Detached Leaf Assays to Simplify Gene Expression Studies in Potato During Infestation by Chewing Insect Manduca sexta
Summary
The presented method creates natural herbivore damaged plant tissue through the application of Manduca sexta larvae to detached leaves of potato. The plant tissue is assayed for expression of six transcription factor homologs involved in early responses to insect herbivory.
Abstract
The multitrophic nature of gene expression studies of insect herbivory demands large numbers of biological replicates, creating the need for simpler, more streamlined herbivory protocols. Perturbations of chewing insects are usually studied in whole plant systems. While this whole organism strategy is popular, it is not necessary if similar observations can be replicated in a single detached leaf. The assumption is that basic elements required for signal transduction are present within the leaf itself. In the case of early events in signal transduction, cells need only to receive the signal from the perturbation and transmit that signal to neighboring cells which are assayed for gene expression.
The proposed method simply changes the timing of the detachment. In whole plant experiments, larvae are confined to a single leaf which is eventually detached from the plant and assayed for gene expression. If the order of excision is reversed, from last in whole plant studies, to first in the detached study, the feeding experiment is simplified.
Solanum tuberosum var. Kennebec is propagated by nodal transfer in a simple tissue culture medium and transferred to soil for further growth if desired. Leaves are excised from the parent plant and relocated to Petri dishes where the feeding assay is conducted with the larval stages of M. sexta. Damaged leaf tissue is assayed for the expression of relatively early events in signal transduction. Gene expression analysis identified infestation-specific Cys2-His2 (C2H2) transcription factors, confirming the success of using detached leaves in early response studies. The method is easier to perform than whole plant infestations and uses less space.
Introduction
Herbivory sets in motion a series of molecular events during which a plant can both identify the attack and mount an appropriate response for its survival. A plant receives two basic cues from chewing insects; one from the physical damage to the tissue and the other from insect-specific substances. Damage-associated molecular patterns (DAMPs) are released in response to damage created by larval mouthparts and trigger a well-defined wound response that results in an increase in the hormone jasmonic acid and the transcription of defense genes1. One of the best-known DAMPs is systemin, a polypeptide that is formed by the cleavage of the larger prosystemin protein after a leaf is wounded2,3. The jasmonic acid wound response is further modulated by herbivore-associated molecular patterns (HAMPs), which can be derived from caterpillar saliva, gut contents (regurgitant) and feces (frass)4. Insects use these substances to either boost or evade the defense response5. Transcription factors then relay the message from hormone signals in the defense response via regulation of downstream defense genes6,7,8.
Some plant-insect interaction studies used in laboratory settings are of the simulated type, with a goal of approximating the natural method of feeding by the insect. Simulated herbivory is usually accomplished by creating artificial damage to plant tissues with various tools that mimic the specific mechanism of insect mouthparts sufficient to cause the release of DAMPs and trigger the production of defense genes. Other insect-specific components such as oral secretions or regurgitant are often added to replicate the contribution from HAMPs9,10,11. The creation of a specific size and type of wound and the application of precise amounts of HAMPs is one advantage to these types of studies and can offer more reproducible results. Natural herbivory studies, where damage to plant tissue is accomplished by the application of field-acquired or laboratory-reared insects, are often more challenging because wound-size and HAMP amounts are governed by insect behavior and add variability to the data. The natural versus simulated methods and their advantages and disadvantages are well debated in the literature12,13,14.
To study early signaling events such as transcription factors, a certain percentage of the leaf must be consumed in a relatively short amount of time, so larvae must begin to chew immediately and maintain consumption until the leaf is frozen for analysis. M. sexta is a voracious feeder on multiple solanaceous plants during many of its larval stages, making it ideal for imparting maximum damage in a relatively short amount of time15. This is convenient when studying early signaling events, as the plant response occurs almost immediately after an insect contacts the leaf surface16,17. The commonly used clip cage method of containment proves clumsy, as multiple cages would require continual adjustments throughout the experiment to allow for the removal or addition of larvae. The leaves must also be large enough and strong enough to support multiple insects feeding at the same time. These types of potato plants require a large amount of space to observe feeding. Larvae will often relocate to the underside of the leaf surface which also makes feeding observations quite difficult. Using whole plants to perform these experiments is clearly cumbersome.
The current study uses detached leaves isolated in Petri dishes rather than whole plants to streamline and simplify the whole plant approach to studying herbivory. The application of the protocol in this study is limited to the observation of a group of C2H2 transcription factors induced early in potato leaves after herbivorous damage by M. sexta larvae.
Protocol
NOTE: The following protocol is designed for one person to set up, make observations and collect samples. Multiple runs of the same setup may be combined to increase biological replication. Any additional repetitions of the experiment should be set up at the same time of day to eliminate possible diurnal influences on gene expression. The protocol is designed to create 3 ‘infested’ leaves for 5 separate harvest time points. Matched control leaves for each time point create a total of 30 samples. The experiment may be performed with a variety of leaf sizes and larval stages, but it is recommended that leaf size, larval stage and infestation time be consistent throughout the procedure.
1. Preparation of the potato plants
NOTE: All steps requiring sterile technique must be performed in a tissue culture hood18,19.
- Prepare Kennebec plantlets from explant source.
- Prepare propagation medium.
- Add 4.43 g of Murashige and Skoog (MS) with vitamins powder, 20 g of sucrose and 2 g of agar substitute to 1 L of reverse osmosis (RO)-purified water in a 2 L flask with a spin bar. Transfer flask to a stir plate and mix. Adjust pH to 5.8 using NaOH while continuing to stir.
NOTE: Agar will not dissolve until autoclaved. - Add 1 mL of preservative/biocide and autoclave on liquid cycle for 20 min (121 °C, 101.3 kPa). Remove medium from autoclave immediately after the cycle is finished and cool it to 50 °C. Transfer 100 mL of sterile and cooled propagation medium to sterile culture vessels (e.g., Magenta or Plantcon) using sterile technique in a tissue culture hood.
- Add 4.43 g of Murashige and Skoog (MS) with vitamins powder, 20 g of sucrose and 2 g of agar substitute to 1 L of reverse osmosis (RO)-purified water in a 2 L flask with a spin bar. Transfer flask to a stir plate and mix. Adjust pH to 5.8 using NaOH while continuing to stir.
- Prepare explant material.
- Obtain explant source as Kennebec seed potatoes and remove all traces of soil by washing with tap H2O. Remove sprouts and cut into 2 cm pieces with a sterile scalpel.
- Sterilize sprout pieces by soaking for 15 s in 70% ethanol, followed by 13 min in 1:1 bleach (1 part RO-water: 1 part concentrated germicidal/commercial grade bleach). Rinse 5 times in sterile H2O.
- Transfer explant material to sterile tissue culture vessels with propagation medium in a tissue culture hood using sterile technique. Transfer vessels to a plant tissue culture chamber and grow for 2‒3 weeks or until plantlets form at 24 °C, 16 h light (140 µmol·m-2·s-1)/8 h dark photoperiod.
- Prepare propagation medium.
- Prepare nodal-propagated tissue culture potato plants.
- Prepare nodal transfer medium.
NOTE: This will make 10 tissue culture vessels which may be used the same day or may be made ahead of time and stored at 4 °C until nodal transfer.- Add 36.43 g of nutrient agar mix to 1 L of RO-purified water in a 2 L flask with a spin bar. Transfer flask to a stir plate and mix. Adjust pH to 5.8 using KOH while continuing to stir.
NOTE: Agar will not dissolve until autoclaved. - Autoclave on liquid cycle for 20 min (121 °C, 101.3 kPa). Remove medium from autoclave immediately after the cycle is finished and cool it to 50 °C. Transfer 100 mL of sterile cooled nodal transfer medium to sterile culture vessels (see the Table of Materials) using sterile technique in a tissue culture hood.
- Add 36.43 g of nutrient agar mix to 1 L of RO-purified water in a 2 L flask with a spin bar. Transfer flask to a stir plate and mix. Adjust pH to 5.8 using KOH while continuing to stir.
- Prepare nodal cuttings.
- Obtain Kennebec plantlets grown from explant material (produced in step 1.1.2). Plantlets should have at least 3 to 4 nodes (branch points). Remove leaves using sterile scissors or scalpel. Cut branches close to the main stem, leaving about 2 mm of branch tissue.
- Remove nodal sections from stem by cutting approximately 2 mm above and below each branch point or node. Arrange nodal cuttings in a sterile tissue culture vessel containing nodal transfer medium (produced in step 1.2.1.2) in the same orientation as in the previous vessel (branch pointing up).
- Transfer vessels with nodal cuttings to a plant tissue culture chamber and grow for 2‒3 weeks at 24 °C, 16 h light (140 µmol·m-2·s-1)/8 h dark photoperiod.
NOTE: Each nodal cutting will grow into a new plantlet. The number of cuttings in each vessel determines leaf size. Fewer cuttings transferred per vessel will result in larger leaves. Three plants per vessel will have 15 mm x 20 mm leaves in 2‒3 weeks.
- Prepare nodal transfer medium.
- (Optional) Prepare soil grown Kennebec potato plants.
NOTE: To produce larger leaves, nodal propagated potato plantlets can be transferred to soil.- Transfer potato plantlets grown in nodal transfer medium to soil by gently pulling the plant from the medium until all the root tissue is released from the agar.
- Transfer to soil just above the 1st node from the roots and lightly pack the soil around the transplant. Water gently to ensure soil contact with the root system.
- Place in a growth chamber with 16 h light (140 µmol·m-2·s-1)/8 h dark photoperiod and 25/20 °C day/night temperatures.
NOTE: Plants are ready when the top two fully expanded leaves have reached the size appropriate for the assay.
- (Optional) Prepare tuber-grown potato plants.
NOTE: Potato plants grown from tubers are larger and more robust and can be useful if rearing larvae on plants or if overnight larval feeding is desired.- Place a potato tuber 6 in deep in a 10 in pot containing soil mix supplemented with 10 mL pelleted slow release fertilizer.
- Place in a growth chamber with 16 h light (140 µmol·m-2·s-1)/8 h dark photoperiod and 25/20 °C day/night temperature. Plants are ready approximately 30‒40 days from tuber planting.
NOTE: Do not use plants that have begun to flower.
2. Preparation of insects for feeding
- Obtain desired larval stage of M. sexta.
NOTE: Larvae for this study were reared on artificial diet through the 5th instar20 and staged by an experienced individual from the in-house insectary. Larvae may also be reared partially or completely on plant tissue. Larvae do not eat immediately before a molt and are most likely to eat right after molt, so appropriate staging is important21. - Transfer larvae to an appropriate containment vessel (e.g., 6-, 12-, 24-well tissue culture dish) depending on larval size. There should be one larva per well in the dish.
NOTE: Larvae may display territorial or cannibalistic behavior without a food source and may become injured if housed together. - (Optional) Starve larvae for up to 2 h as this may improve larval feeding.
NOTE: Larvae should be in a containment vessel stored in the growth chamber during this time.
3. Components setup for the infestation experiment
NOTE: See the schematic summary in Figure 1.
- Make placement templates for each harvest time point.
NOTE: It is helpful to set up 5 different trays for each harvest time point. This keeps samples organized and allows the dishes to be moved around more efficiently as a set without changing their arrangement. If percentage-of-damage calculations will be performed, this is essential as before- and after-infestation images must be captured at the same focal length.- Obtain 5 sturdy trays capable of holding a set of six appropriately sized Petri dishes and line with white paper.
NOTE: The size of the Petri dish is based on the leaf size chosen for the feeding assay. The leaf should fit easily in the dish without touching the sides. For instance, a 60 mm x 15 mm dish is suitable for leaves up to 50 mm in length or width. - Trace a set of six circles using the appropriately sized Petri dish on the paper in each tray. Label one set of circles ‘control’ A, B and C and the other ‘infested’ A, B, and C. Also label each placement template with the appropriate harvest time.
- Obtain 5 sturdy trays capable of holding a set of six appropriately sized Petri dishes and line with white paper.
- (Optional) Set up a camera for ‘before infestation’ and ‘after infestation’ image capture.
- Secure a camera on a stand at the appropriate focal length for image capture of all Petri dishes in the placement template.
- Label the harvest time point tubes.
- Label a set of 30, 1.7 mL microcentrifuge tubes corresponding to each circle in the placement template. Label the tubes to appropriately identify the perturbation (control/infested), the plant replication letter (A, B or C) and the harvest time point (number of minutes post infestation period).
- Prepare Petri dishes chosen in step 3.1.1.
- Place a sterile filter paper disc in each of the 30 Petri dishes from step 3.1.1. Add sterile water to moisten the discs; do not allow excess water to pool in the dish. Place each dish in each of the six circles in each placement template.
- Position three potato plants next to each placement template. Ensure that plants are all the same age and relative size.
4. Performing infestation
NOTE: One harvest time point/placement template is set up at a time.
- Remove the top two size-matched leaves from each plant with sterile scissors and place one leaf in the control Petri dish and one in the infested Petri dish for each plant (A, B and C). Carry out this process as quickly as possible.
- (Optional) Transfer the placement template to the camera stand to capture a ‘before infestation’ image.
- Transfer larvae to each infested dish using soft touch forceps as quickly as possible. Set the timer for desired ‘infestation’ time.
NOTE: The period of time when larvae are consuming the leaf tissue is the ‘infestation time’. This is something that can be determined empirically using a few test leaves/larvae before the start of the actual infestation. The chosen ‘infestation time’ should be consistent for all infested leaves.
NOTE: Larvae should be handled with care by soft touch forceps. 2nd through 4th instar may be grasped gently by their horn or midsection. - Observe feeding to make sure all larvae are eating. Larvae should be added/removed based on feeding behavior. Keep lids on the Petri dishes as much as possible.
NOTE: Multiple larvae may be used per leaf. - Remove larvae from leaves at the end of the infestation time. Start the timer for the harvest time.
- (Optional) Transfer the placement template to the camera stand to capture the ‘after infestation’ image.
NOTE: All six leaves in the placement template are harvested at the specific harvest time.
5. Harvesting leaves
- Transfer each leaf to the corresponding labeled tube at the end of each harvest time point and immediately freeze by dropping the tube into liquid N2. Store the harvested plant tissue at -80 °C until the isolation of RNA.
- Repeat steps 4‒5.1 for each harvest time point.
6. Processing leaf tissue for gene expression analysis
- Grind the frozen leaf tissue to a powder with a micropestle.
- Isolate total RNA and process for gene expression as previously described22.
7. (Optional) Estimating leaf damage
- Visually estimate percent of leaf damage or calculate leaf area in the before- and after-infestation images.
- Measure leaf area with software tools (e.g., Phenophyte)23.
- From leaf area measurements, calculate percentage of damage as
% damage = [(leaf area before – leaf area after)/leaf area before] x 100.
Representative Results
Leaf consumption defines success of the protocol. Healthy, accurately staged larvae should begin feeding immediately after placement on the leaf surface and feeding should continue in a fairly consistent manner throughout the infestation time. In Video 1, the larva at the top begins to chew immediately after placement and maintains a consistent rate while feeding. This is especially important if assaying early gene expression events after infestation. The larva at the bottom did not consume any leaf material and is an example of an unsuccessful infestation.
Visual approximations during leaf consumption are monitored to ensure enough damage is produced. The amount of leaf material consumed can be calculated as the percentage of damage by using images of leaves before and after the infestation. Figure 2 illustrates the variable rates of consumption by different developmental stages of M. sexta larvae on different types of potato plants. The leaves in Figure 2A were detached from 2-week-old nodal-propagated tissue culture plants. All the leaves in this figure are of the same size but were subjected to infestation by different stages of larvae for 5 min. Figure 2A: I-IV, Video 2, Video 3, Video 4, and Video 5 illustrate the different rates of consumption and feeding styles for each larval stage from a portion of the infestation. This is helpful in determining how much damage is possible using each leaf and larval stage combination and illustrates the voracious nature of the older larvae. The use of 1st instar larvae is not recommended as their mandibles are not developed enough to produce damage in the 5 min infestation window. It is important to note that younger more tender leaves derived from tissue-culture-grown plants are often more palatable to larvae. Based on these results, 4th instar larvae were chosen to further assess damage to leaves from more mature, soil grown potato plants. The soil grown plants more closely approximate those acquired in the field. Figure 2B illustrates the range of damage when 3rd and 4th instar larvae were applied to leaves detached from soil-grown potato plants. Two-week-old nodal propagated plants were transplanted to soil and grown for an additional 3 weeks. Damaged leaves from soil grown plants fell into three groups; 15-20%, 20-30%, and 30-40%. The three leaves in each group are shown to represent the different levels of damage for each range. The leaves from more mature soil-grown plants needed more larvae applied and a longer infestation time to reach the same level of damage observed in the leaves from younger nodal-propagated plants. These results illustrate the range of outcomes possible from successful herbivory from different types of plants.
After successful herbivory is complete and percent damage is assessed, leaf tissue is assayed for gene expression. Gene expression results should indicate a robust induction or repression of transcripts involved in the response to infestation. Figure 3 illustrates the gene expression profiles of six C2H2 transcription factors from a successful herbivory experiment in detached leaves. C2H2 zinc finger StZFP2 was induced by M. sexta herbivory in potato in previous whole plant studies24. Although StZFP2 was induced by herbivory in the detached leaf assay, infestation was not significant when compared to the effect of the detachment itself. However, two other StZFP2 homologs, StZFP5 and StZFP7, were significantly induced at 40 and 80 min post herbivory when compared to detached controls. StLOX3 and StMYC2 are marker genes induced by both wounding and herbivory and indicate the involvement of jasmonic acid regulated defense pathways. The goal of this particular study was to identify infestation-responsive transcription factors among a panel of candidate genes. The identification of two C2H2 zinc finger transcription factors that are robustly responsive to M. sexta herbivory supports the use of detached leaves for infestation assays.
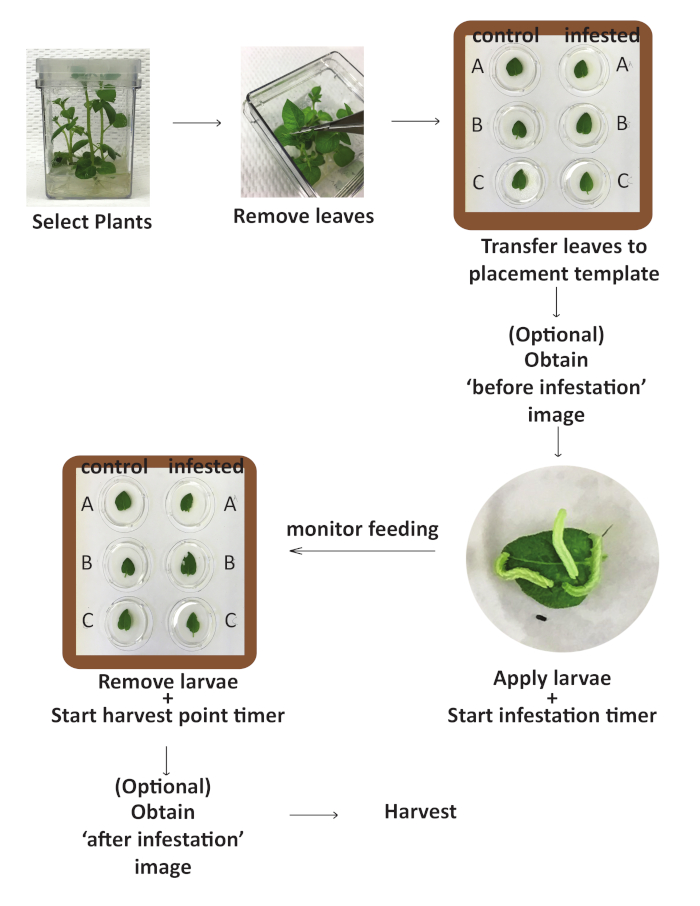
Figure 1: Flowchart for the infestation protocol. Schematic representation of the steps included in the experimental workflow of the infestation section of the protocol. Please click here to view a larger version of this figure.
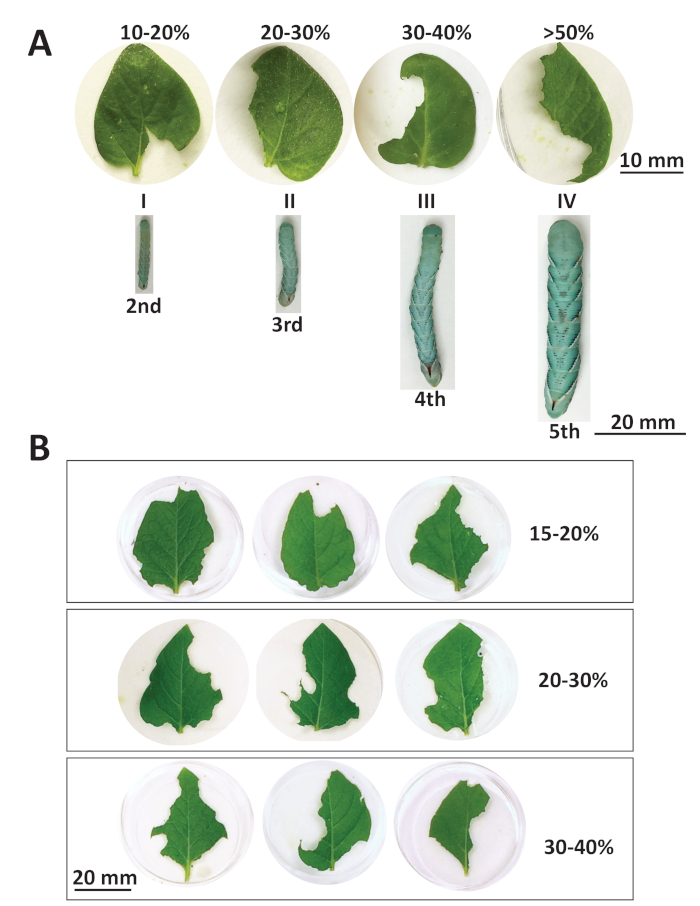
Figure 2: Leaf damage caused by M. sexta larvae. (A) Percentage damage to tissue culture leaves at each larval stage over 5 min. Roman numerals I-IV refer to videos 2-5 respectively that show each instar feeding on the leaf pictured. (B) Range of damage to soil-grown leaves by 4th instar over 20 min. Please click here to view a larger version of this figure.
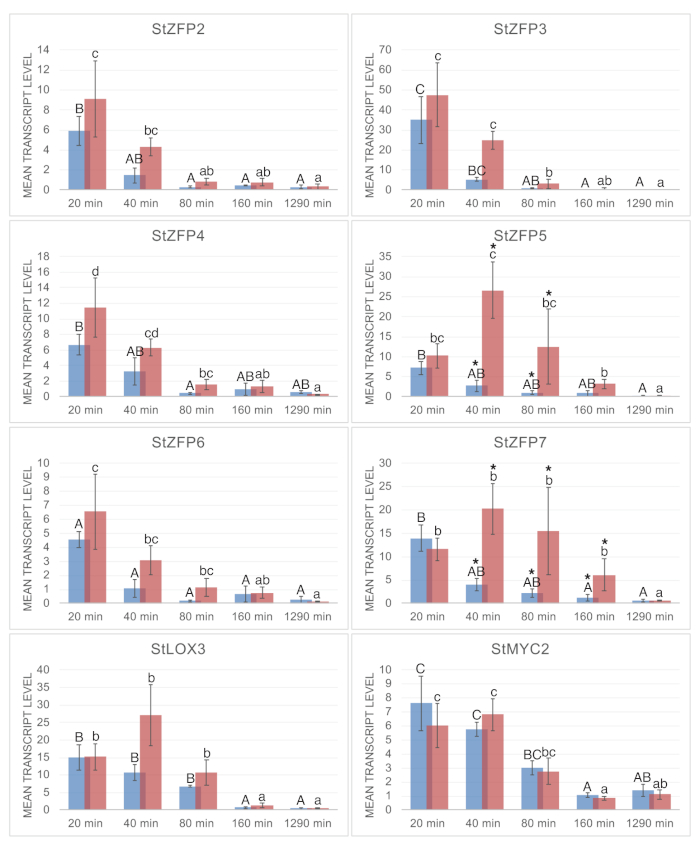
Figure 3: Gene expression analysis of leaves. Real-time quantitative polymerase chain reaction (RT-qPCR) gene expression analysis of C2H2 zinc finger transcription factors is shown. Mean transcript level is, 2−ΔCT with (ΔCT = CT of test gene – CT of exogenous control gene). Excised control leaves in (blue) and excised infested leaves in (red) are shown at each time point. Each value is the average of three biological replicates. Three-way ANOVA was conducted on the ΔCt values. Significant differences in control leaves over time are indicated with capital letters. Significant differences in infested leaves over time are indicated in lower case letters. Significant differences between control and infested leaves at the same time point is shown with an asterisk (*). Pr > F values were all less than 0.001, except for StZFP6 control treatment with 0.0086. Error bars represent standard deviation. NCBI accession numbers are: StLOX3-X96406.1, StZFP2- MK809525, StZFP4-CV500970.1, StZFP6-DN587601.1, StZFP7-DN590005.1. Spud DB accession numbers from <http://solanaceae.plantbiology.msu.edu> are StMYC2-PGSC0003DMT400045204, StZFP3-PGSC0003DMT400040144, StZFP5-PGSC0003DMT400040141. This figure has been modified from22. Please click here to view a larger version of this figure.

Video 1: Video clip of larval feeding. Second instar M. sexta larvae feeding (top) and not feeding (bottom) on a leaf from two week old tissue culture grown potato plant. Please click here to view this video. (Right-click to download.)

Video 2: Figure 2A video clip (I) of larval feeding. Second instar M. sexta larva feeding on a leaf from two week old tissue culture grown potato plant. Please click here to view this video. (Right-click to download.)

Video 3: Figure 2A video clip (II) of larval feeding. Third instar M. sexta larva feeding on a leaf from two week old tissue culture grown potato plant. Please click here to view this video. (Right-click to download.)

Video 4: Figure 2A video clip (III) of larval feeding. Fourth instar M. sexta larva feeding on a leaf from two week old tissue culture grown potato plant. Please click here to view this video. (Right-click to download.)

Video 5: Figure 2A video clip (IV) of larval feeding. Fifth instar M. sexta larva feeding on a leaf from two week old tissue culture grown potato plant. Please click here to view this video. (Right-click to download.)
Discussion
The use of existing whole plant herbivory methodologies is unnecessary to achieve the goal of this particular study (i.e., screen a set of candidate genes for their response to infestation). The obvious benefit of the detached leaf refinement is shortening the time it takes to perform herbivory assays. The unwieldy nature of whole plants with clip cages is eliminated and assays are performed sooner, since plants as young as 2 weeks can be used to harvest leaves. It also requires a much smaller footprint during feeding and less growth chamber space; both important when these resources are limited.
The limitation of this assay, namely detachment, is encountered when defense-gene expression is assayed. Detachment itself causes a wound response and it is therefore important to assess what effect detachment has on basal levels of defense-gene expression. In one study involving simulated herbivory, transcript levels of a well-known defense gene protease inhibitor II(PIN2) were unexpectedly high in the control leaves of detached potato leaves likely due to "wounding caused during collection of the leaf"25. However, application of insect regurgitant from M. sexta greatly enhanced levels of PIN2 gene expression. This illustrates the need to use controls to tease out the effect of detachment from the infestation response.
Detached leaf assays are often performed to save time, space and resources. They have been used in screening for resistance to fungal diseases in wheat26,27, and insect resistance in soybean28 and chickpea29. The assay is widely accepted in the study of the pathogen that causes late blight in potato30,31. A recent study found that detached leaf assays were equivalent to whole plant assays in determining late blight resistance in the field32.
Scant evidence exists in the literature, however, for the use of detached leaves to assay for defense-gene expression. One study profiled six defense genes in response to spider mite infestation of detached lima bean33. The leaves also produced volatiles that induced defense responses in nearby uninfested leaves, a well-known strategy of defense-gene expression in plants34. To our knowledge, no chewing insect herbivory studies to date utilize detached leaves to assay for gene expression.
The previously defined infestation responsive C2H2 zinc finger, StZFP2 was not significantly induced by infestation in the current detached leaf assay. Aside from the obvious difference of whole plant versus detached leaves, the previous study utilized a continuous type of infestation in which plant tissue was assayed for gene expression immediately following the removal of the larvae24. This is different from the discontinuous type of infestation utilized in the current study, where larval removal was followed by a resting period before leaf harvest. During this recovery period, StZFP2 levels decrease quickly and may result in a lower level of StZFP2 induction. There could also be other so-far undefined systemic signaling components that may contribute to the augmented induction observed in the whole plant study. Another possibility is that StZFP2 transcripts could have peaked before the 20 min harvest time. It is clear, however, that the impressive induction of StZFP5 and StZFP7 transcripts make this type of infestation protocol relevant. Another limitation of the present method would be the inability to use root feeding larvae such as corn rootworm. It would also not be suitable when whole plant communication such as root to leaf signaling35 or signaling from systemic leaves36,37 is being studied.
Success of this protocol depends heavily on the quality and accurate staging of larva used in the experiment. Rearing skills and basic knowledge of M. sexta behavior are helpful but not critical. However, obtaining healthy, appropriately staged larvae can directly affect feeding success. Larvae enter a quiescent period that precedes each larval molt during which they do not feed21. Clearly, such larvae will not damage leaf tissue. The ideal time to use larvae is just after a molt. A developmentally staged cohort of insects of a particular larval stage will also improve the reproducibility of gene expression as plants may respond differently to each developmental stage.
Access to an insectary would be ideal for the supply of larvae. M. sexta eggs or larvae can also be ordered from commercial sources38,39 and reared on diet or plant material to the appropriate stage. There are also several online tools that can help identify each instar40,41,42. Larvae reared on diet will not contain plant specific components such as microbial symbionts acquired by larvae reared on plants43. Gene expression profiles may be altered if these components are missing. Larvae may be partially or completely reared on their host plant if these effects are important to the study. Withholding food from insects a few hours prior to feeding experiments can also improve feeding behavior. Field-collected larvae could also be robust and advantageous as they have been exposed to proper trophic levels, but that adds another variable which may or not be suitable for all studies.
The production of healthy, insect- and pesticide-free plants is also critical. Plants with viruses, insect pests and fungal organisms will introduce confounding factors and present a challenge in obtaining reproducible data.
Many larval instars, potato plant ages and leaf sizes can be combined to customize the protocol for a specific type of study. The length of the infestation and harvest times can also be adjusted. Potentially, many other plant-insect interactions could be studied using detached leaves if whole plant observations are used as a baseline. The detached leaf assay is a relevant and valuable alternative to whole plant herbivory studies to analyze gene expression.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Bob Farrar and Alexis Park for providing insects used in this study and for their expertise in larval staging. Additional thanks to Michael Blackburn and Saikat Ghosh for critical review of the manuscript.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
USDA is an Equal Opportunity Provider and Employer.
Materials
| agar substitute | PhytoTechnology Laboratories | G3251 | product is Gelzan |
| containment vessel (6,12 or 24 well dish) | Fisher Scientific | 08-772-49, 08-772-50, 08-72-51 | many other companies sell these products |
| manduca eggs | Carolina Biological Supply Company | 143880 | 30-50 eggs |
| manduca eggs | Great Lakes Hornworm | NA | 50, 100, 250 or 500 eggs |
| manduca larvae | Carolina Biological Supply Company | call for specific larval instar requests | any instar |
| manduca larvae | Great Lakes Hornworm | call for specific larval instar requests | any instar |
| microcentrifuge tubes, 1.7 ml | Thomas Scientific | 1158R22 | these have been tested in liquid N2 and will not explode |
| Murashige & Skoog (MS) Basal Medium w/Vitamins | PhytoTechnology Laboratories | M519 | used to make propagation medium |
| nutrient agar mix | PhytoTechnology Laboratories | M5825 | product is Murashige & Skoog Basal Medium with vitamins, sucrose, and Gelzan |
| paper filter discs | Fisher Scientific | 09-805A | Whatman circles-purchase to fit in petri dish |
| petri dish, 60X15 mm or 100X15 mm | Fisher Scientific | FB0875713A or FB0875712 | purchase size appropriate for leaf size |
| potato tubers | any | B size (not organic) | suggest Maine Farmer’s Exchange |
| pots, 10" | Griffin Greenhouse Supplies, Inc. | 41PT1000CN2 | |
| preservative/biocide | Plant Cell Technology | NA | product is PPM (Plant Preservative Mixture) |
| seed potatoes for explant source | any | B size (not organic) | suggest Maine Farmer’s Exchange |
| slow release fertilizer (14-14-14 ) | any | NA | Osmocote is a popular brand name |
| soft touch forceps | BioQuip | 4750 | |
| soil mix | Griffin Greenhouse Supplies, Inc. | 65-51121 | product is Sunshine LC1 mix |
| sterile culture vessel | PhytoTechnology Laboratories | C2100 | Magenta-type vessel, PTL-100 |
| sterile culture vessel | Fisher Scientific | ICN2672206 | product is MP Biomedicals Plantcon |
Referências
- Choi, H. W., Klessig, D. F. DAMPs, MAMPs, and NAMPS in plant innate immunity. BMC Plant Biology. 16, 1-10 (2016).
- Pearce, G., Strydom, D., Johnson, S., Ryan, C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 253, 895-897 (1991).
- Savatin, D. V., Gramegna, G., Modesti, V., Cervone, F. Wounding in the plant tissue: the defense of a dangerous passage. Frontiers in Plant Science. 470 (5), 1-11 (2014).
- Basu, S., Varsanit, S., Louis, J. Altering Plant Defenses: Herbivore-Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores. Molecular Plant-Microbe Interactions. 31, 13-21 (2018).
- Chung, S. H., et al. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proceedings of the National Academy of Sciences, USA. 110, 15728-15733 (2013).
- Chen, M. -. S. Inducible direct plant defense against insect herbivores: A review. Insect Science. 15, 101-114 (2008).
- Howe, G. A., Major, I. T., Koo, A. J. Modularity in jasmonate signaling for multistress resilience. Annual Review of Plant Biology. 69, 387-415 (2018).
- War, A. R., et al. Plant defence against herbivory and insect adaptations. AoB PLANTS. 10 (4), 1-19 (2018).
- McCloud, E. S., Baldwin, I. T. Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta. 203, 430-435 (1997).
- Schittko, U., Hermsmeier, D., Baldwin, I. T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuate: II. Accumulation of plant mRNAs responding to insect-derived cues. Plant Physiology. , 701-710 (2001).
- Halitschke, R., Schittko, U., Pohnert, G., Boland, W., Baldwin, I. T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuate. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiology. 125, 711-717 (2001).
- Lortzing, T., et al. Transcriptomic responses of Solanum dulcamara to natural and simulated herbivory. Molecular Ecology Resources. 17, 1-16 (2017).
- Hjältén, J. Simulating herbivory: problems and possibilities. Ecological Studies. 173, 243-255 (2004).
- Lehtilä, K., Boalt, E. The use and usefulness of artificial herbivory in plant-herbivore studies. Ecological Studies. 173, 257-275 (2004).
- Schittko, U., Preston, C. A., Baldwin, I. T. Eating the evidence? Manduca sexta larvae can not disrupt specific jasmonate induction in Nicotiana attenuata by rapid consumption. Planta. 210, 343-346 (2000).
- Zebelo, S. A., Maffei, M. E. Role of early signalling events in plant-insect interactions. Journal of Experimental Botany. 66, 435-448 (2015).
- Maffei, M. E., Mithofer, A., Boland, W. Before gene expression: early events in plant-insect interaction. Trends in Plant Science. 12, 310-316 (2007).
- Goodwin, P. B., Adisarwanto, T. Propagation of potato by shoot tip culture in Petri dishes. Potato Research. 23, 445-448 (1980).
- Goodwin, P. B. Rapid propagation of potato by single node cuttings. Field Crops Research. 4, 165-173 (1981).
- Martin, P. A. W., Blackburn, M. B. Using combinatorics to screen Bacillus thuringiensis isolates for toxicity against Manduca sexta and Plutella xylostella. Biological Control. 42, 226-232 (2007).
- Bell, R. A., Joachim, F. G. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Annals of the Entomological Society of America. 69 (2), 365-373 (1976).
- Lawrence, S. D., Novak, N. G. The remarkable plethora of infestation-responsive Q-type C2H2 transcription factors in potato. BMC Research Notes. 11, 1-7 (2018).
- Green, J. M., et al. PhenoPhyte: a flexible affordable method to quantify 2D phenotypes from imagery. Plant Methods. 8 (45), 1-12 (2012).
- Lawrence, S. D., Novak, N. G., Jones, R. W., Farrar, R. R., Blackburn, M. B. Herbivory responsive C2H2 zinc finger transcription factor protein StZFP2 from potato. Plant Physiology and Biochemistry. 80, 226-233 (2014).
- Korth, K. L., Dixon, R. A. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiology. 115, 1299-1305 (1997).
- Browne, R. A., Cooke, B. M. Development and evaluation of an in vitro detached leaf assay for pre-screening resistance to Fusarium head blight in wheat. European Journal of Plant Pathology. 110, 91-102 (2004).
- Browne, R. A., et al. Evaluation of components of fusarium head blight resistance in soft red winter wheat germ plasm using a detached leaf assay. Plant Disease. 89, 404-411 (2005).
- Michel, A. P., Rouf Mian, M. A., Davila-Olivas, N. H., Canas, L. A. Detached leaf and whole plant assays for soybean aphid resistance: differential responses among resistance sources and biotypes. Journal of Economic Entomology. 103, 949-957 (2010).
- Sharma, H. C., Pampapathy, G., Dhillon, M. K., Ridsdill-Smith, J. T. Detached leaf assay to screen for host plant resistance to Helicoverpa armigera. Journal of Economic Entomology. 98, 568-576 (2005).
- Vivianne, G. A. A., et al. A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. European Journal of Plant Pathology. 105, 241-250 (1999).
- Kamoun, S., et al. A gene encoding a protein elicitor of Phytophthora infestans is down-regulated during infection of potato. Molecular Plant-Microbe Interactions. 10, 13-20 (1997).
- Nowakowska, M., Nowicki, M., Kłosińska, U., Maciorowski, R., Kozik, E. U. Appraisal of artificial screening techniques of tomato to accurately reflect field performance of the Late Blight resistance. Plos One. 9, e109328 (2014).
- Arimura, G., et al. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature. 406, 512-515 (2000).
- Erb, M. Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Current Opinion in Plant Biology. 44, 117-121 (2018).
- Hasegawa, S., et al. Gene expression analysis of wounding-induced root-to-shoot communication in Arabidopsis thaliana. Plant, Cell and Environment. 34, 705-716 (2011).
- Ryan, C. A., Moura, D. S. Systemic wound signaling in plants: A new perception. Proceedings of the National Academy of Sciences, USA. 99, 6519-6520 (2002).
- Hilleary, R., Gilroy, S. Systemic signaling in response to wounding and pathogens. Current Opinion in Plant Biology. 43, 57-62 (2018).
- . Hornworms Available from: https://www.carolina.com/hornworm/hornworms/FAM_143880.pr (2018)
- . Products Available from: https://www.greatlakeshornworm.com/products/ (2018)
- . Raising Manduca sexta Available from: https://acad.carleton.edu/curricular/Biol/resources/rlink/description2.html (2018)
- . Teach life cycles with the tobacco hornworm Available from: https://www.carolina.com/teacher-resources/Interactive/teach-life-cycles-with-the-tobacco-hornworm/tr30179.tr (2018)
- Chung, S. H., et al. Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Scientific Reports. 7, 1-13 (2017).

