Identification of Novel CK2 Kinase Substrates Using a Versatile Biochemical Approach
Summary
The objective of this protocol is to label, enrich, and identify substrates of protein kinase CK2 from a complex biological sample such as a cell lysate or tissue homogenate. This method leverages unique aspects of CK2 biology for this purpose.
Abstract
The study of kinase-substrate relationships is essential to gain a complete understanding of the functions of these enzymes and their downstream targets in both physiological and pathological states. CK2 is an evolutionarily conserved serine/threonine kinase with a growing list of hundreds of substrates involved in multiple cellular processes. Due to its pleiotropic properties, identifying and characterizing a comprehensive set of CK2 substrates has been particularly challenging and remains a hurdle in the study of this important enzyme. To address this challenge, we have devised a versatile experimental strategy that enables the targeted enrichment and identification of putative CK2 substrates. This protocol takes advantage of the unique dual co-substrate specificity of CK2 allowing for specific thiophosphorylation of its substrates in a cell or tissue lysate. These substrate proteins are subsequently alkylated, immunoprecipitated, and identified by liquid chromatography/tandem mass spectrometry (LC-MS/MS). We have previously used this approach to successfully identify CK2 substrates from Drosophila ovaries and here we extend the application of this protocol to human glioblastoma cells, illustrating the adaptability of this method to investigate the biological roles of this kinase in various model organisms and experimental systems.
Introduction
Protein kinases are key components of signal transduction cascades. Phosphorylation of substrate proteins by these enzymes elicits biological responses that regulate critical events controlling cell division, metabolism, and differentiation, among others. CK2 is a ubiquitously expressed, acidophilic serine/threonine kinase that is conserved from yeast to humans and that plays important roles in many cellular processes ranging from transcriptional regulation to cell cycle progression to apoptosis1,2,3. The enzyme is a heterotetramer composed of two catalytic α (or α') subunits and two regulatory β subunits4. In addition to being highly pleiotropic, CK2 exhibits two other unusual characteristics that complicate its analysis, namely constitutive activity5 and dual co-substrate specificity6. This latter property endows CK2 with the ability to use GTP as well as ATP for phosphorylation of substrate proteins.
Genetic deletion of the catalytic or regulatory subunits of CK2 in mice results in embryonic lethality indicating that it plays crucial roles during development and organogenesis7,8. CK2 is also overexpressed in several types of cancer and thus represents a promising therapeutic target9,10,11. Indeed, specific inhibitors that target CK2 kinase activity are currently under investigation for this purpose12,13,14. While inhibition of CK2 is a viable option, given its pleiotropic nature, an alternative and perhaps more rational approach would be to target critical CK2 substrates that underlie the progression of certain cancers. Therefore, the comprehensive identification and characterization of CK2 substrate proteins would be of significant benefit for elucidating the specific function(s) of this kinase within a particular tissue or tumor type.
Here, we describe a versatile biochemical method for identifying CK2 substrates from a complex biological sample such as a cell or tissue lysate. This protocol takes advantage of the dual co-substrate specificity of CK2 by use of the GTP analogue GTPγS (guanosine 5'-[γ-thio]triphosphate) that other endogenous kinases cannot use. This effectively allows the kinase to "label" its substrates within this sample for subsequent isolation and identification.
Protocol
NOTE: Ensure that the required materials are available and properly prepared (see Table of Materials).
1. Preparation
- Mechanically lyse tissue sample (1-2 mg of tissue in 100 µL of lysis buffer, Table 1) or cultured cells (10 cm plate that is 80-90% confluent in 350 µL of lysis buffer), with the goal being to collect a total of 900 µL of sample for the experiment. Note that this volume is in slight excess of what is required for the experiment described below.
- Spin down the samples by centrifugation at 17,500 x g for 3 minutes at 4 °C. Upon completion, transfer 270 µL of the supernatant to each of three new 1.7 mL microcentrifuge tubes. There will be approximately 90 µL remaining. Remove 40 µL to be used as an “input control” and place in a new tube. Place all samples on ice.
2. Kinase assay: thiophosphorylation and alkylation
- Label the three tubes containing 270 µL each as follows: “kinase rxn”, “GTPγS only”, and “PNBM (p-nitrobezyl mesylate) only”. Prepare the kinase reactions.
- To the “kinase rxn” tube, add 2.7 µL (equivalent to 1,350 U) of CK2, then add 2.7 µL of 2.5 mM GTPγS.
- To the “GTPγS only” tube, add 2.7 µL of 2.5 mM GTPγS, and then add 2.7 µL of lysis buffer.
- To the “PNBM only” tube, add 5.4 µL of lysis buffer. Flick all tubes to mix and then immediately place on ice.
- Incubate all three tubes for 1 min in a 30 °C water bath. Following incubation, add 13.5 µL of 12 mg/mL PNBM to all three tubes. Invert to mix samples. Incubate these samples at room temperature for 1 h.
- After starting the incubation in step 2.2, prepare the desalting columns as soon as possible as the process takes approximately 45 min.
3. Preparation of desalting columns
- Initially prepare columns (3 for this example experiment) by inverting each column several times to re-suspend the Sephadex G-25 resin in the storage buffer. Allow the resin to settle by attaching each column to a clamp stand and letting it sit undisturbed for approximately 5 min.
- Following settling of the Sephadex G-25 resin, remove the caps from both the top and bottom of the column to allow the storage buffer to drain by gravity and have a tube placed below the bottom opening to collect the flow-through for discard.
- Once storage buffer is depleted, add approximately 2.7 mL of lysis buffer in order to equilibrate the columns. Collect the flow-through and discard. Repeat 3 times. Following the final equilibration, the columns are ready for step 4.1.
4. Removal of PNBM
- After the 1 h incubation (step 2.2) and column preparation (step 3) are complete, apply the samples to the columns. Label each column as follows: “kinase rxn”, “GTPγS only”, and “PNBM only”. Load all of each sample onto its respective column. Collect and discard the flow-through.
- Wash samples by adding 420 µL of lysis buffer to each column. Allow lysis buffer to filter through the column and collect the flow-through for discard. Following this wash step, place tubes in position for collection of samples.
- Elute samples by adding 500 µL of lysis buffer to each column. Collect the flow-through that now contains thiophosphorylated and alkylated CK2 substrates.
5. Immunoprecipitation: Part I
- Prepare samples for immunoprecipitation by first removing 80 µL for an “elution input control” sample from each of the respective elutions (“kinase rxn”, “GTPγS only”, and “PNBM only”) collected in step 4.3. Following removal of 80 µL from each sample, there will be approximately 420 µL remaining per sample.
- Split each sample into 2 tubes containing 200 µL each. Label each respective tube: “kinase rxn anti-thiophosphate ester”, “kinase rxn IgG”, “GTPγS anti-thiophosphate ester”, “GTPγS IgG”, “PNBM anti-thiophosphate ester”, and “PNBM IgG”.
- Add 2.8 µg of anti-thiophosphate ester antibody to each of the anti-thiophosphate ester-labeled tubes and 2.8 µg of isotype control antibody to each of the IgG-labeled tubes. Place tubes on a rotator at 4 °C for 2 h.
- Begin preparation of protein A/G bead during the last 15 min of the 2 h incubation in step 5.2.
6. Protein A/G agarose bead preparation
- Briefly vortex the storage tube to ensure beads are completely re-suspended. Cut off the end of a P200 pipette tip using a clean razor blade in order to increase gauge size. Pipet 100 µL of the bead slurry per immunoprecipitation into a new 1.7 mL microcentrifuge tube. For this example, a total of 6 tubes are needed.
- Centrifuge the tubes at 17,500 x g for 1 min at 4 °C. Remove the supernatant and discard. Re-suspend the beads in 200 µL of lysis buffer and briefly vortex. Repeat the spin and wash steps 3 times.
- Following the final wash, place the beads on ice until the incubation in step 5.2 is complete.
7. Immunoprecipitation: Part II
- After the 2 h incubation from step 5.2, spin down the samples at 17,500 x g for 3 min at 4 °C. Following centrifugation add 200 µL from each sample to the tubes with the washed beads. Place the tubes on a rotator at 4 °C for 1 h.
- Following the 1 h incubation, centrifuge the tubes at 17,500 x g for 1 min at 4 °C. Next remove 40 µL of supernatant from each sample and save as a “depletion control” (total 6 tubes). Remove the remainder of the supernatant and discard. Take care not to disturb the beads.
- Wash the samples by adding 200 µL of lysis buffer and vortexing briefly. Then centrifuge at 17,500 x g for 1 min at 4 °C. Remove the supernatant and discard. Repeat the wash and spin steps 3 times. Take care not to disturb the beads during these steps.
- After completing the wash steps, add 50 µL of 2x sample buffer to each sample containing beads. For all other samples, add 8 µL of 6x sample buffer: “input control”, “elution input controls”, and “depletion controls”.
- Once buffer is added to the tubes, pipet up and down to mix, and heat all samples at 95 °C for 5 min before proceeding with SDS-PAGE.
8. Analysis/Validation of results
- Validate successful CK2-dependent thiophosphorylation and alkylation.
- To determine the efficacy of CK2-mediated thiophosphorylation in step 2.2, perform SDS-PAGE and Western blotting by running 15-20 µL of the “elution input controls” collected in step 5.1 on a 12.5% polyacrylamide gel.
- Probe membranes with the following antibodies: anti-thiophosphate ester, anti-CK2α, and anti-GAPDH (or other appropriate loading control). If this step was successful, an enhanced anti-thiophosphate ester signal should be apparent in the “kinase rxn” lane compared to the other two lanes (Figure 2).
- Visualize enriched putative substrates of CK2 and determine protein identity.
- To assess if the immunoprecipitation steps were successful, run 25-30 µL of the samples eluted from the beads in step 7.4 on a separate 12.5% polyacrylamide gel. Ensure that all equipment is clean and wear gloves at all times during this step to minimize contamination.
- Stain the gel with Coomassie blue to visualize enriched proteins from various stages of the experimental protocol (Figure 3). Using new razorblades, carefully excise unique bands present in the “kinase rxn anti-thiophosphate ester IP” lane, noting their approximate molecular weights.
- Submit these bands for protein identification by liquid chromatography/tandem mass spectrometry (LC-MS/MS) (Figure 4). If antibodies directed against the identified proteins are available, confirm the results of mass spectrometry by SDS-PAGE and immunoblotting of input, depleted, and IP fractions collected during the course of the protocol (Figure 4).
Representative Results
A schematic diagram of the experimental procedure is provided in Figure 1. The underlying basis of the technique is the unusual ability of CK2 to use GTP for phosphoryl group transfer. Addition of exogenous CK2 holoenzyme along with the GTP analogue, GTPγS, to a cell lysate results in thiophosphorylation of endogenous CK2 substrates. Subsequent treatment of the lysate with the alkylating reagent p-nitrobenzyl mesylate (PNBM) generates a thiophosphate ester moiety on these specific substrate proteins that can then be immunoprecipitated using an anti-thiophosphate ester antibody and ultimately identified by mass spectrometry. Figure 2 depicts a positive result following the addition of CK2 and GTPγS and then PNBM to T98G (glioblastoma) cell lysate. These results demonstrate that CK2-dependent thiophosphorylation and subsequent alkylation were successful. As expected, an enhanced anti-thiophosphate ester signal by Western blotting is observed only in the lane containing the complete kinase reaction and not in the GTPγS only- and PNBM only-treated samples. Shown in Figure 3 is a Coomassie blue-stained gel of the immunoprecipitated and eluted proteins using isotype control IgG or anti-thiophosphate ester antibodies in the presence or absence of excess CK2 and/or GTPγS. These data also demonstrate a positive result as multiple unique bands are evident only in the anti-thiophosphate ester IP lane in which the lysate was incubated with exogenous CK2 and GTPγS. The band indicated with an asterisk was excised from the gel and submitted for protein identification by mass spectrometry. Figure 4 illustrates representative data obtained by mass spectrometric analysis including protein identification, percent coverage, and number of unique peptides identified per protein within the band. Shown are the top ten hits from the submitted band (Figure 3) and information regarding whether or not the protein has been previously identified as a substrate of CK215,16,17. The identity of one of the known immunoprecipitated CK2 substrates, nucleolin15, was confirmed by SDS-PAGE and immunoblotting of the indicated fractions using an anti-nucleolin antibody.
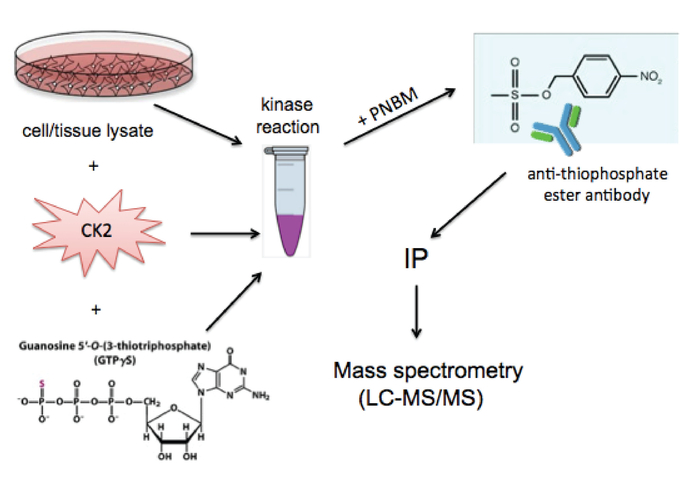
Figure 1: Schematic diagram of the experimental strategy. The GTP analogue, GTPγS, along with excess recombinant CK2 holoenzyme is added to a cell or tissue lysate and allows for thiophosphorylation of substrates by CK2 but not by other endogenous kinases. Thiophosphorylated substrates are next alkylated with PNBM, generating a thiophosphate ester moiety on these proteins, which are then captured via immunoprecipitation (IP) for subsequent identification by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Please click here to view a larger version of this figure.
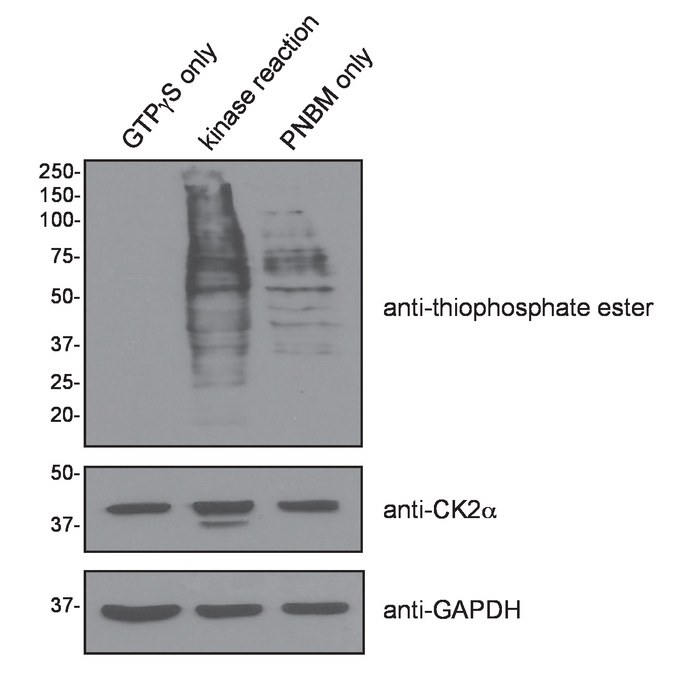
Figure 2: Validation of CK2-dependent thiophosphorylation in whole cell lysate. Whole cell lysates prepared from T98G cells were incubated with GTPγS in the presence (kinase reaction) or absence (GTPγS only) of exogenous recombinant CK2 holoenzyme. PNBM was subsequently added to the indicated reactions, and samples were resolved by SDS-PAGE followed by immunoblotting with the indicated antibodies. Protein molecular weight markers are indicated in kDa. Please click here to view a larger version of this figure.
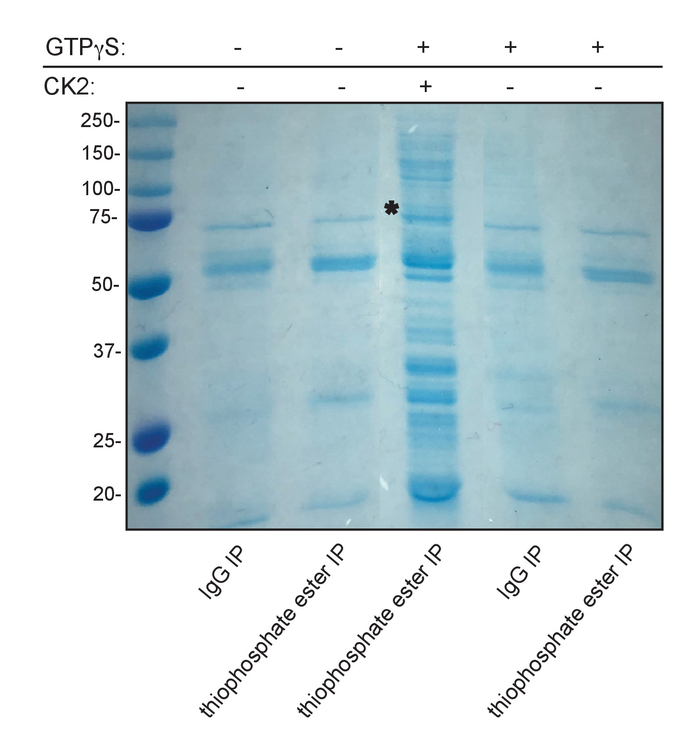
Figure 3: Immunoprecipitation of putative CK2 substrate proteins. Enrichment and visualization of putative CK2 substrates (third lane) is evident as multiple unique bands following immunoprecipitation with anti-thiophosphate ester antibodies. Immunoprecipitates were resolved by SDS-PAGE and the gel was stained with Coomassie blue. The band marked with an asterisk was excised from the gel and submitted for protein identification by mass spectrometry. Protein molecular weight markers are indicated in kDa. IP=immunoprecipitation. Please click here to view a larger version of this figure.
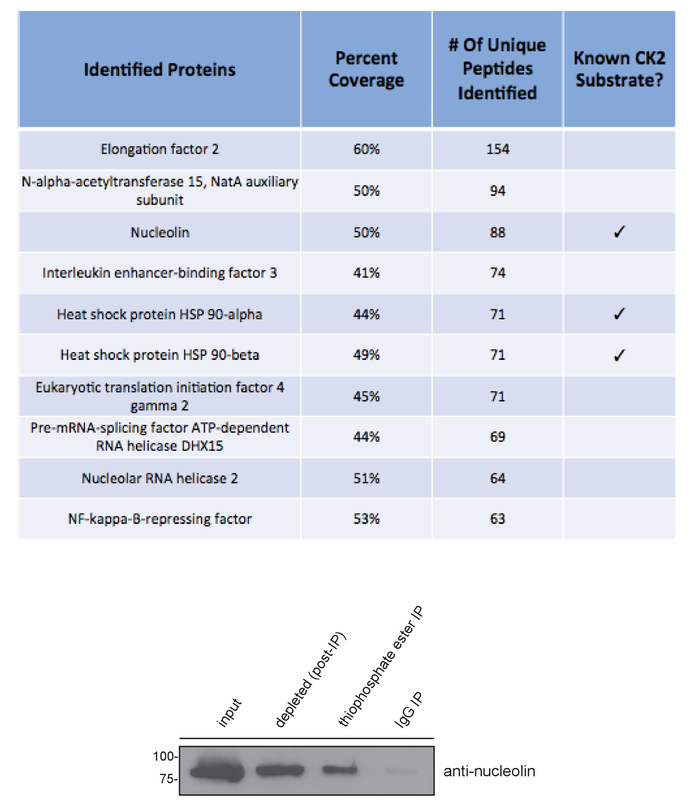
Figure 4: Identification and confirmation of proteins as substrates of CK2 in vitro. Data obtained by mass spectrometry demonstrates that both previously known15,16,17 as well as putative novel CK2 substrates were identified using this experimental approach. Shown are the top ten proteins identified from the excised band (top). The identity of nucleolin, a known CK2 substrate, was confirmed by immunoblotting of the indicated fractions using an anti-nucleolin antibody (bottom). Protein molecular weight markers are indicated in kDa. IP=immunoprecipitation. Please click here to view a larger version of this figure.
| Reagent Stock Concentration | Reagent Final Concentration | Example volumes added based on stock concentrations |
| 1 M Tris pH 7.4 | 20.0 mM | 200 µL |
| 4 M NaCl | 20.0 mM | 50 µL |
| 20% Triton X-100 | 0.50% | 250 µL |
| 1 M MgCl2 | 10.0 mM | 100 µL |
| 1 M DTT | 0.5 mM | 5 µL |
| 200 mM Na3VO4 | 1.0 mM | 50 µL |
| 500 mM NaF | 10.0 mM | 200 µL |
| 500 mM β-glycerol phosphate | 10.0 mM | 200 µL |
| H2O | 8.945 mL | |
| +1 cOmplete Mini tab/10 mL | 1 tablet |
Table 1. Lysis buffer recipe (10 mL, 1X).
Discussion
Here, we describe a relatively simple biochemical method for identifying substrates of protein kinase CK2 from a complex biological sample. The critical steps of this protocol are based on the unusual enzymatic properties of CK2 and include CK2-dependent thiophosphorylation of specific substrate proteins using GTPγS and their subsequent immunoprecipitation and identification. With these results, we have demonstrated the utility and versatility of this approach as we have now applied this strategy in both human glioblastoma cells and Drosophila ovaries18.
A number of previously published studies using quantitative phosphoproteomics approaches have indeed proven successful in identifying novel CK2 substrates19,20,21,22,23. However, some of these strategies make use of immobilized substrate arrays, and it is possible that the conformation of an immobilized protein may render a potential phosphorylation site inaccessible to the kinase. The technique described here permits phosphorylation within a more physiological or native environment (i.e., a cell lysate), thereby reducing the probability of site inaccessibility. Another benefit of this strategy is that once protein identification is determined by mass spectrometry, validation of putative CK2 substrates can easily be performed on samples collected during the procedure if antibodies directed against the substrate protein(s) of interest are available. For example, using standard western blotting, one should observe a reduction in the level of the relevant protein in the depleted (post-IP) samples and its presence in the anti-thiophosphate ester immunoprecipitates as we have demonstrated for the known CK2 substrate nucleolin (Figure 4).
A notable limitation of this method is that the final step of the procedure prior to analysis by mass spectrometry relies on the ability to discern discrete differences in banding pattern on a gel. Thus, it is certainly possible that specific CK2 substrates may be missed if they are of low abundance and therefore below the limit of visual detection. If one is concerned about this possibility, a more sensitive method such as silver staining can be used to visualize these proteins instead of using Coomassie blue. An additional consideration that should be acknowledged is that a number of CK2 substrates may not be identified using this strategy since the physiologically relevant sites will already be phosphorylated in vivo. This is almost certainly to be the case given the constitutive activity of CK2. Finally, it should also be noted that this methodology only identifies proteins as putative substrates of CK2 in vitro. Subsequent assays to validate that these are physiologically relevant substrates of CK2 in vivo are required and should entail identification of CK2-dependent phosphorylation sites and assessing if phosphorylation of these particular residues is altered in response to manipulation of CK2 kinase activity.
In summary, this strategy coupled with downstream experimental approaches (phosphorylation site mapping by truncation/deletion analysis, site-directed mutagenesis, functional in vitro and in vivo assays, etc.) will facilitate the study of this unusual kinase and will increase our understanding of the various roles that CK2 plays in multiple biological systems.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by a Commonwealth Universal Research Enhancement grant from the Pennsylvania Department of Health to T.I.S.
Materials
| 12 mg/mL PNBM | Abcam | ab138910 | 40.5 µL |
| 2.5 mM GTPγS | Sigma-Aldrich | G8634-1MG | 5.4 µL |
| Anti-CK2α (E-7) mouse monoclonal antibody | Santa Cruz Biotechnology | sc-373894 | 1:1000 for Western blotting |
| Anti-GAPDH (6C5) mouse monoclonal antibody | Santa Cruz Biotechnology | sc-32233 | 1:1000 for Western blotting |
| Anti-nucleolin rabbit polyclonal antibody | Abcam | ab22758 | 1:1000 for Western blotting |
| Anti-thiophosphate ester [51-8] rabbit monoclonal antibody | Abcam | ab92570 | Varies (final concentration 2.8 µg for each sample) |
| Centrifuge pre-set to 4ºC | ThermoScientific | Sorvall Legend Micro 21R Cat# 75-772-436 | |
| cOmplete Mini EDTA-Free Protease Inhibitor | Roche | 11836170001 | |
| Lysis Buffer | See recipe below | See recipe below | 30 mL |
| Normal rabbit IgG antibody (isotype control) | Cell Signaling Technology | 2729S | Varies (final concentration 2.8 µg for each sample) |
| PD MiniTrap Column | GE Healthcare | 28-9180-10 | 3 columns |
| Protein A/G Plus Agarose Beads | Santa Cruz Biotechnology | sc-2003 | 600 µL |
| Recombinant human CK2 holoenzyme | New England Biolabs | P6010S | 2.7 µL |
| Rotator | Labnet: Mini Labroller | Mini Labroller SKU# H5500 | |
| T98G human glioblastoma cells | ATCC | CRL-1690 | |
| Water bath pre-set to 30ºC | Shel Lab | H20 Bath Series Model# SWB15 |
Referências
- Litchfield, D. W. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochemical Journal. 369 (Pt 1), 1-15 (2003).
- Ahmed, K., Gerber, D. A., Cochet, C. Joining the cell survival squad: an emerging role for protein kinase CK2). Trends in Cell Biology. 12 (5), 226-230 (2002).
- Meggio, F., Pinna, L. A. One-thousand-and-one substrates of protein kinase CK2. The FASEB Journal. 17 (3), 349-368 (2003).
- Niefind, K., Guerra, B., Ermakowa, I., Issinger, O. G. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. The EMBO Journal. 20 (19), 5320-5331 (2001).
- Sarno, S., Ghisellini, P., Pinna, L. A. Unique activation mechanism of protein kinase CK2. The N-terminal segment is essential for constitutive activity of the catalytic subunit but not of the holoenzyme. Journal of Biological Chemistry. 277 (25), 22509-22514 (2002).
- Niefind, K., Putter, M., Guerra, B., Issinger, O. G., Schomburg, D. GTP plus water mimic ATP in the active site of protein kinase CK2. Nature Structural & Molecular Biology. 6 (12), 1100-1103 (1999).
- Lou, D. Y., et al. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Molecular and Cellular Biology. 28 (1), 131-139 (2008).
- Buchou, T., et al. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Molecular and Cellular Biology. 23 (3), 908-915 (2003).
- Chua, M. M., et al. CK2 in Cancer: Cellular and Biochemical Mechanisms and Potential Therapeutic Target. Pharmaceuticals (Basel). 10 (1), (2017).
- Tawfic, S., et al. Protein kinase CK2 signal in neoplasia. Histology and Histopathology. 16 (2), 573-582 (2001).
- Hanif, I. M., Hanif, I. M., Shazib, M. A., Ahmad, K. A., Pervaiz, S. Casein Kinase II: an attractive target for anti-cancer drug design. The International Journal of Biochemistry & Cell Biology. 42 (10), 1602-1605 (2010).
- Siddiqui-Jain, A., et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Pesquisa do Câncer. 70 (24), 10288-10298 (2010).
- Chon, H. J., Bae, K. J., Lee, Y., Kim, J. The casein kinase 2 inhibitor, CX-4945, as an anti-cancer drug in treatment of human hematological malignancies. Frontiers in Pharmacology. 6, 70 (2015).
- Perea, S. E., et al. CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Molecular and Cellular Biochemistry. (1-2), 163-167 (2008).
- Xiao, S., et al. Induced expression of nucleolin phosphorylation-deficient mutant confers dominant-negative effect on cell proliferation. PLoS One. 9 (10), e109858 (2014).
- Shi, Y., Brown, E. D., Walsh, C. T. Expression of recombinant human casein kinase II and recombinant heat shock protein 90 in Escherichia coli and characterization of their interactions. Proceedings of the National Academy of Sciences of the United States of America. 91 (7), 2767-2771 (1994).
- Mollapour, M., Tsutsumi, S., Kim, Y. S., Trepel, J., Neckers, L. Casein kinase 2 phosphorylation of Hsp90 threonine 22 modulates chaperone function and drug sensitivity. Oncotarget. 2 (5), 407-417 (2011).
- McMillan, E. A., et al. The protein kinase CK2 substrate Jabba modulates lipid metabolism during Drosophila oogenesis. Journal of Biological Chemistry. 293 (8), 2990-3002 (2018).
- Turowec, J. P., et al. Protein kinase CK2 is a constitutively active enzyme that promotes cell survival: strategies to identify CK2 substrates and manipulate its activity in mammalian cells. Methods in Enzymology. , 471-493 (2010).
- Rusin, S. F., Adamo, M. E., Kettenbach, A. N. Identification of Candidate Casein Kinase 2 Substrates in Mitosis by Quantitative Phosphoproteomics. Frontiers in Cell and Developmental Biologyl. 5, 97 (2017).
- Bian, Y., et al. Global screening of CK2 kinase substrates by an integrated phosphoproteomics workflow. Scientific Reports. 3, 3460 (2013).
- Franchin, C., et al. Quantitative analysis of a phosphoproteome readily altered by the protein kinase CK2 inhibitor quinalizarin in HEK-293T cells. Biochimica et Biophysica Acta. 1854 (6), 609-623 (2015).
- Franchin, C., et al. Re-evaluation of protein kinase CK2 pleiotropy: new insights provided by a phosphoproteomics analysis of CK2 knockout cells. Cellular and Molecular Life Sciences. 75 (11), 2011-2026 (2018).

