Affinity Purification of Chloroplast Translocon Protein Complexes Using the TAP Tag
Summary
We here present a proven and tested protocol for the purification of chloroplast protein import complexes (TOC-TIC complex) using the TAP-tag. The one-step affinity-isolation protocol can potentially be applied to any protein and be used to identify new interaction partners by mass spectrometry.
Abstract
Chloroplast biogenesis requires the import of thousands of nucleus-encoded proteins into the plastid. The import of these proteins depends on the translocon at the outer (TOC) and inner (TIC) chloroplast membranes. The TOC and TIC complexes are multimeric and probably contain yet unknown components. One of the main goals in the field is to establish the complete inventory of TOC and TIC components. For the isolation of TOC-TIC complexes and the identification of new components, the preprotein receptor TOC159 has been modified N-terminally by the addition of the tandem affinity purification (TAP) tag resulting in TAP-TOC159. The TAP-tag is designed for two sequential affinity purification steps (hence “tandem affinity”). The TAP-tag used in these studies consists of a N-terminal IgG-binding domain derived from Staphylococcus aureus Protein A (ProtA) followed by a calmodulin-binding peptide (CBP). Between these two affinity tags, a tobacco etch virus (TEV) protease cleavage site has been included. Therefore, TEV protease can be used for gentle elution of TOC159-containing complexes after binding to IgG beads. In the protocol presented here, the second Calmodulin-affinity purification step was omitted. The purification protocol starts with the preparation and solubilization of total cellular membranes. After the detergent-treatment, the solubilized membrane proteins are incubated with IgG beads for the immunoisolation of TAP-TOC159-containing complexes. Upon binding and extensive washing, TAP-TOC159 containing complexes are cleaved and released from the IgG beads using the TEV protease whereby the S. aureus IgG-binding domain is removed. Western blotting of the isolated TOC159-containing complexes can be used to confirm the presence of known or suspected TOC and TIC proteins. More importantly, the TOC159-containing complexes have been used successfully to identify new components of the TOC and TIC complexes by mass spectrometry. The protocol that we present potentially allows the efficient isolation of any membrane-bound protein complex to be used for the identification of yet unknown components by mass spectrometry.
Introduction
Plants depend on chloroplasts for photosynthesis and photoautotrophic growth1. The vast majority of chloroplast proteins are encoded in the nucleus, synthesized in the cytosol and imported into the chloroplast via the TIC and TOC complexes in the envelope membranes2. The core of the TOC complex consists of the Toc75 protein-conducting channel and the two receptor GTPases Toc33 and Toc1593,4,5. TOC159 is essential for the biogenesis of chloroplasts and mediates the massive accumulation of photosynthesis-associated proteins6. The TIC complex consists of the TIC20 protein-conducting channel as well as TIC110 and TIC407,8. Recently, a 1MD protein translocation complex at the inner envelope membrane was isolated and contained previously unidentified components9, one of which was TIC56. We recently co-isolated TIC56 of the 1 MDa complex using the TAP-TOC159 affinity purification protocol. The data indicated a structural overlap between the "canonical" TOC/TIC complexes and the 1 MDa complex10. Previously unknown components such as KOC1 were also identified as new interaction partners of the TOC and TIC complexes using the TAP-method described here10,11.
Thus, the TAP-tag purification is an efficient method for the isolation of protein complexes and the identification of interacting partners by subsequent mass spectrometric analysis12. The TAP tag consists of two IgG binding repeats separated from a calmodulin-binding peptide (CBP) by a tobacco etch virus (TEV) protease cleavage site. The original method, consisting of an IgG-affinity purification step followed by TEV cleavage and subsequent calmodulin-affinity chromatography permitted the native purification of large and highly pure protein complexes13,14. We have simplified the procedure and demonstrated that the TOC159-containing protein complexes can also be purified efficiently using only the IgG-affinity purification step followed by TEV-cleavage for elution15. In our experience, the omission of the calmodulin-affinity step resulted in higher yields and may therefore be appropriate for low abundance proteins.
In short, we engineered stable transgenic A. thaliana lines expressing TAP-TOC159 in the ppi2 (toc159 KO mutant) background and established it as a reliable source for the purification of TOC-TIC complexes. The protocol for the isolation of the TOC-TIC complex starts with the homogenization of the plant material in a detergent-free buffer. After centrifugation, the supernatant is discarded. The pellet containing the total membrane fraction is solubilized using a detergent-containing buffer. After an ultra-centrifugation step, the supernatant containing solubilized TOC-TIC complexes is applied to the IgG-resin for affinity purification. After several washing steps, elution is carried out using a TEV protease-containing buffer to selectively cleave downstream of the IgG-binding domains and gently release native TOC159-containing complexes. The TEV eluate can be analyzed directly by Western blotting or mass spectrometry to identify the interaction partners of TOC15910,11,15. The method has also been used to identify post-translational modifications of TOC15915. In the future, native TOC159-containing complexes may be used for structural studies using cryoelectron microscopy.
Protocol
1. Preparation of Arabidopsis Plants
- Prepare 1 L of half strength of Murashige and Skoog (MS) medium including vitamins with 1% sucrose and adjust the pH to 5.7 with potassium hydroxide (KOH). Add 0.8% of phytoagar and autoclave for 20 min at 120 °C.
- Pour 75 mL of MS medium per Petri plate (diameter 14.5 cm, height 3 cm) and allow the solidification for 1 h.
- Add 30 mg of Arabidopsis thaliana seeds to a 1.5 mL microfuge tube, and surface sterilize with 70% (v/v) ethanol containing 0.05% (v/v) Triton X-100 for 5 min, followed by 100% (v/v) ethanol for 10 min. Remove the ethanol.
- Transfer the seeds to sterile filter paper for drying. Sprinkle the sterilized seeds evenly onto a MS plate (each genotype requires at least 8-10 plates), and seal each plate with surgical tape.
- Incubate the plates at 4 °C for two days in the dark to synchronize seed germination. Transfer to a growth chamber with the following light cycle: 8 h of 120 µmol m−2 s−1 light, and 16 h of dark at 22-20 °C.
2. Preparation of HsIgG Agarose Beads
- Suspend CNBr-activated agarose beads (4 g) in 100 mL of 1 mM HCl and incubate for 30 min at room temperature.
- Transfer the agarose beads into a sintered glass filter, wash under vacuum with 100 mL of 100 mM HCl and repeat 2-3 times.
NOTE: Precool the wash solution to 4 °C. - Resuspend the agarose beads in 1 mL of cold 100 mM HCl and transfer to a 50 mL conical centrifuge tube. Wash the sintered glass filter with 3 mL of 100 mM HCl and transfer the liquid to the same tube.
- Spin at 400 × g for 5 min at 4 °C and remove the supernatant with a pipette.
NOTE: Do not decant the tube. - Resuspend the beads in 50 mL of coupling buffer (Table 1); mix briefly, and then spin at 400 × g for 5 min at 4 °C.
- Dissolve 50 mg of lyophilized Homo sapiens IgG (Hs-IgG) in 10 mL of coupling buffer, mix the agarose beads gently, and incubate in rotary shaker overnight at 4 °C.
- Spin at 400 × g for 5 min at 4 °C and remove the supernatant with a pipette.
- Wash the IgG-agarose beads with 50 mL of coupling buffer and spin at 400 × g for 5 min at 4°C.
- Resuspend the IgG-agarose beads with 50 mL of blocking buffer (Table 1) and spin at 400 × g for 5 min at 4 °C. Repeat once.
- Resuspend IgG-agarose beads in 50 mL of blocking buffer, rotate the mixture for 2 h at RT and spin at 400 × g for 5 min at 4 °C.
- Remove the supernatant and resuspend the IgG-agarose beads in 200 mL of NaCl coupling buffer (Table 1).
- To block any remaining cross-linking CNBr groups, wash the IgG-agarose beads with 100 mL of 0.1 M glycine-HCl, pH 2.8. Spin at 400 × g for 5 min at 4 °C and remove the supernatant. Wash with 0.2 M glycine-HCl, pH 2.8, spin at 400 × g for 5 min at 4 °C and remove the supernatant. Finally, wash the IgG-agarose beads with 200 mL of ultrapure water.
- Wash the IgG-agarose beads with 200 mL of ultrapure water and then 200 mL of PBS buffer (Table 1).
- Resuspend the IgG-agarose beads in 20 mL of PBS buffer with 0.01% NaN3 (sodium azide) and store at 4 °C.
3. Isolation and Solubilization of the Membrane Fraction
- Grind 10 g of three weeks old A. thaliana seedlings in liquid nitrogen using a cold mortar and pestle with sand.
- Add 20 mL of cold grinding buffer (Table 1) to 10 g of ground tissue, mix well, and thaw on ice.
- Filter the homogenate through two layers of quick filtration material into a 50mL conical centrifuge tube. Soak the quick filtration material with grinding buffer before filtration.
- Centrifuge the filtrate for 10 min at 1,500 × g at 4 °C and transfer the supernatant to a chilled 50 mL conical centrifuge tube, repeat the same step one more time and collect the supernatant. Retain a 200 µL sample of the "total fraction" and store at -80 °C for later analysis
- Transfer the supernatant to cold 38.50 mL ultracentrifuge tubes and top up to 35 mL with cold grinding buffer. Centrifuge for 1 h at 100,000 × g at 4 °C in an ultracentrifuge.
- Transfer the supernatant to a 50 mL conical centrifuge tube. Retain a 200 µL sample of the "soluble fraction" and store at -80 °C for later analysis.
- Re-suspend the green pellet using a glass teflon homogenizer, in grinding buffer. Centrifuge for 1 h at 100,000 × g at 4 °C in an ultracentrifuge and discard the supernatant.
- Resuspend the green pellet in 18.75 mL of 1x grinding Buffer using a glass teflon homogenizer and add 9.375 mL of 1x grinding buffer.
- Add 9.375 mL of 4x solubilisation solution (Table 1) (containing the TX-100 detergent) to give 37.5 mL and incubate for 30 min on a "rotating shaker" at 4 °C.
- Centrifuge for 1 h at 100,000 × g at 4 °C in an ultracentrifuge. Transfer the supernatant to a 50 mL conical centrifuge tube. Retain a 200 µL sample of the supernatant (future "load fraction") and store at -80 °C for later analysis.
- Resuspend the pellet in 37.5 mL of grinding buffer. Retain the sample of insoluble part and store at -80 °C
4. Immunoprecipitation
- Equilibrate 100 µL of packed IgG-agarose resin in Buffer A (Table 1) and spin at 100 × g for 5 min at 4 °C.
- Remove the supernatant and resuspend the IgG-agarose resin in 100 µL of Buffer A at 4 °C.
- Transfer the washed IgG-agarose resin to the 37.5 mL of solubilized membranes and incubate overnight on a rotating shaker in a 50 mL conical centrifuge tube at 4 °C.
- Sediment the IgG-agarose resin at 100 × g for 5 min at 4 °C and remove the supernatant with a pipette. Retain a 200 µL sample of the "flow through" and store at -80 °C for later analysis.
- Wash the IgG-agarose resin in 37.5 mL of Buffer A and incubate for 10 min on a rotating shaker.
- Sediment the IgG-agarose resin at 100 × g for 5 min at 4 °C, remove the supernatant with a pipette, add 5 mL of grinding Buffer A to a 15 mL centrifuge tube and centrifuge at 100 × g, 4 °C, 5 min. Retain a 200 µL sample of the "Wash 1" and store at -80 °C for later analysis.
- Repeat the 5 mL washing step three times with Buffer A.
- Carry out the last two washing steps without the inhibitors (NaF, Protease inhibitor and PMSF)
NOTE: Inhibitors are no longer required at this stage and omitted to reduce cost. Retain a 200 µL sample of the last washing step "final wash (W6)" and store at -80 °C for later analysis. - Transfer the IgG-agarose resin to a 500 µL spin column with a 35 µm filter and wash the beads twice with 300 µL of TEV elution buffer (Table 1) (not containing AcTEV) for equilibration. Close the spin column.
- Add 300 µL of TEV elution buffer containing 50 units (10 µL) of AcTEV. Prepare the mix in a tube before adding to the resin.
- Incubate the spin column with beads in a thermomixer at 16 °C, 350 rpm for 2 h. Invert the column gently several times every 30 min.
- Open the spin column, place it in a new clean 1.5 mL tube and spin at 100 × g for 5 min at 4 °C to collect the eluate.
- Dry (for instance) 10% (v/v) of the eluted sample (~25 µL) in a centrifugal evaporator and use for mass spectrometry or other further analysis.
- Aliquot the remaining eluate (~275 µL) divided in to eleven 1.5 mL centrifuge tubes (25 µL each), dry in a centrifugal evaporator, and store at -80 °C.
- Analyze the samples retained throughout the procedure as well as the eluates by standard SDS-PAGE followed by immunoblotting. For sample preparation, precipitate 50 µg of Total (T), Load (L), Flow through (FT), Wash 1 (W1) and 200 µL of Wash 6 (W6) by chloroform/methanol extraction16.
- Add 10 µL of 2x SDS-PAGE loading buffer (Table 1) to the precipitated samples and 25 µL of eluted dried sample. Vortex and boil for 10 min at 95 °C in a heat block.
- Analyze Total (T), Load (L), Flow through (FT), Wash 1 (W1), Wash 6 (W6) and Elution (E) by immunoblotting using anti-TOC159 antibodies to determine the efficiency of the isolation procedure.
Representative Results
We here described a protocol for the purification of a TAP-tagged chloroplast envelope protein complex from transgenic A. thaliana plants. As shown in Figure 1A, plants expressing the 35S:NTAP-TOC159 construct were used to isolate this complex while plants expressing the 35S:NTAP construct were used as a control. Figure 1B shows detailed steps of the purification of TAP-TOC159 protein complexes followed by mass spectrometry to allow the identification of interacting proteins. The immunoblot analysis (Figure 2, right side) confirms that isolated TAP-TOC159 interacts with TOC75 and TOC33 of the TOC complex. The chloroplast outer membrane kinase KOC1 known to phosphorylate TOC159 also co-isolated with the TAP-TOC159 complex (Figure 2B, right side). The presence of TIC110 reveals that the isolated TAP-TOC159 complex also contains the components of the TIC complex. The FBN1A antibody raised against a plastoglobule protein unrelated to protein import did not recognize the TAP-TOC159 complex indicating the absence of contaminations. In the negative control, immunoprecipitated NTAP protein did not co-isolate with any of the TOC-TIC proteins (Figure 2, left side) and confirms the specificity of the TAP-TOC159 purification.
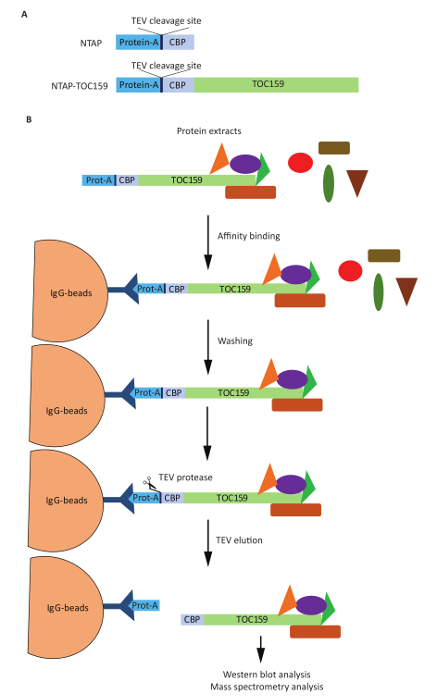
Figure 1. Scheme of the constructs and affinity purification procedure. (A). The 35S:NTAP-TOC159 construct, encoding NTAP-TOC159 was stably expressed in Arabidopsis thaliana. Negative control plants expressed the 35S:NTAP construct, encoding the TAP-tag alone. (B). The step wise affinity purification protocol consists of membrane isolation and solubilization, IgG agarose immuno-purification, washes, TEV protease cleavage and elution. Please click here to view a larger version of this figure.
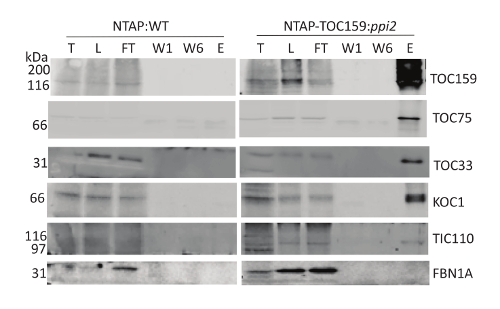
Figure 2. Tandem affinity purification of the TOC159 protein. N-terminally TAP-tagged TOC159 was purified from NTAP-TOC15:ppi2 Arabidopsis plants and TAP protein purified from TAP:WT plants was used as a negative control. Total protein extracts (T), solubilized membrane proteins = load fraction (L), flow through (FT), first and last wash fractions (W1 and W6) and 10% of the TEV eluate were analyzed by Western blotting. The membrane was probed with the antibodies against TOC159 (AT4G02510), TOC75 (AT3G46740), TOC33 (AT1G02280), KOC1 (AT4G32250), TIC110 (AT1G06950), and FBN1A (AT4G04020). Please click here to view a larger version of this figure.
| Table of buffers | ||
| Coupling Buffer | NaHCO3 Adjust to pH 8.5 adjust pH with 0.1 M Na2CO3 |
100 mM |
| Blocking Buffer | Tris–HCl Adjust to pH 8 with HCl. |
100 mM |
| PBS Buffer | Na2HPO4 KH2PO4 NaCl KCl Adjust to pH 7.3 with HCl. |
4.3 mM 1.4 mM 137 2.7 |
| NaCl coupling Buffer | Coupling buffer NaCl |
1 M |
| 2X grinding Buffer | Tris-HCl, pH 7.5 with HCl. NaCl NaF PMSF Plant protease inhibitor cocktail |
100 mM 200 mM 5 mM 1 mM 0.20% |
| 4X solubilisation solution | Glycerol Triton-X100 |
40% 3% |
| Buffer A | 2X grinding Buffer 1X grinding Buffer 4X solubilisation solution |
100 ml 50 ml 25 ml 25% |
| TEV elution Buffer | Tris-HCl, pH 8 with HCl EDTA, pH 8 with HCl NaCl Glycerol Triton-X100 DTT |
50 mM 0.5 mM 100 mM 10% 0.75% 1 mM |
| 2x SDS-PAGE loading Buffer | Tris-HCl pH 6.8 with HCl SDS Glycerol Bromophenol blue DTT |
0.1 M 4% 20% 0.20% 0.2 M |
Table 1. Buffer recipes.
Discussion
We demonstrated that a single step TAP tag purification is a specific and efficient method for the purification of the Arabidopsis TOC-TIC complex. Although the TAP-tag would allow two successive purification steps (IgG- followed by calmodulin affinity purification after TEV protease-cleavage), we believe that in many cases the first affinity step followed by TEV protease cleavage will be sufficient. Our target, TOC159, is low abundant and the inclusion of the second calmodulin-affinity step excessively diminished the protein yield which was the main reason for excluding it from our present protocol. The TEV-elution in itself is a highly specific and gentle purification step that will only release the TAP-tagged target together with associated interaction partners while contaminating the proteins sticking non-specifically to the IgG resin will remain there. Thus, in combination with the IgG-affinity step, the TEV elution results in a sufficiently efficient purification for our and probably many others' purposes.
Care must be taken that the TAP-tagged construct is fully functional in vivo. TAP-tagging could have potentially deleterious effects on protein activity, stability or localization. TOC159 consists of three domains, the N-terminal acidic domain, the central GTP-binding domain and C-terminal membrane domain, that anchors TOC159 in the outer chloroplast membrane2. To avoid potential interference with membrane insertion, we fused the TAP-tag to the N-terminus of TOC159. Fusion to the N-terminus is rather the exception than the rule. Many proteins contain N-terminal targeting information and should therefore by tagged at the C-terminus. We assured that TOC159 is functional in vivo by complementation of the ppi2 mutant (an albino mutant lacking TOC159) with TAP-TOC159. The rescue of the green, wild type phenotype indicated that TAP-TOC159 was functional15.
The purification protocol was used to identify new interaction partners of TOC159, that included KOC1 and TIC5610,11. In total, around 30 proteins were associated with the complex suggesting that new interaction partners will be isolated and characterized in the near future. While we highly recommend the TAP-tag for complex purification, we would still like to point out that additional versions to the one presented here exist and may be very useful for some applications.
Declarações
The authors have nothing to disclose.
Acknowledgements
The work was supported by grants from the Swiss National Science Foundation (31003A_156998 and 31003A_176191) and by the University of Neuchâtel.
Materials
| NaHCO3 | PanReac AppliChem | A0384 | |
| Tris | Biosolve | 0020092391BS | |
| Na2HPO4 | Sigma | S7909 | |
| KH2PO4 | PanReac AppliChem | A2946 | |
| NaCl | PanReac AppliChem | A2942 | |
| NaF | Sigma | S1509 | Toxic |
| PMSF | PanReac AppliChem | A0999 | Toxic |
| Plant protease inhibitor cocktail | Sigma | P9599 | |
| Glycerol | PanReac AppliChem | A0970 | |
| Triton X100 | Roche | 10789704001 | |
| Glycine | PanReac AppliChem | A1067 | |
| EDTA | Carl Roth | 8043 | |
| Dithiothreitol (DTT) | PanReac AppliChem | A1101 | |
| SDS | PanReac AppliChem | A0675 | |
| Bromophenol blue | Sigma | B0126 | |
| HCl | VWR | 20252-290 | Corrosive |
| Sucrose | PanReac AppliChem | A3935 | |
| Murashige and Skoog medium with vitamins | Duchefa Biochemie | M0222 | |
| Phytoagar | Duchefa Biochemie | P1003 | |
| Petri plate | Greiner Bio-one | 639102 | |
| 1.5 mL microfuge tubes | Sarstedt | 72.706 | |
| Ethanol | Fisher chemical | E/0600DF/15 | |
| Filter paper | Whatman | 10311804 | |
| Surgical tape | 3M | 1530-1 | |
| CNBr-Activated agarose beads (sepharose 4B) | GE Healthcare | 17-0430-01 | |
| Sintered glass filter | DURAN | 2515703 | |
| Centrifuge tube 50 mL | TPP | 91050 | |
| Purified immunoglobulin G (HsIgG) | MP Biomedicals | 855908 | |
| Rotating shaker | IKA | 4016000 | |
| NaN3 (sodium azide) | Sigma | 71290 | Toxic |
| Quick filtration material (Miracloth) | Merk-Millipore | 475855 | |
| Ultracentrifube XNP-80 | Beckman Coulter | A99839 | |
| Rotor SW 32 Ti | Beckman Coulter | 369650 | |
| Ultracentrifuge tubes | Beckman Coulter | 326823 | |
| Glass teflon homogenizer (Potter) | Wheaton | 357426 | |
| Spin column (Mobicol) | Mo Bi Tec | M1003 | |
| Filter 35µm for spin column (Mobicol) | Mo Bi Tec | M513515 | |
| AcTEV | Invitrogen | 12575-015 | |
| Centrifugal evaporator (Speed Vac) | Eppendorf | 5305000100 | |
| FBN1A(PGL35) antibody | AgriSera | AS06 116 | RRID:AB_2247012 |
| Sand, Fontainebleau | VWR | 27460.295 |
Referências
- Jarvis, P., López-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nature Reviews Molecular Cell Biology. 14 (12), 787-802 (2013).
- Kessler, F., Schnell, D. Chloroplast biogenesis: diversity and regulation of the protein import apparatus. Current opinion in cell biology. 21 (4), 494-500 (2009).
- Schnell, D. J., Kessler, F., Blobel, G. Isolation of components of the chloroplast protein import machinery. Science. 266 (5187), 1007-1012 (1994).
- Kessler, F., Blobel, G., Patel, H. A., Schnell, D. J. Identification of two GTP-binding proteins in the chloroplast protein import machinery. Science. 266 (5187), 1035-1039 (1994).
- Jarvis, P., et al. An Arabidopsis mutant defective in the plastid general protein import apparatus. Science. 282 (5386), 100-103 (1998).
- Bauer, J., et al. Essential role of the G-domain in targeting of the protein import receptor atToc159 to the chloroplast outer membrane. The Journal of cell biology. 159 (5), 845-854 (2002).
- Kovacs-Bogdan, E., Soll, J., Bolter, B. Protein import into chloroplasts: the Tic complex and its regulation. Biochimica et biophysica acta. 1803 (6), 740-747 (2010).
- Nakai, M. The TIC complex uncovered: The alternative view on the molecular mechanism of protein translocation across the inner envelope membrane of chloroplasts. Biochimica et biophysica acta. 1847 (9), 957-967 (2015).
- Kikuchi, S., et al. Uncovering the protein translocon at the chloroplast inner envelope membrane. Science. 339 (6119), 571-574 (2013).
- Kohler, D., et al. Characterization of chloroplast protein import without Tic56, a component of the 1-megadalton translocon at the inner envelope membrane of chloroplasts. Plant physiology. 167 (3), 972-990 (2015).
- Zufferey, M., et al. The novel chloroplast outer membrane kinase KOC1 is a required component of the plastid protein import machinery. The Journal of biological chemistry. 292 (17), 6952-6964 (2017).
- Rigaut, G., et al. A generic protein purification method for protein complex characterization and proteome exploration. Nature biotechnology. 17 (10), 1030-1032 (1999).
- Rohila, J. S., Chen, M., Cerny, R., Fromm, M. E. Improved tandem affinity purification tag and methods for isolation of protein heterocomplexes from plants. The Plant journal. 38 (1), 172-181 (2004).
- Rohila, J. S., et al. Protein-protein interactions of tandem affinity purified protein kinases from rice. PloS one. 4 (8), 6685 (2009).
- Agne, B., et al. The acidic A-domain of Arabidopsis TOC159 occurs as a hyperphosphorylated protein. Plant physiology. 153 (3), 1016-1030 (2010).
- Wessel, D., Flugge, U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Analytical biochemistry. 138 (1), 141-143 (1984).

