Microfocus X-ray CT (microCT) Imaging of Actinia equina (Cnidaria), Harmothoe sp. (Annelida), and Xenoturbella japonica (Xenacoelomorpha)
Summary
Here, protocols for performing microfocus X-ray computed tomography (microCT) imaging of three marine invertebrate animals are explained in detail. This study describes steps such as sample fixation, staining, mounting, scanning, image reconstruction, and data analyses. Suggestions on how the protocol can be adjusted for different samples are also provided.
Abstract
Traditionally, biologists have had to rely on destructive methods such as sectioning in order to investigate the internal structures of opaque organisms. Non-destructive microfocus X-ray computed tomography (microCT) imaging has become a powerful and emerging protocol in biology, due to technological advancements in sample staining methods and innovations in microCT hardware, processing computers, and data analysis software. However, this protocol is not commonly used, as it is in the medical and industrial fields. One of the reasons for this limited use is the lack of a simple and comprehensible manual that covers all of the necessary steps: sample collection, fixation, staining, mounting, scanning, and data analyses. Another reason is the vast diversity of metazoans, particularly marine invertebrates. Because of marine invertebrates’ diverse sizes, morphologies, and physiologies, it is crucial to adjust experimental conditions and hardware configurations at each step, depending on the sample. Here, microCT imaging methods are explained in detail using three phylogenetically diverse marine invertebrates: Actinia equina (Anthozoa, Cnidaria), Harmothoe sp. (Polychaeta, Annelida), and Xenoturbella japonica (Xenoturbellida, Xenacoelomorpha). Suggestions on performing microCT imaging on various animals are also provided.
Introduction
Biological researchers generally have had to make thin sections and perform observations by light or electron microscopy in order to investigate the internal structures of opaque organisms. However, these methods are destructive and problematic when applied to rare or valuable specimens. Furthermore, several steps in the method, such as embedding and sectioning, are time consuming, and it can take several days to observe a sample, depending on the protocol. Moreover, when handling numerous sections, there is always a possibility of damaging or losing some sections. Tissue-clearing techniques are available for some specimens1,2,3,4,5 but are not yet applicable for many animal species.
To overcome these problems, some biologists have started using microfocus X-ray computed tomography (microCT) imaging6,7,8,9,10,11,12,13,14,15. In X-ray CT, the specimen is irradiated with X-rays from various angles that are generated from an X-ray source moving around the sample, and the transmitted X-rays are monitored by a detector that also moves around the sample. The X-ray transmission data obtained are analyzed to reconstruct cross-sectional images of the specimen. This method enables the observation of internal structures without destruction of the sample. Because of its safety and ease, it is commonly used in medical and dental applications, and CT systems can be found in hospitals and dental centers worldwide. Moreover, industrial X-ray CT is frequently used for observing non-medical samples for inspection and metrology in the industrial field. In contrast to medical CT, in which the X-ray source and the detectors are mobile, the two parts are fixed in industrial CT, with the sample rotating during scanning. Industrial CT generally produces higher resolution images than medical CT and is referred to as microCT (micrometer-level resolution) or nanoCT (nanometer-level resolution). Recently, research using microCT has rapidly increased in various fields of biology14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34.
Biological studies using CT initially targeted internal structures that mainly consist of hard tissue, such as bone. Advances in staining techniques using various chemical agents enabled the visualization of soft tissues in various organisms6,7,8,9,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34. Of these reagents, iodine-based contrast agents are relatively safe, inexpensive, and can be used for the visualization of soft tissues in various organisms7,14. Concerning marine invertebrates, microCT has been widely used on such animals as molluscs6,25,32,33, annelids18,19,20,28, and arthoropods21,23,29,31. However, there have been few reports on other animal phyla, such as bryozoans6, xenacoelomorphs26, and cnidarians24,30. In general, there have been fewer studies using microCT on marine invertebrates than those on vertebrates. One major reason for this limited use on marine invertebrates is the vast diversity observed in these animals. Because of their diverse sizes, morphologies, and physiologies, each species reacts differently to different experimental procedures. Therefore, it is crucial during sample preparation to choose the most appropriate fixation and staining reagent, and to set conditions at each step, adjusted for each species. Similarly, it is also necessary to set the scanning configurations, such as the mounting method, voltage, current, mechanical magnifying rate, and the space resolution power, appropriately for each sample. To overcome this problem, a simple and comprehensible manual that covers all of the necessary steps, explains how each step can be adjusted depending on the specimen, and shows detailed examples from multiple samples is essential.
In the present study, we describe the microCT imaging protocol step-by-step, from sample fixation to data analysis, using three marine invertebrate species. Specimens of the sea anemone Actinia equina (Anthozoa, Cnidaria) were collected near the Misaki Marine Biological Station, University of Tokyo. They had a spherical, soft body that was about 2 cm in diameter (Figure 1A-C). Harmothoe sp. (Polychaeta, Annelida) samples were also collected near Misaki Marine Biological Station. They were slender worms that were about 1.5 cm in length, with tough chaetae present along the whole body (Figure 1D). A Xenoturbella japonica35 (Xenoturbellida, Xenacoelomorpha) specimen was collected near Shimoda Marine Research Center, University of Tsukuba, during the 13th JAMBIO Coastal Organism Joint Survey. It was a soft-bodied worm that was about 0.8 cm in length (Figure 1E). Adjustments made for the conditions and configurations of each sample are explained in detail. Our study provides several suggestions on how to perform microCT imaging on marine invertebrates, and we hope that it will inspire biologists to utilize this protocol for their research.
Protocol
1. Fixation
- For Actinia equina, relax the animals in 10% MgCl2 seawater for about 15 min at room temperature. Transfer to 70% ethanol and store at room temperature.
- For Harmothoe sp., anesthetize the animals by placing them in ice-cold seawater for about 15 min. Transfer to 10% (v/v) formalin solution with seawater and store at room temperature.
- For Xenoturbella japonica, relax the animal using 7% MgCl2 in freshwater. Fix in 4% paraformaldehyde (PFA) in filtered seawater overnight. Place in 70% ethanol and store at 4 °C.
CAUTION: PFA is hazardous and must be handled with care.
2. Staining
- Transfer the samples to 50% ethanol and store at room temperature for 15 h. Replace the 50% ethanol with 25% ethanol and store at room temperature for 2 h.
NOTE: This is not necessary for the Harmothoe sp. sample in 10% (v/v) formalin solution with seawater. - Replace the solution with distilled water (DW) and store the samples in DW at room temperature for 2 h. Repeat this step three times.
- Prepare 25% Lugol solution by diluting the stock solution (below) to 25% with DW. Stock solution (100% Lugol solution) contains 10 g of KI and 5 g of I2, adjusted to 100 mL with DW.
NOTE: Lugol solution is light-sensitive, so store and handle the solution protected from light. Follow the regulations of each country and institution for iodine handling and waste disposal. - Decant the DW from the samples and pour in the 25% Lugol solution. Stain for 24 h at room temperature.
3. Stage Mounting
- Prepare 0.5% agarose by dissolving 500 mg agarose in 100 mL of DW in a 250 mL conical flask in a microwave (800 W, about 1-3 min). Cool to about 30-40 °C by keeping at room temperature.
CAUTION: Do not overheat or completely seal the flask when heating to prevent the agarose from boiling over. - Mount large samples such as A. equina using a 50 mL tube.
NOTE: Use 50 mL tubes for mounting large samples that do not fit in a 1,000 μL micropipette 'blue' tip.- Place the stained sample in a 60-mm dish with DW to wash off excessive staining solution from the surface.
- Gently pour 5 mL of 0.5% agarose into a 50 mL tube and harden the agarose on ice. Be careful not to make bubbles in the agarose.
- Gently add 20 mL of 0.5% agarose to the 50 mL tube and place on ice until the agarose begins to harden. Place the specimen within the 0.5% agarose using forceps. Be careful not to make bubbles in the agarose.
- Adjust the position and orientation of the sample with forceps and harden the agarose on ice.
- Place clay on the microCT mounting stage and set the 50 mL tube on the clay (Figure 2A).
- Mount small samples such as Harmothoe sp. and X. japonica using a 1,000 µL micropipette 'blue' tip.
NOTE: For smaller samples, 200 μL micropipette ‘yellow’ tip can be used. In this case, use 30 μL of agarose for the plug and add 200 μL of agarose or distilled water.- Draw up 100 µL of 0.5% agarose into a 1,000 µL micropipette 'blue' tip and harden the agarose by holding the tip over ice, making a plug in the tip (Figure 2B-a).
- Decant the stained sample into a 60-mm dish without using forceps.
- Gently transfer the sample using ring tweezers into another 60-mm dish with DW to wash off excessive staining solution from the surface.
- Add 1,000 µL of either 0.5% agarose or DW into the plugged tip (step 3.3.1) using a micropipette.
NOTE: Use of 0.5% agarose is recommended, but use DW for fragile or precious samples in which agarose should be avoided. - Gently transfer the sample from the 60-mm dish into the agarose or DW in the plugged tip using ring tweezers.
- Gently adjust the position and orientation of the sample with a petiolate needle or precision tweezers. Be careful not to make bubbles in the mounting medium. Make sure the sample is stable between the walls of the tip when DW is used as mounting medium (Figure 2D-b). Place the tip on ice to harden the agarose if it is used as mounting medium.
- Cut the tip off a new 1,000 µL micropipette 'blue' tip (Figure 2B-b,c) and insert the tip of the plugged tip into the new tip.
- Place clay on the microCT mounting stage and set the tips with the sample inside on the clay (Figure 2C,D).
NOTE: The staining solution will start to wash off the sample once it is placed in DW, so proceed to the next scanning step promptly.
4. MicroCT scanning
- Turn on the X-ray beam at 80 kV, 100 µA.
- While observing the X-ray transmission image at the center of the screen (Figure 3A), move the stage so that the whole sample can be seen by clicking on the X and Z axis buttons (Figure 3A), and manually adjusting the Y axis knob on the mounting stage (Figure 3B). Set the contrast of the image so that the internal structures can be observed by adjusting the contrast conditions (Figure 3A: Image contrast).
- Adjust the orientation of the sample by changing the angle of the tube/tip in the clay (Figure 2A). Rotate the stage 90° by setting the rotation axis (Figure 3A) to 90 and clicking on the relative movement button (Figure 3A). Perform the same maneuver four times to complete a full rotation.
NOTE: Manually turn off the X-ray beam each time the sample door is opened, unless the system turns it off automatically. - Move the stage so that the sample is at the center of the view by clicking on the Z axis button (Figure 3A) and by manually adjusting the Y axis knob on the mounting stage (Figure 3B). Turn the stage by 90° and do the same. Turn the stage 360° and check that the sample is at the center of the view from all directions.
- Move the stage along the x-axis toward the X-ray beam source by clicking on the X axis button (Figure 3A) to enlarge the sample and adjust as needed so that it just fits in the view (Figure 3C).
- Turn the stage 360° and adjust as needed so that the sample fits within view from all directions.
- Adjust the scanning conditions as shown in Table 1.
- Start scanning; it takes about 10 min.
5. Image reconstruction
- Start up the microCT system's accessory software (see the Table of Materials) and open the scanned data.
- Adjust differences in the rotation axis of the sample during scanning by clicking on the automatic shift value calculation button (Figure 4A: green box).
- Adjust the orientation of the image by rotating the orange arrows (Figure 4B). If the orientation was changed, repeat step 5.2.
- Click on the area tab (Figure 4C: magenta box) and trim areas where samples are not present (Figure 4C: yellow box).
- Click on the reconfiguration tab (Figure 4D: magenta box) and set the filters as follows to remove noise. Ring artifact reduce filter: Median filter -3; Noise elimination filter: Average filter-1.
- Perform reconfiguration by clicking on the reconfiguration button (Figure 4D: green box).
- Adjust image brightness and contrast by setting the black and white values as black value 0, white value 250 (Figure 4D: blue box).
- Save the reconstructed TIFF image dataset as an 8-bit TIFF by clicking on the save button. Rename TIFF files as following: Date_sample_resolution (µm)_number.tiff.
NOTE: The original microCT datasets from this study are available in the Figshare repository, doi:10.6084/m9.figshare.767083736.
6. Data analyses
- Start up the data analysis software (see the Table of Materials) and enable importing of TIFF files by clicking on the Database icon (Figure 5A: magenta box) and turning off the box shown in Figure 5B.
- Click on the import icon (Figure 5C: magenta box), select the dataset saved in section 5, and click open.
- Click on the copy links button (Figure 5D) to import the data.
- Display the 2D cross-section by clicking on the 2D viewer icon (Figure 5C: blue box).
- Calibrate the dataset by clicking on the 3D viewer tab (Figure 5E: magenta box) and entering the resolution value at scanning (which was 0.018 in this study [Figure 5F]).
- Click on the brightness/contrast icon (Figure 5E green box). Adjust the brightness and contrast by moving the cursor inside the displayed 2D image and changing the window level and window width (Figure 5G).
- Check other cross-section images by moving the scrollbar (Figure 5G: box).
- Change the orientation of the cross-section by clicking on the orientation icon (Figure 5E: blue box) and check images at all orientations (Figure 5H).
- Click on the displayed image, and choose the export tab to store cross-section images.
Representative Results
We performed microCT imaging on A. equina (Anthozoa, Cnidaria), Harmothoe sp. (Polychaeta, Annelida), and X. japonica (Xenoturbellida, Xenacoelomorpha) after staining the samples with 25% Lugol solution. The staining successfully enhanced the contrast of the internal structures in all specimens, enabling observations of internal soft tissues (Figure 6). Together with past reports6,7,16,19,22,23,24,25,26,28,30,31,32,33, this shows that microCT can be used on various marine invertebrates for visualizing their morphology, including soft internal tissues. Clear images were obtained even with the X. japonica specimen, whose epidermis was badly damaged (Figure 6F,G), showing that this method is applicable to fragile specimens with external damage.
Scanning only the region of interest, in contrast to a wider area, greatly increased the clarity and resolution of the image (compare Figure 6F and Figure 6G). However, a single high-resolution dataset of a whole specimen was reconstructed for Harmothoe sp. (Figure 6C) and X. japonica (Figure 6F) from multiple scans performed on different (but overlapping) parts of the specimen. The seams between each scan were inconspicuous in the reconstructed images. Our study shows that single high-resolution images can be obtained with cone-beam microCT systems. By scanning a larger area at high resolution, there is a smaller risk of overlooking small structures. Another advantage is that it is easier to locate the relative positions of structures that are situated far apart, such as the anterior and posterior tips of an elongated annelid.
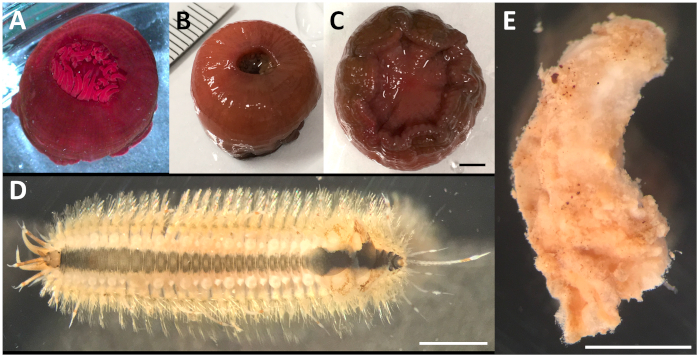
Figure 1: Marine invertebrate animals observed in this study. (A–C) Actinia equina (Anthozoa, Cnidaria). (A) Distal end of a live animal relaxed in 10% MgCl2 seawater. Distal (B) and proximal (C) ends after fixation in 70% ethanol. (D) Live anesthetized Harmothoe sp. (Polychaeta, Annelida), dorsal view with anterior to the left. Most of the elytra were already missing at this stage, with only four remaining near the posterior end. (E) Xenoturbella japonica (Xenoturbellida, Xenacoelomorpha) fixed in 70% ethanol. Right view, with anterior to the top. Because of circumstances at collection, its epidermis was starting to come off. Scale bars = 3 mm. Please click here to view a larger version of this figure.
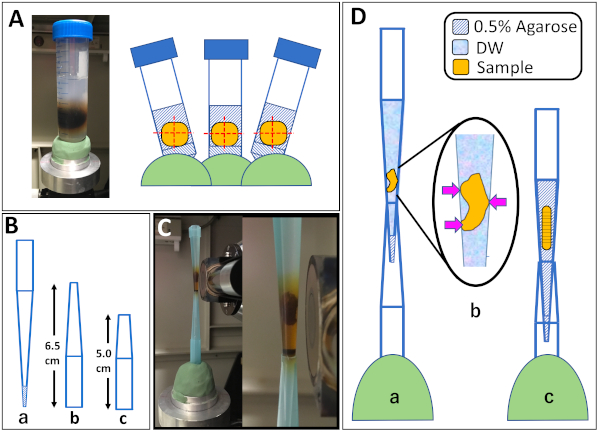
Figure 2: Mounting samples on the microfocus X-ray computed tomography system. (A) Mounting samples in a 50 mL tube using clay. The orientation of the sample could be adjusted using the clay. (B) Preparation of a 1,000 µL micropipette 'blue' tip for mounting small samples. a: Tip with its end plugged with 100 µL of 0.5% agarose (diagonal lines). The samples were placed in this tip. The tip with the sample was inserted into another 1,000 µL micropipette 'blue' tip (b, c) for mounting. b was used for Xenoturbella japonica, and c was used for Harmothoe sp. (C) Mounted X. japonica sample, overview (left) and close up (right). X-ray source can be seen to the right of the sample. (D) Diagrams for mounting samples in a 1,000 µL micropipette 'blue' tip. a: X. japonica sample in distilled water. b: the sample was in contact with the tip wall (arrows), so that it does not move while scanning. c: Harmothoe sp. sample in 0.5% agarose. Please click here to view a larger version of this figure.
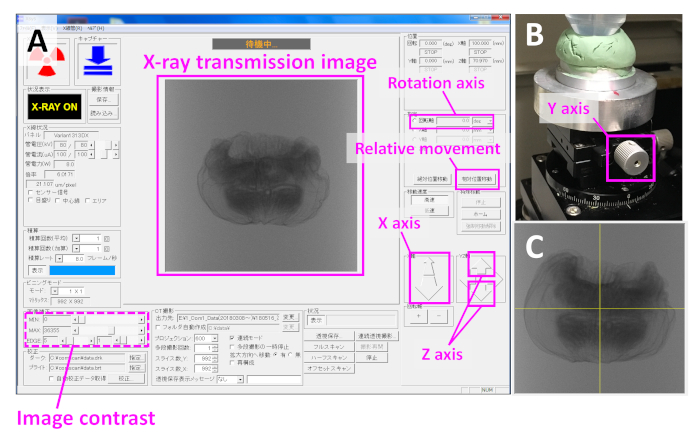
Figure 3: Scanning samples on the microfocus X-ray computed tomography system. (A) Operating screen during scanning of the microfocus X-ray computed tomography system showing an X-ray transmission image of an Actinia equina specimen. Adjust the contrast and brightness with the 'Image contrast' at the lower left. (B) View of the mounting stage showing the Y axis knob. (C) X-ray transmission image of the A. equina specimen after the mounting stage was moved closer to the X-ray beam source. Notice it is enlarged when compared to the image at the center of (A). Please click here to view a larger version of this figure.
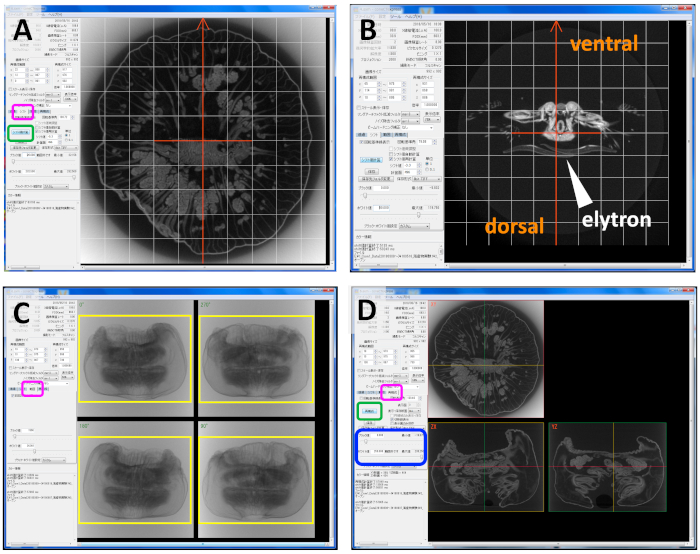
Figure 4: Operating screen of the image reconstruction system. (A) Screen for adjusting differences in the rotation axis of the sample during scanning, showing an Actinia equina specimen. Magenta box: shift tab; green box: automatic shift value calculation button. (B) Screen for adjusting the orientation of the image, with Harmothoe sp. shown. (C) Screen during the image reconstruction of A. equina, trimming the area outside the yellow box where no samples are present. Magenta box: area tab. (D) Screen during image reconstruction, showing the reconstructed image of A. equina. Magenta box: reconfiguration tab; green box: reconfiguration button; blue box: black and white value adjustment. Please click here to view a larger version of this figure.
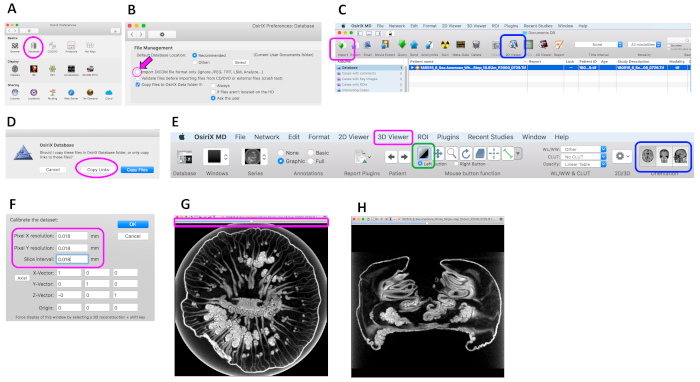
Figure 5: Operating screen of the image analysis system. (A) Preference window. The Database icon (magenta circle) was clicked to open the database file management window. (B) Database file management window. In this software, the box shown with an arrow needs to be off to enable importing TIFF files. (C) Menu and tool bars of the Database screen. Magenta box = import icon; blue box = 2D viewer icon. (D) Dataset import window. Magenta circle = copy links button. (E) Menu and tool bars of the 2D viewer screen. Magenta box = 3D viewer tab; green box = brightness/contrast icon; blue box = orientation icon. (F) Calibration setting window. Enter the desired resolution values within the columns in the magenta box. (G) Cross-section of an Actinia equina specimen displayed in the 2D viewer window for adjusting brightness and contrast. Magenta box: scrollbar for checking other cross-sections. (H) Cross-section of A. equina displayed in the 2D viewer window with a different orientation to (G). Please click here to view a larger version of this figure.
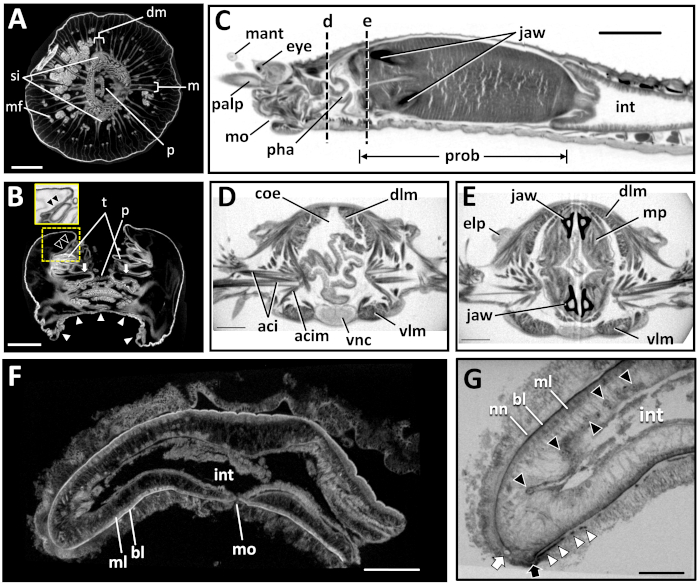
Figure 6: Scanned and reconstructed images of marine invertebrates. (A) Transverse and (B) longitudinal sections of Actinia equina. The area inside the dotted yellow box in (B) is enlarged in the inset. Abbreviations: dm, pair of directive mesenteries; m, pair of perfect mesenteries; mf, mesenterial filament; p, pharynx; si, siphonoglyphs; t, tentacle; arrows, oral disc; white arrowheads, pedal disc; black arrowheads, sphincter muscle. Scale bars in A, B = 3 mm. (C-E) Harmothoe sp. (C) Sagittal section of the anterior part. (D, E) Transverse section at the dotted lines d and e in (C). Abbreviations: aci = aciculum; acim = acicular muscle; coe = coelom; dlm = dorsal longitudinal muscle; elp = elytrophore; eye = eye; int = intestine; jaw = jaw; mant = median antenna; mo = mouth; mp = muscles of proboscis; palp = palp; pha = pharynx; prob = proboscis; vlm = ventral longitudinal muscle; vnc = ventral nerve cord. Scale bars: C = 1 mm; D, E = 0.3 mm. (F, G) Xenoturbella japonica. (F) Sagittal section of the whole sample. (G) Sagittal section of the anterior part. bl = basal lamina; int = intestine; ml = muscle layer; mo = mouth; nn = intraepidermal nerve net; white arrow = statocyst; black arrow = frontal pore; white arrowheads = ventral glandular network; black arrowheads = oocytes. Scale bars: F = 1 mm, G = 0.5 mm. Please click here to view a larger version of this figure.
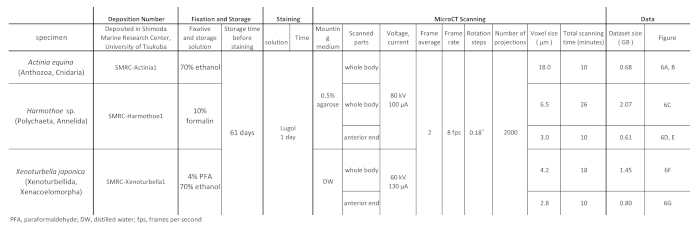
Table 1: Sample preparation and scanning protocol for each specimen.
Discussion
Fixatives using formalin, such as the 10% (v/v) formalin solution in seawater used in this study, are known to preserve the morphology of diverse marine invertebrates and are often used for microCT imaging18,24,25,26,28,30,33. However, restrictions on the use of this chemical have become strict in some countries in recent years, and substitutes such as paraformaldehyde or glutaraldehyde may be used. If there are plans to extract DNA after scanning, it is better to avoid using formalin as a fixative, because it is known to fragment DNA. In this case, the use of fixatives that preserve DNA, such as 70% ethanol, is recommended. In this study, the cnidarian A. equina was fixed using 70% ethanol, and clear microCT images were obtained from the 70% ethanol-fixed samples (Figure 6A, B).
In a previous study that performed microCT scanning of various cnidarian taxa, many samples were dehydrated in 100% ethanol, and some were critical-point-dried prior to scanning24. Although soft internal organs such as tentacle clusters, muscles, and gonads were successfully observed in their study, dehydration and drying processes are known to result in major artefacts such as the deformation and contraction of soft tissues11,21. In the present study, we were able to observe the internal structures of the cnidarian A. equina fixed in 70% ethanol and stained with 25% Lugol solution (Figure 6A,B). Our protocol, without any dehydration or drying steps, is preferable, and should be performed whenever possible to reduce the risk of damage to the specimens and artefacts during scanning.
Lugol solution, iodine solution, and phosphotungstic acid (PTA) are staining solutions that are often used on biological samples in microCT imaging6,7,9,14,16,17,20,26,27,38. From our experience of using various biological samples, Lugol solution provided the best results for many samples, with dark staining in a relatively short amount of time. Iodine solution yielded only very weak stains, and PTA required a long time for sufficient staining and the stained specimens showed strong contractions. Therefore, all specimens were stained with Lugol solution in this study. However, although Lugol solution is recommended, the appropriate staining solution differs between specimens, and we suggest that trials using other staining solutions be performed if there are enough specimens. Irrespective of the staining solution, samples do contract during staining37,38, so it is important to keep the staining time short.
A critical step in microCT scanning is to mount the sample so as to prevent it from moving. In this study, this was performed in two steps, first using agarose as the direct mounting medium, and then using clay to mount the tube that contained the sample to the stage. For the first step, various low-density mounting media have been used in previous studies, including ethanol6,17,20,25,30, agarose9,29, and floral foam15,22,31. Agarose was selected in this study as it is a low-cost chemical that is accessible worldwide. A disadvantage of agarose is that it may be difficult to retrieve the sample from the hardened agarose after scanning but using low-melting-point agarose makes this retrieval step easier. For the second step, jaw clamps or screws are often used6,9,17. Clay was selected in this study as it enables fine adjustments in the orientation and angle of the sample. Caution is needed for experiments with long scanning times, as the possibility of the sample moving is higher when using clay rather than jaw clamps or screws.
A previous study conducted microCT scanning on seven polychaete species with body lengths of 2-8 mm, smaller than the Harmothoe sp. used in this study16. They were able to generate high-resolution images, and showed organs such as vascular systems and individual chaeta clearer than in the present study. The main cause of this difference was not the protocol, but the specifications of the microCT systems used. The system used in the previous study was equipped with an 11-megapixel charge-coupled device camera (4000 x 2672 pixels) with a maximum resolution of <0.8 µm/pixel16. The active image matrix size of the system used in this study was 992 x 992 pixels, with a maximum resolution of >5 µm/pixel. Therefore, the spatial resolution of the microCT system used in this study was inferior to the high performance microCT system used in Faulwetter et al.16. This difference was particularly noticeable when scanning specimens smaller than 8 mm, in which we experienced a lack of resolution (data not shown). However, because fewer data were obtained during scanning in this study, the scanning time was much shorter than in the previous study16 (data: 992 x 992 and 4000 x 2672 pixels, respectively; scanning time: 10 to 26 min and 30 min to several hours, respectively). A short scanning time reduces the discoloration of the iodine staining, allowing the use of Lugol solution, which is a good staining solution with a high penetration rate, but easily diffuses in DW34. A short scanning time also decreases the possibility of the sample moving during scanning, which enabled the use of a simple mounting method using agarose or DW (Figure 2). Longer scanning times also have the disadvantage of possible sample shrinkage blur in images. Several other mechanical and hardware problems that can occur during long scans have also been reported39. Therefore, when using microCT systems, it is important to accurately understand the specification of each system, and to choose the right system in terms of specimen size or research aim. In some cases, a microCT system with low resolution may be sufficient.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank Toshihiko Shiroishi for his assistance and for providing the research environment during this study. We are grateful to Kensuke Yanagi and Takato Izumi for advice on A. equina, and Masaatsu Tanaka for advice on the Harmothoe sp. specimen. We would like to thank the staff at Shimoda Marine Research Center, University of Tsukuba, and Misaki Marine Biological Station, The University of Tokyo for their help in sample collections. We would like to thank Editage (www.editage.jp) for English language editing. This work was supported by the JSPS Grant-in-Aid for Young Scientists (A) (JP26711022) to HN, and JAMBIO, Japanese Association for Marine Biology.
Materials
| 250-ml Erlenmeyer flask | Corning | CLS430183 | |
| 5-ml Sampling tube ST-500 | BIO-BIK | 103010 | |
| 50-ml Polypropylene tube | Greiner Bio One International | 227261 | |
| 60-mm Non-treated Dish | IWAKI | 1010-060 | |
| Agarose | Promega | V3125 | |
| Ecological grade tip (blue) 1000 µl | BMBio | BIO1000RF | |
| Ethanol | Wako Pure Chemical Industries | 057-00451 | |
| Formalin | Wako Pure Chemical Industries | 061-00416 | |
| Iodine | Wako Pure Chemical Industries | 094-05421 | |
| Magnesium chloride hexahydrate | Wako Pure Chemical Industries | 135-00165 | |
| OsiriX DICOM Viewer | Pixmeo SARL | OsiriX MD v10.0 | https://www.osirix-viewer.com |
| Paraformaldehyde | Wako Pure Chemical Industries | 163-25983 | |
| Petiolate needle | AS ONE | 2-013-01 | |
| Pipetman P200 Micropipette | GILSON | F123601 | |
| Pipetman P1000 Micropipette | GILSON | F123602 | |
| Potassium iodide | Wako Pure Chemical Industries | 166-03971 | |
| Precision tweezers 5 | DUMONT | 0302-5-PS | |
| QuickRack MultI fit tip (yellow) 200 ul | Sorenson | 10660 | |
| Razor blades | Feather | FA-10 | |
| Ring tweezers | NAPOX | A-26 | |
| Stereoscopic microscope | Leica | MZ95 | |
| X-ray Micro-CT imaging system | Comscantechno | ScanXmate-E090S105 |
Referências
- Susaki, E. A., Tainaka, K., Perrin, D., Yukinaga, H., Kuno, A., Ueda, H. R. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nature Protocols. 10, 1709-1727 (2015).
- Susaki, E. A., Ueda, H. R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: toward organism-level systems biology in mammals. Cell Chemical Biology. 23, 137-157 (2016).
- Silvestri, L., Costantini, I., Sacconi, L., Pavone, F. S. Clearing of fixed tissue: a review from a microscopist’s perspective. Journal of Biomedical Optics. 21, 081205 (2016).
- Greenbaum, A., et al. Bone CLARITY: clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Science Translational Medicine. 9, (2017).
- Konno, A., Okazaki, S. Aqueous-based tissue clearing in crustaceans. Zoological Letters. 4, 13 (2018).
- Metscher, B. D. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology. 9, 11 (2009).
- Metscher, B. D. MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Developmental Dynamics. 238 (3), 632-640 (2009).
- Degenhardt, K., Wright, A. C., Horng, D., Padmanabhan, A., Epstein, J. A. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circulation Cardiovascular Imaging. 3 (3), 314-322 (2010).
- Metscher, B. D. X-ray microtomographic imaging of intact vertebrate embryos. Cold Spring Harbor Protocols. 12, 1462-1471 (2011).
- Boistel, R., Swoger, J., Kržič, U., Fernandez, V., Gillet, B., Reynaud, E. G. The future of three-dimensional microscopic imaging in marine biology. Marine Ecology. 32, 438-452 (2011).
- Mizutani, R., Suzuki, Y. X-ray microtomography in biology. Micron. 43, 104-115 (2012).
- Merkle, A. P., Gelb, J. The ascent of 3D X-ray microscopy in the laboratory. Microscopy Today. 21, 10-15 (2013).
- Ziegler, A., Menze, B. H., Zander, J., Mosterman, P. J. Accelerated acquisition, visualization, and analysis of zooanatomical data. Computation for humanity. Information technology to advance society. , 233-260 (2013).
- Gignac, P. M., et al. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. Journal of Anatomy. 228 (6), 889-909 (2016).
- du Plessis, A., Broeckhoven, C., Guelpa, A., le Roux, S. G. Laboratory x-ray micro-computed tomography: a user guideline for biological samples. GigaScience. 6 (6), 1-11 (2017).
- Faulwetter, S., Vasileiadou, A., Kouratoras, M., Dailianis, T., Arvanitidis, C. Micro-computed tomography: Introducing new dimensions in taxonomy. ZooKeys. 263, 1-45 (2013).
- Staedler, Y. M., Masson, D., Schonenberger, J. Plant tissues in 3D via X-ray tomography: simple contrasting methods allow high resolution imaging. PLoS One. 8 (9), 75295 (2013).
- Fernández, R., Kvist, S., Lenihan, J., Giribet, G., Ziegler, A. Sine Systemate Chaos? A Versatile Tool for Earthworm Taxonomy: Non-Destructive Imaging of Freshly Fixed and Museum Specimens Using Micro-Computed Tomography. PLoS One. 9 (5), 96617 (2014).
- Paterson, G. L. J., et al. The pros and cons of using micro-computed tomography in gross and microanatomical assessments of polychaetous annelids. Memoirs of Museum Victoria. 71, 237-246 (2014).
- Faulwetter, S., Dailianis, T., Vasileiadou, K., Kouratoras, M., Arvanitidis, C. Can micro-CT become an essential tool for the 21st century taxonomist? An evaluation using marine polychaetes. Microscopy and Analysis. 28, 9-11 (2014).
- Sombke, A., Lipke, E., Michalik, P., Uhl, G., Harzsch, S. Potential and limitations of X-ray micro-computed tomography in arthropod neuroanatomy: a methodological and comparative survey. Journal of Comparative Neurology. 523, 1281-1295 (2015).
- Landschoff, J., Plessis, A., Griffiths, C. L. A dataset describing brooding in three species of South African brittle stars, comprising seven high-resolution, micro X-ray computed tomography scans. GigaScience. 4 (1), 52 (2015).
- Keiler, J., Richter, S., Wirkner, C. S. The anatomy of the king crab Hapalogaster mertensii Brandt, 1850 (Anomura: Paguroidea: Hapalogastridae) – new insights into the evolutionary transformation of hermit crabs into king crabs. Contributions to Zoology. 84 (2), 149-165 (2015).
- Holst, S., Michalik, P., Noske, M., Krieger, J., Sötje, I. Potential of X-ray micro-computed tomography for soft-bodied and gelatinous cnidarians with emphasis on scyphozoan and cubozoan statoliths. Journal of Plankton Research. 38, 1225-1242 (2016).
- Moles, J., Wägele, H., Ballesteros, M., Pujals, &. #. 1. 9. 3. ;., Uhl, G., Avila, C. The End of the Cold Loneliness: 3D Comparison between Doto antarctica and a New Sympatric Species of Doto (Heterobranchia: Nudibranchia). PLoS One. 11 (7), 0157941 (2016).
- Nakano, H., et al. A new species of Xenoturbella from the western Pacific Ocean and the evolution of Xenoturbella. BMC Evolutionary Biology. 17, 245 (2017).
- Tsuda, K., et al. KNOTTED1 Cofactors, BLH12 and BLH14, Regulate Internode Patterning and Vein Anastomosis in Maize. Plant Cell. 29 (5), 1105-1118 (2017).
- Parapar, J., Candás, M., Cunha-Veira, X., Moreira, J. Exploring annelid anatomy using micro-computed tomography: A taxonomic approach. Zoologischer Anzeiger. 270, 19-42 (2017).
- Akkari, N., Ganske, A. S., Komerički, A., Metscher, B. New avatars for Myriapods: Complete 3D morphology of type specimens transcends conventional species description (Myriapoda, Chilopoda). PLoS One. 13 (7), 0200158 (2018).
- Gusmao, L. C., Grajales, A., Rodriguez, E. Sea anemones through X-rays: visualization of two species of Diadumene (Cnidaria, Actiniaria) using micro-CT. American Museum Novitates. 3907, (2018).
- Landschoff, J., Komai, T., du Plessis, A., Gouws, G., Griffiths, C. L. MicroCT imaging applied to description of a new species of Pagurus Fabricius, 1775 (Crustacea: Decapoda: Anomura: Paguridae), with selection of three-dimensional type data. PLoS One. 13 (9), 0203107 (2018).
- Machado, F. M., Passos, F. D., Giribet, G. The use of micro-computed tomography as a minimally invasive tool for anatomical study of bivalves (Mollusca: Bivalvia). Zoological Journal of the Linnean Society. , (2018).
- Sasaki, T., Endo, K., Kogure, T., Nagasawa, H., et al. 3D visualization of calcified and non-calcified molluscan tissues using computed tomography. Biomineralization. , 83-93 (2018).
- Maeno, A., Tsuda, K. Micro-computed Tomography to Visualize Vascular Networks in Maize Stems. Bio-protocol. 8 (1), 2682 (2018).
- Nakano, H., et al. Correction to: A new species of Xenoturbella from the western Pacific Ocean and the evolution of Xenoturbella. BMC Evolutionary Biology. 18, 83 (2018).
- Maeno, A., Kohtsuka, H., Takatani, K., Nakano, H. MicroCT files from ‘Microfocus X-ray computed tomography (microCT) imaging of Actinia equina (Cnidaria), Harmothoe sp. (Annelida), and Xenoturbella japonica (Xenacoelomorpha)’. figshare. , (2019).
- Vickerton, P., Jarvis, J., Jeffery, N. Concentration-dependent specimen shrinkage in iodine-enhanced microCT. Journal of Anatomy. 223 (2), 185-193 (2013).
- Buytaert, J., Goyens, J., De Greef, D., Aerts, P., Dirckx, J. Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro-CT and light sheet fluorescence microscopy (LSFM). Microscopy and Microanalysis. 20 (4), 1208-1217 (2014).
- Sasov, A., Liu, X., Salmon, P. L. Compensation of mechanical inaccuracies in micro-CT and nano-CT. Proceedings of SPIE. 7078, 70781 (2008).

