception of the cardboard box incubator where the set temperatures were never reached (Figure 13b and Figure 14b). In the warmer environments, the heat loss was limited and the set temperatures were recovered in under 10 minutes (Figure 13a,c and Figure 14a,c). In an ambient temperature of 39 °C and set temperature of 37 °C, opening the door did not cause nor reduce overheating of the incubators (Figure 13c).
The power consumption increased with cold environments and with an increase in the set temperature. Better insulating incubator shells (polystyrene foam and cooler box) showed a reduced power consumption as compared to the cardboard box incubator. In similar environments (ambient temperature of about 27 °C), the three incubator set-ups consumed 0.22 to 0.52 kWh/24h less energy than the standard incubators tested (Table 3).
The temperature in the incubator remained stable over 24 hours with all type of incubator shells and ambient temperature tested (Figure 13 and Figure 14). Slight variations of the measured temperature compared to the set temperature were observed according to the position of the temperature logging device in the incubator. With the exception of the tests with the ambient temperature (39 °C) warmer than the set temperature (37 °C) (Figure 13c), the variations in temperature were all within the 2 °C acceptable range for E. coli incubation.
All tests were performed in the presence of E. coli and total coliform measurement materials (membrane filter placed on growth plate). Replicates of a sample were placed in each incubator set-up and in a standard incubator for comparison. In all set-ups and conditions, the growth of E. coli and total coliform was successful and comparable to the growth observed in the standard incubator. A summary of the incubator configurations and ambient temperature conditions tested with results are shown in Table 3.
| Test 1: Time to set temperature |
Test 2: Side door opening one minute |
Test 3: Power consumption over 24-hour period |
Test 4: Temperature variation over 24-hour period |
Test 5: E. coli growth observed |
|||
| Ambient temperature | Set temperature | (min) | Maximal loss of temperature (°C); time to recover set temperature (min) | (kWh/24 h) | Absolute maximal temperature (°C); absolute minimal temperature (°C) * | (Yes / No) | |
| Polystyrene foam box | 3.5 °C | 37 °C | 45 | 10 °C; 17 min | 0.78 | 37; 35.5 | Yes |
| 7.5 °C | 44.5 °C | 74 | 16.5 °C; 31 min | 0.89 | 44.5; 42.5 | ND† | |
| 27 °C | 37 °C | 12 | 2.5 °C; 3 min | 0.28 | 37.5; 36.5 | Yes | |
| 44.5 °C | 20 | 4.5 °C; 7 min | 0.43 | 44.5; 43.5 | ND† | ||
| 39 °C | 37 °C | 0 (overheat) | 2 °C; 0 min (overheat) | 0.11 | 42.5; 42 | Yes | |
| 44.5 °C | 7 | 3.5 °C; 5 min | 0.17 | 45; 43.5 | ND† | ||
| Hard cooler box | 3.5 °C | 37 °C | 54 | 8 °C; 10 min | 0.86 | 37.5; 36 | Yes |
| 7.5 °C | 44.5 °C | 96 | 12 °C; 30 min | 1.05 | 45; 43 | ND† | |
| 27 °C | 37 °C | 13 | 1.5 °C; 0 min | 0.27 | 37.5; 36.5 | Yes | |
| 44.5 °C | 25 | 2 °C; 4 min | 0.50 | 45; 43.5 | ND† | ||
| 39 °C | 37 °C | 0 (overheat) | 1 °C; 0 min (overheat) | 0.11 | 43; 42.5 | Yes | |
| 44.5 °C | 9 | 4 °C; 3 min | 0.19 | 45.5; 44.5 | ND† | ||
| Cardboard box with survival blanket | 3.5 °C | 37 °C | Never reached (stable temperature after 109 min) | 6.5 °C; stable temperature after 30 min | 1.24 | 33.5; 30.5 | Yes |
| 7.5 °C | 44.5 °C | Never reached (stable temperature after 120 min) | 8 °C; stable temperature after 20 min | 1.28 | 36.5; 32 | ND† | |
| 27 °C | 37 °C | 15 | 2.5 °C; 6 min | 0.42 | 36.5; 35.5 | Yes | |
| 44.5 °C | 24 | 3 °C; 8 min | 0.70 | 44.5; 42.5 | ND† | ||
| 39 °C | 37 °C | 0 (overheat) | 1.5 °C; 0 min (overheat) | 0.11 | 41.5; 40 | Yes | |
| 44.5 °C | 9 | 2 °C; 0 min | 0.20 | 45; 43.5 | ND† | ||
| Standard incubator | 27 °C | 37 °C | 18 | 1 °C; 0 min (overheat) | 0.64 | 38.5; 36 | ND† |
| 44.5 °C | 23 (overheat) | 2.5 °C; 0 min | 0.95 | 47.5; 43.5 | ND† |
Table 3: Results summary for the incubator configurations and ambient temperature conditions tested. *Test 4: Absolute maximal and minimal temperatures recorded during the stable periods, i.e., from 10 minutes after the end of a disruptive event (time to reach set temperature, opening the door). †ND: No data, test not run.

Figure 10: Incubator shells tested. Open (upper row) and closed (bottom row). Polystyrene foam box (left), thickness of 3.5 cm, outer dimensions 39 x 56 x 36 cm; hard plastic cooler box (middle), thickness of 2.5 cm, outer dimensions 32 x 41 x 47 cm; cardboard box (right) covered with a standard survival blanket of a thickness of 12 µm folded twice, outer dimensions 30 x 42 x 37 cm. Please click here to view a larger version of this figure.
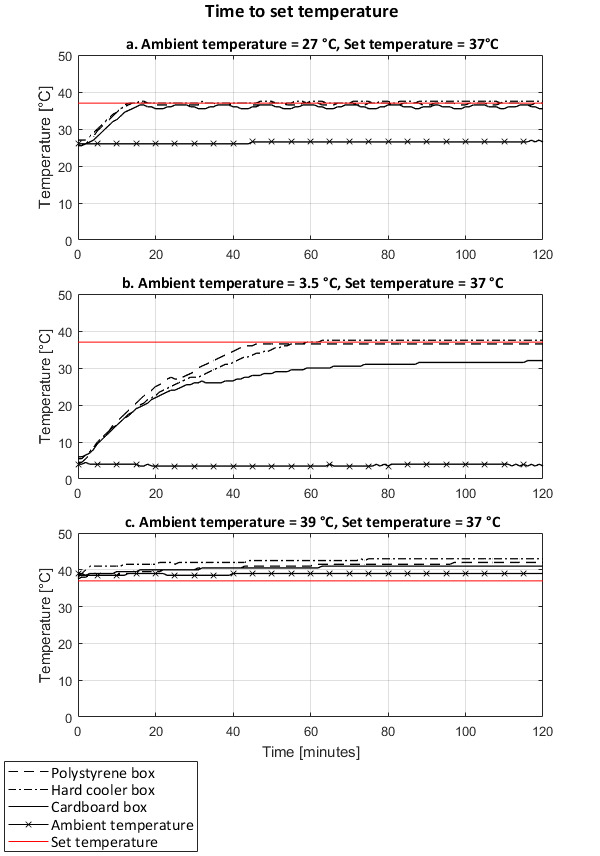
Figure 11: Time to reach set temperature (37 °C) of the incubator set-ups under different ambient temperature conditions. Performances of incubators with a shell made of a polystyrene foam box, a hard cooler box, and a cardboard box covered with a survival blanket. At room ambient temperature (a), cold ambient temperature (b), and warm ambient temperature (c). Temperatures recorded on the support rack of the incubators. Please click here to view a larger version of this figure.
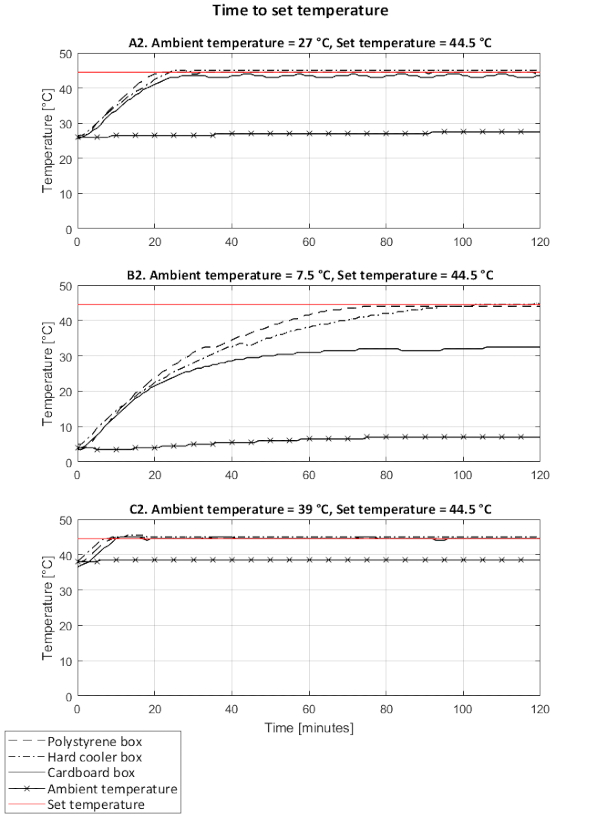
Figure 12: Time to reach set temperature (44.5 °C) of the incubator set-ups under different ambient temperature conditions. Performances of incubators with a shell made of a polystyrene foam box, a hard cooler box, and a cardboard box covered with a survival blanket. At room ambient temperature (a), cold ambient temperature (b), and warm ambient temperature (c). Temperatures recorded on the support rack of the incubators. Please click here to view a larger version of this figure.
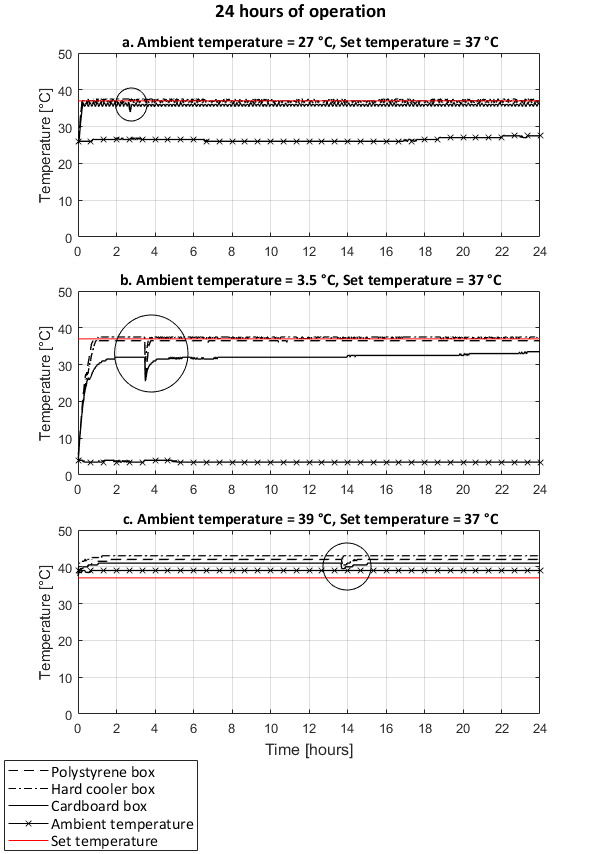
Figure 13: Temperature variations over 24-hour period and effect of door opening under different ambient temperature conditions. Set temperature of 37 °C. Performances of incubators with a shell made of a polystyrene foam box, a hard cooler box, and a cardboard box covered with a survival blanket. At room ambient temperature (a), cold ambient temperature (b), and warm ambient temperature (c). Circled areas show the temperature variations due to the door opening for one minute. Temperatures recorded on the support rack of the incubators. Please click here to view a larger version of this figure.
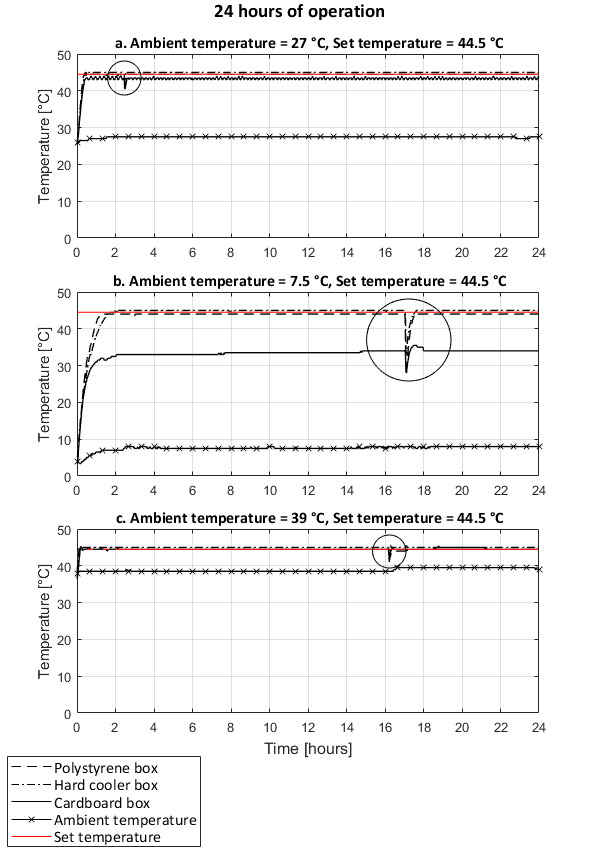
Figure 14: Temperature variations over 24-hour period and effect of door opening under different ambient temperature conditions. Set temperature of 44.5 °C. Performances of incubators with a shell made of a polystyrene foam box, a hard cooler box, and a cardboard box covered with a survival blanket. At room ambient temperature (a), cold ambient temperature (b), and warm ambient temperature (c). Circled areas show the temperature variations due to the door opening for one minute. Temperatures recorded on the support rack of the incubators. Please click here to view a larger version of this figure.
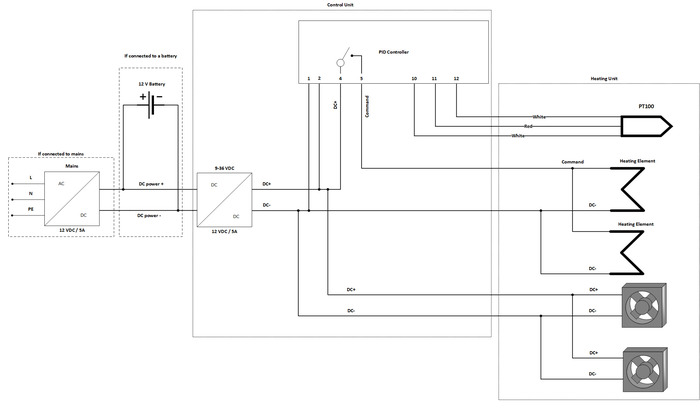
Figure S1: Electrical diagram of incubator electrical core wiring. Alternatives for mains operation and battery operation are indicated. Please click here to view a larger version of this figure.
| Parameter | Selected value | |||||
| 1 | Type of control output | Control Q1 / Alarm Q2 | ||||
| 2 | Type of connected sensor | Pt100 (-200 to 140°C) | ||||
| 3 | Lower limit selectable for setpoint value | 0 | ||||
| 4 | Upper limit selectable for setpoint value | 50 | ||||
| 5 | Type of control | Heating | ||||
| 6 | ON/OFF hysteresis or dead band for P.I.D. control | 0 | ||||
| 7 | Proportional bandwidth of the process expressed as units (°C if temperature) | 1 | ||||
| 8 | Integral time. Intertia of the process expressed as seconds | 80.0 | ||||
| 9 | Derivative time for P.I.D. | 20.0 | ||||
| 10 | Cycle time for time-proportioning output | 10 | ||||
| 11 | Allow/deny modification of setpoint values by frontal keyboard | Allow modification of all setpoints | ||||
| 12 | Software filter. Number of readings to calculate the comparison value PV-SPV | 10 | ||||
| 13 | Type of degree | °C | ||||
| 14 | Type of cooling liquid | Air | ||||
Table S1: PID temperature controller settings. Display of set values; other parameters not necessary to run the incubator were left to default values.
Discussion
Under Sustainable Development Goal 6.1, the demand for water quality sampling is increasing, especially in remote rural areas where monitoring practices are less established14. A major barrier to implementing regular water quality testing in these settings is poor access to laboratories capable of supporting microbial methods6. This paper presents a method for a reliable incubator constructed from materials that are relatively cheap and widely available. The electrical components are relatively easy to source and assemble, requiring only limited expertise. Moreover, the incubator shell design is flexible and therefore can be constructed from locally available materials. This is especially desirable for those traveling to remote locations, since luggage space is not needed for a heavy and bulky shell. Depending on the shell used, the volume of the incubator is also adaptable and can be sized to accommodate a specific sample size. The presented set-up can be used on- and off-grid, which makes it robust to power cuts or absence of reliable electrical supply. While certain design limitations were observed, this set-up up generally proved to be effective under a range of ambient temperature conditions (3.5 °C to 39 °C).
There are several steps in the protocol that are critical for achieving an incubator design suitable to one's needs. The first is the selection of the electrical components of the incubator. Alternative components can be chosen based on the price or the local availability. Depending on the material selected and their technical specifications, the incubator may have altered performances as compared to the results presented. Another critical step in the protocol is the choice of shell material, which should be made based on the expected range of ambient temperatures, local power supply, and availability of materials. At lower ambient temperatures (< 25 °C), a shell constructed of polystyrene foam or a hard cooler box is recommended to achieve a set temperature of 37 °C to 44.5 °C. Based on the experimental data presented, these set ups can be expected to reach the set temperature in 45 – 96 minutes and consume 0.78 – 1.05 kWh/24h in cold environments (3.5 to 7.5 °C). The cardboard box with survival blanket is not recommended for use at lower ambient temperatures since this set up never reached a stable set temperature during the experimental observation period. At moderate ambient temperature (27 °C) any of the shell types tested are acceptable, with similar to slightly greater power consumption observed for the cardboard box set up. At higher ambient temperatures (39 °C), the incubator designs presented here were prone to overheating if unless the set temperature was even higher (i.e., 44.5 °C). Therefore, such conditions would require a cooling device or use in a climate controlled space.
The cost of constructing the incubator presented here was about 300 USD when materials were sourced in Switzerland. However, these costs may be considerably lower in different locations, especially if shipping fees for the electronic core components can be kept to a minimum. Modification of the various components described in the protocol can further reduce costs. The protocol presented here is limited in that it compares only three shell material types at two set temperatures, as well as verification of microbial growth for E. coli only. Future research should test the suitability of this incubator design under a greater range of temperature parameters and using additional microbial indicator species (e.g., Enterococcus) and pathogens (e.g., salmonella, Vibrio cholerae). Future research should also focus on development of effective cooling techniques within the incubator, which would allow for its use in extremely warm environments (> 40 °C).
To our knowledge, there is no other known field incubator that offers adaptable volume capacity and is easily dismountable, while remaining transportable and low-cost. This innovative alternative to commercially available incubators fulfills a need for governments and organizations with water quality and other culture-based testing objectives where few laboratory facilities are available. When paired with simple water quality testing equipment, this incubator can help practitioners with limited capacities to establish permanent or seasonal laboratories at a reasonable cost. By increasing the number of laboratories in remote areas, efforts to conduct regular water quality surveillance or achieve punctual monitoring of system operations will become increasingly feasible.
Declarações
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Swiss Agency for Development Cooperation and the REACH programme funded by UK Aid from the UK Department for International Development (DFID) for the benefit of developing countries (Aries Code 201880). The views expressed and information contained in it are not necessarily those of or endorsed by these agencies, which can accept no responsibility for such views or information or for any reliance placed on them. The authors also thank Arnt Diener for his contributions to early iterations of the polystyrene foam incubator prototype.
Materials
| Heating foil | Thermo | 2115337 | Self-adhesive 10×20 cm; Operating voltage 12V; Power 20W |
| Axial fan | Yen Sun Technology Corp. | FD126025MB | 6x6x2.5 cm; Operating voltage 12VDC; Power 1.44W; Max. current consumption 60mA |
| PID Temperature Controller | Wachendorff Automation GmbH & Co. KG | UR3274S | PID controller 32×74 mm; Universal input for process signals, thermocouples, Pt100; Operating voltage 24 VDC; Outputs (thermostats) 10 A relay, 5 A relay, SSR, RS 485 |
| Temperature sensor Pt100 | Conrad | 198466 | Temperature range -100°C to 200°C; Sensor Pt100, Type FS-400P |
| Universal enclosure | OKW Gehäuse System | C2012201 | Dimensions 200 x 120 x 60 mm |
| ON/OFF Switch | SHIN CHIN INDUSTRIAL CO. | R13-70A-01 | Connection Type C CEE 7/16 plug 6.3 mm; Contact resistance Max 50 mΩ; Switching voltage 24 VDC; Switching current (mx.) 10A; Insulation resistance Min 100 MΩ/500 Vdc |
| DC/DC converter | Traco Power | TMDC 60-2412 | Nominal voltage 24 VDC; Input voltage 9-36 VDC; Output voltage 12 VDC; Max. output current 5 A; Power 60W |
| AC power adapter | Bicker Elektronik | BET-0612 | Output voltage 12 VDC; Max. output current 5 A; Input voltage 115-230 VAC |
| Spacer | Schäfer Elektromechanik | 20/4 | Without thread; Thread size M4; Polystyrene; Distance 20 mm |
| Cable gland | WISKA | 10066410 | M12 x 1.5 cm; clamping range 3 – 7 mm |
| Luster terminal | Adels Contact | 125312 | Nominal current 25 A; Nominal Voltage 500V |
| Screw M4 x 50 | Bossard | 1579010 | M4 x 50 mm |
| Screw nut M4 | Bossard | 1241478 | M4 |
| Washer M4 | Bossard | 1887505 | M4 |
| Screw M3 x 25 | Bossard | 1211099 | M3 x 25 mm |
| Screw nut M3 | Bossard | 1241443 | M3 |
| Washer M3 | Bossard | 1887483 | M3 |
| Support plate | - | - | Insulating material (plastic or other); 28 x 25 cm |
Referências
- Bain, R., et al. A summary catalogue of microbial drinking water tests for low and medium resource settings. International Journal of Environmental Research and Public Health. 9 (1609-1625), (2012).
- Köster, W., et al. Analytical methods for microbiological water quality testing. Assessing Microbial Safety of Drinking Water. , 237-277 (2003).
- World Health Organization (WHO). . Guidelines for Drinking Water Quality. , (2011).
- World Health Organization (WHO). . Safely Managed Drinking Water – Thematic Report on Drinking Water. , (2017).
- Peletz, R., Kumpel, E., Bonham, M., Rahman, Z., Khush, R. To what extent is drinking water tested in sub-Saharan Africa? A comparative analysis of regulated water quality monitoring. International Journal of Environmental Research and Public Health. 13 (3), 275 (2016).
- Diener, A., et al. Adaptable drinking-water laboratory unit for decentralised testing in remote and alpine regions. 40th WEDC International Conference. , 1-6 (2017).
- Malkin, R. A. Design of health care technologies for the developing world. Annual Review of Biomedical Engineering. 9 (1), 567-587 (2007).
- Rahman, Z., Khush, R., Gundry, S. Aquatest: Expanding Microbial Water Quality Testing for Drinking Water Management. Drinking Water Safety International. 1 (4), 15-17 (2010).
- DelAgua Water Testing Ltd. . DelAgua Portable Water Testing Kit: User Manual Version 5.0. , (2015).
- Aquagenx LLC. . Portable Incubator Fabrication Instructions. , (2015).
- Nair, J., Mathew, K., Ho, G. E. Experiences with implementing the H2S method for testing bacterial quality of drinking water in remote aboriginal communities in Australia. Water for all life: A decentralized infrastructure for a sustainable future. , (2007).
- Kandel, P., Kunwar, R., Lamichhane, P., Karki, S. Extent of fecal contamination of household drinking water in Nepal: Further analysis of Nepal Multiple Indicator Cluster Survey 2014. American Journal of Tropical Medicine and Hygiene. 96 (2), 446-448 (2017).
- Edberg, S. C., Rice, E. W., Karlin, R. J., Allen, M. J. Escherichia coli: the best biological drinking water indicator for public health protection. Journal of Applied Microbiology. 88 (51), 1065-1165 (2000).
- Taylor, D. D. J., Khush, R., Peletz, R., Kumpel, E. Efficacy of microbial sampling recommendations and practices in sub-Saharan Africa. Water Research. 134, 115-125 (2018).

