Localization of SUMO-modified Proteins Using Fluorescent Sumo-trapping Proteins
Summary
SUMO is an essential and highly conserved, small ubiquitin-like modifier protein. In this protocol we are describing the use of a stress-tolerant recombinant SUMO-trapping protein (kmUTAG) to visualize native, untagged SUMO conjugates and their localization in a variety of cell types.
Abstract
Here we are presenting a novel method to study the sumoylation of proteins and their sub-cellular localization in mammalian cells and nematode oocytes. This method utilizes a recombinant modified SUMO-trapping protein fragment, kmUTAG, derived from the Ulp1 SUMO protease of the stress-tolerant budding yeast Kluyveromyces marxianus. We have adapted the properties of the kmUTAG for the purpose of studying sumoylation in a variety of model systems without the use of antibodies. For the study of SUMO, KmUTAG has several advantages when compared to antibody-based approaches. This stress-tolerant SUMO-trapping reagent is produced recombinantly, it recognizes native SUMO isoforms from many species, and unlike commercially available antibodies it shows reduced affinity for free, unconjugated SUMO. Representative results shown here include the localization of SUMO conjugates in mammalian tissue culture cells and nematode oocytes.
Introduction
The purpose of this method is to facilitate the study and analysis of SUMO-conjugated proteins using the recombinant SUMO-trapping UTAG (Ulp domain Tag) protein. As detailed below, UTAG can be used in lieu of other reagents and approaches to purify, detect, and visualize SUMO-modified proteins. Depending on growth conditions, cells may contain hundreds or thousands of proteins that are modified with SUMO or SUMO chains (for review see Kerscher et al. 20061 and Kerscher 20162). This represents a considerable difficulty for the functional analyses of specific SUMO-modified proteins, especially since only a fraction of a particular sumoylation target is actually modified3. In addition to their roles in essential cellular processes such as transcriptional regulation, protein homeostasis, the response to cellular stress, and chromatin remodeling during mitosis and meiosis; it has now become sufficiently clear that SUMO, SUMO-modified proteins, and SUMO pathway components also have potential as biomarkers for pathologies such as cancer and neurodegenerative disorders4,5,6,7. This underscores the need for robust, reliable, and readily available tools and innovative approaches for the detection and functional analysis of SUMO-modified proteins in a variety of cells and samples.
In many systems, SUMO-specific antibodies are the reagents of choice for the detection, isolation and functional analyses of SUMO-modified proteins8,9. However, some commercially available SUMO-specific antibodies are expensive, limited in quantity or availability, prone to exhibit wildly variable affinities and cross-reactivity, and in some instances lack reproducibility10. One alternative approach is the expression of epitope-tagged SUMO in transformed cells and organisms, but linking epitope tags to SUMO may artificially lower its conjugation to protein targets11. Additionally, epitopes are not useful when untransformed cells or tissues are evaluated.
Ulp1 is a conserved SUMO protease from S. cerevisiae that both processes the SUMO precursor and desumoylates SUMO-conjugated proteins12. We developed the UTAG reagent based on the serendipitous observation that a mutation of the catalytic Cysteine (C580S) in Ulp1's SUMO processing Ulp domain (UD) not only prevents SUMO cleavage but also traps SUMO-conjugated proteins with high avidity12. For simplicity, we referred to this carboxy-terminal SUMO-trapping Ulp1(C580S) fragment as UTAG (short for UD TAG). UTAG is a recombinant pan-SUMO trapping protein that represents a useful alternative to anti-SUMO antibodies used for the isolation and detection of SUMO-modified proteins. Importantly, it specifically recognizes natively-folded, conjugated SUMO and not just one or several epitopes on SUMO. To improve both the protein stability and SUMO binding strength of UTAG, we generated a variant of UTAG from the stress-tolerant yeast Kluyveromyces marxianus (Km). KmUTAG tightly binds SUMO-conjugates with nanomolar affinity13. Additionally, kmUTAG is resistant to elevated temperatures (42 °C), reducing agents (5 mM TCEP – Tris(2-carboxyethyl)phosphine hydrochloride), denaturants (up to 2 M Urea), oxidizing agents (0.6% hydrogen peroxide), and non-ionic detergents. This stress tolerance is beneficial during harsh purification condition and prolonged incubation times, ensuring its stability and SUMO-trapping activity. Not surprisingly, however, KmUTAG's SUMO-trapping activity is incompatible with cysteine-modifying reagents, ionic detergents and fully denatured protein extracts. The remarkable affinity and properties of KmUTAG indicate that this reagent may become part of the standard repertoire for the study of sumoylated proteins in multiple species.
Here we provide a simple method to detect SUMO-modified proteins in mammalian cells and nematodes using a recombinant fluorescent mCherry-KmUTAG fusion protein (kmUTAG-fl).
Protocol
1. SUMO detection in fixed tissue culture cells using recombinant KmUTAG-FL SUMO-trapping protein
- Grow tissue culture cells of choice on 22 mm round cover slips in 6-well TC plates until 70%–80% confluent. Perform steps 1.2–1.8 in the 6-well plate.
- Wash cells briefly with 1 mL of DPBS (Dulbecco's phosphate-buffered saline)
- For fixation, prepare a fresh solution of 4% Paraformaldehyde (PFA) solution (diluted in DPBS). To fix cells, add 2 mL 4% PFA in DPBS to each well. Incubate the cells for 20 min at room temperature. NOTE: All steps using PFA should be performed in a laboratory safety hood and PFA must be disposed properly.
- Wash the fixed cells 3x in 1 mL DPBS while nutating the plate, 5 min each wash.
- To permeabilize cells, incubate for 15 min with 0.1% Triton X-100 in DPBS
- Wash the cells 3x in 1 mL DPBS while nutating the plate, 5 min each wash
- Incubate the cells with 500 µL of 0.1 M Glycine-HCL (pH 2.0) for 10 s, then neutralize pH immediately with 500 µL of 10x SUMO Protease Buffer (SPB).
- Wash the cells 3x in 1 mL DPBS while nutating plate, 5 min each wash
- Remove the coverslips from the well and place them in a humidity chamber. Then proceed with incubations on the coverslip as follows:
- For UTAG-fl staining only: mix 1 µg UTAG-fl with 100 µL of 1x SPB containing 5 mM TCEP in a tube, pipette the mix onto the cells on the coverslip, and incubate at room temperature for 1 h in the humidity chamber.
- Optionally, for UTAG-FL and anti SUMO1 antibody co-staining, proceed with the following:
- Mix in a tube 1 µg UTAG-FL and 0.5 µL SUMO2/3 8A2 (obtained for Developmental Studies Hybridoma Bank9) with 100 µL of blocking buffer, pipette the mix onto the coverslip, and incubate in room temperature for 1 h.
- Wash cells on the coverslip 3 times with 200 µL DPBS, 5 min each wash.
- Mix in a tube 0.5 µL anti-mouse Alexa Fluor 488 conjugated antibody with 100 µL Blocking buffer, pipette the mix onto the coverslip, and incubate in room temperature for 1 h.
- To wash coverslips, pipette 200 µL DPBS on each coverslip and leave in place for 10 min. Repeat the wash 2 more times.
- Remove coverslip from the last wash and invert it onto a pre-cleaned microscopy slide with a drop of mounting medium (see Table of Materials).
- Store overnight in a -20°C freezer before viewing under the microscope. Visualize using the appropriate filter sets for DAPI (DNA), Texas Red (kmUTAG-fl), and Alexa Fluor 488 (if optional SUMO2/3 co-staining is performed).
2. SUMO detection in fixed nematode gonads using UTAG-fl
- Transfer adult hermaphrodites to an 8 µL droplet of egg buffer14 on a plus-charged slide that has been coated with poly-L-lysine. Release gonads from the worms using 27.5 G needles. Proceed with either antibody labeling or UTAG-fl labeling.
- For antibody labeling samples, proceed as follows:
- Freeze-crack samples in liquid nitrogen and then fix overnight in -20 °C methanol in a Coplin jar.
- Also in Coplin jars, wash slides for 3 times in 1x PBS, then block for 20 min in PBS containing 0.5% BSA and 0.1% Tween 20.
- Add 30 µL of anti-SUMO 6F2 antibody (1:10) to each slide covering the specimens. Incubate overnight in a humidity chamber at 4 °C.
NOTE: SUMO 6F2 was obtained from the Developmental Studies Hybridoma Bank15. - In Coplin jars, wash slides for 2 min in 1x PBS, then cover and incubate specimens with 30 µL of DyLight 488 goat-anti mouse secondary antibody (1:200) for 1.5 hours in a humidity chamber at room temperature.
- In Coplin jars, wash slides for 2 min in 1x PBS, perform a quick dip in dH20, and then mount the slides with mounting medium (see Table of Materials).
- For KmUTAG-fl labeling, proceed with the following:
- To fix cells, add 1 volume of 8% PF to samples for a final concentration of 4% PF. Fix for 10 min in a humidity chamber and then quench the reaction by transferring slides to a Coplin jar containing 1x PBS with 0.1 M glycine for at least 5 min.
- In Coplin jars, wash cells for 5 min in 1x PBS, then permeabilize the samples in 1x PBS containing 0.1% Triton-X for 10 min.
- Wash in a Coplin jar for at least 5 min in 1x PBS, then add 200 µL of 0.1 M Glycine-HCl (pH = 2.0) to the samples on the slide for 10 seconds. Immediately add 200 µL of 10x SPB to neutralize the pH.
- Wash the slide in 1x PBS in a Coplin jar for 5 min.
- Remove slide from wash and pipette 100 µL of 1x SPB + 5mM TCEP containing 2 µg of UTAG-fl to the nematodes on the slides, and incubate in humidity chamber for 1 h without rocking.
- Return the slide to the Coplin jar and wash for 15 min in 1x PBS
- Remove the slide from the wash, use a laboratory wipe to dry around the sample, and then mount the slides with 5 µL of mounting medium (see Table of Materials). Store the slides at 4 °C. Visualize using the appropriate filter sets for DAPI (DNA), Texas Red (kmUTAG-fl), and DyLight 488 (SUMO2/3).
Representative Results
KmUTAG-fl is a recombinant, mCherry-tagged SUMO-trapping protein. To produce kmUTAG-fl, we cloned a codon-optimized mCherry-kmUTAG into the pSPOT1 bacterial overexpression plasmid (Figure 1). After induction, the kmUTAG-fl protein was purified on Spot-TRAP, eluted, and frozen until further use. To ensure the SUMO-trapping activity of KmUTAG-fl, we confirmed binding to SUMO1-conjugated beads and precipitation of a SUMO-CAT fusion protein (data not shown, but see previous work13).
KmUTAG-fl incubated with fixed PNT2 cells showed a distinct nuclear staining when observed using the appropriate filter set (Chroma) and a 100x oil-immersion objective on a Epifluorescent Zeiss Axioplan Microscope (Figure 2– Top right panel). Both diffuse nuclear staining and distinct nuclear foci were visible. Nuclear localization was confirmed using co-staining with DAPI (Figure 2– top left panel). Consistent with the SUMO-trapping activity of kmUTAG-fl the nuclear localization pattern was reminiscent of SUMO2/3 staining. Co-staining with anti SUMO2/3 8A2 antibody (Figure 2 – bottom left panel) confirmed the co-localization of kmUTAG-fl with the SUMO2/3 signal (Figure 2– merge, bottom right). This validates the efficacy of KmUTAG-fl to detect SUMO2/3 in mammalian cells.
Since kmUTAG-fl exhibits pan-SUMO specificity, we also tested it on C. elegans nematodes to determine if the localization pattern of kmUTAG-fl would match the previously reported patterns of anti-SUMO antibodies and mCherry::SUMO fusion proteins 8,15. Isolated gonads from oocyte-producing adult hermaphrodites were processed for labeling with either anti-SUMO antibody or kmUTAG-fl. Consistent with previous reports 8, anti-SUMO antibody initially localized to the nucleoplasm of late meiotic prophase oocytes (Figure 3A,C). It then redistributed to the central ring complex (RC) of the paired homologs (bivalents) as the nuclear envelope broke down and the chromosomes congress towards the metaphase plate (Figure 3B,D). Components of the ring complex, which is situated between the DAPI-stained homologs, include both GEI-17(a E3 SUMO ligase) and SUMO-conjugated chromokinesin 8. In parallel preparations, gonads labeled with KmUTAG-fl revealed similar patterns (Figure 3E,F). KmUTAG-fl shifted from labeling the nucleoplasm to concentrating on the ring complex, as oocytes began (-1 oocyte in Figure 3E) and completed (-1 oocyte in Figure 3F) the process of nuclear envelope breakdown. These results validate KmUTAG-fl as a useful tool for the analysis of meiosis and SUMO-related processes in C. elegans and possibly other nematodes.

Figure 1. Schematic representation of the kmUTAG-fl SUMO-trapping fusion protein used in this method. The Spot-tag vector pSPOT1 is used for the expression of KmUTAG-fl. Please click here to view a larger version of this figure.
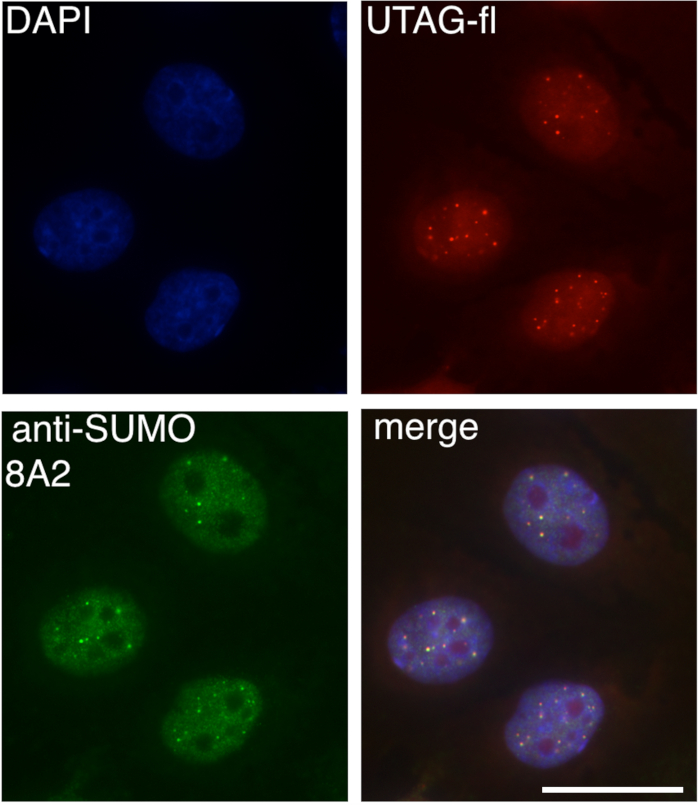
Figure 2. kmUTAG-fl colocalization with SUMO2/3 in mammalian cells. PNT2 cells were grown on coverslips, fixed, and stained with both KmUTAG-fl and anti-SUMO2 8A2 antibody, before applying mounting media containing DAPI. Slides were visualized using a confocal microscope and appropriate filters for DAPI (DNA), mCherry (kmUTAG-fl), and GFP (anti SUMO2/3). kmUTAG-fl colocalized with SUMO2/3 in PNT2 cells. Scale bar = 20 µm. Please click here to view a larger version of this figure.
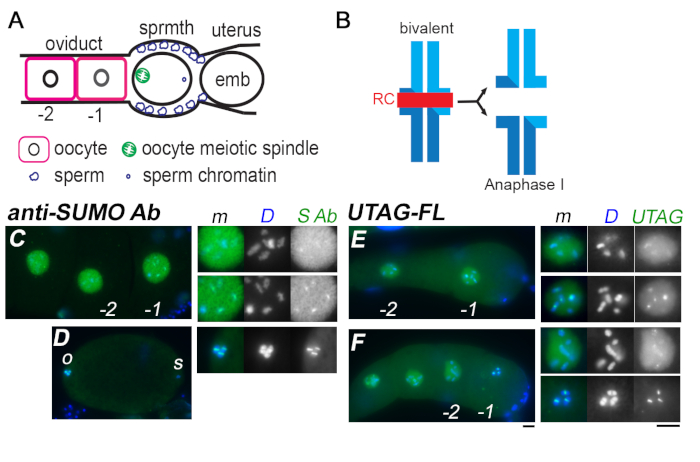
Figure 3. Comparison of SUMO labeling by anti-SUMO antibodies and KmUTAG-fl during C. elegans oocyte maturation. (A) Schematic of the proximal C. elegans gonad. Developing oocytes enter the spermatheca (sprmth) one-by-one where they are fertilized before entering the uterus. Oocytes in the -1 position, immediately adjacent to the spermatheca undergo nuclear envelope breakdown prior to fertilization. After fertilization, the sperm chromatin remains in a condensed state until the oocyte chromosomes complete their meiotic divisions. (B) Schematic showing the structure of a chromosomal bivalent that forms after homologous chromosomes have recombined, disassembled their synaptomenal complex, and have condensed in preparation for metaphase. A central ring complex (RC) between the homologs is enriched not only in aurora kinase and the checkpoint protein BUB-1 but also the E3 SUMO ligase GEI-17 and chromokinesin. (C-D) A line of developing oocytes within the oviduct (C) and a newly fertilized oocyte in metaphase of meiosis I (D) labeled with anti-SUMO antibody. (C-D) Full-sized images at the right show the merged (m) and single channel DAPI (D) and anti-SUMO antibody (S Ab) images of the -2 and -1 nuclei and the metaphase I spindle region. o = metaphase I chromosomes of oocyte, s = sperm chromatin mass. (E-F) Developing oocytes within the oviduct labeled with KmUTAG-fl. Concentration of KmUTAG-fl at the ring complex begins shortly before nuclear envelope breakdown (-1 oocyte in E) and becomes largely restricted to the ring complex after nuclear envelope breakdown (-1 oocyte in F). Blue = DAPI. Scale bar = 5 µm. Please click here to view a larger version of this figure.
Discussion
Here we introduce the use of kmUTAG-fl, a recombinant protein, for functional studies of SUMO in fixed mammalian cells and dissected nematode gonads. KmUTAG-fl is a stress-tolerant pan-SUMO specific reagent that recognizes and traps native SUMO-conjugated proteins and SUMO chains. Since SUMO's tertiary structure is highly conserved it is very likely that SUMO variants from additional model and non-model systems can be analyzed with the kmUTAG-fl reagent. As such, KmUTAG-fl may represent a useful alternative or secondary reagent for traditional antibody staining protocols.
There is ample precedent for the use of non-antibody alternatives, including lectins for carbohydrate detection as well as aptamer nucleotides and affimer peptides for in vivo labeling of proteins 16,17,18,19. Additionally, affimers and monobodies have been generated that bind SUMO and prevent the interaction with SUMO-interacting motifs (SIMs) on other protein18,20,21. However, to our knowledge, KmUTAG-fl is the first recombinant fluorescent pan-SUMO-trapping protein for the direct visualization of SUMO conjugates in fixed cells. KmUTAG-fl is derived from Ulp1 of a stress-tolerant yeast, K. marxianus, and this may explain its remarkable stability 13. Unlike most antibodies, KmUTAG does not require a secondary antibody for visualization, and stained cells are readily visible under the fluorescence microscope. Both our analyses of SUMO conjugates in mammalian cells and nematode gonads suggest that KmUTAG-based imaging compares favorably to SUMO antibody-staining and shows little noise (e.g. compare Figure 3A,B versus Figure 3D,E).
Critical steps in the protocol include the use of fresh paraformaldehyde and a short fixation time to keep SUMO natively-folded so it can be recognized by kmUTAG. Also, the brief treatment of fixed cells with the low pH glycine solution increases its sensitivity for SUMO. However, not all samples may lend themselves to analysis with KmUTAG-fl. As with antibody-based protocols, when attempting to localize SUMO in new cell types or tissues, detergent choice and detergent concentrations may be important variables. Ultimately, we plan to generate additional KmUTAG-fl variants including a cell-penetrating kmUTAG protein that can be used to detect SUMO dynamics in living cells, organoids, and tissue biopsies.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank all members of the Kerscher lab for their support, Nathalie Nguyen for critical reading of the manuscript, and Lidia Epp for sequencing. This work has been supported by the Commonwealth Research Commercialization fund MF16-034-LS to OK. Research support for W&M students was provided by the Bailey-Huston Research fund, and Charles Center Honors Fellowships to RY and CH.
Materials
| 16% Paraformaldehyde (formaldehyde) aqueous solution | Electron Microscopy Sciences | 30525-89-4 | |
| 6-Well Cell Culture Plates | Genesee Scientific/Olympus Plastics | 25-105 | |
| Alexa Fluor 488 AffiniPure Goat Anti-Mouse IgG (H+L) | Jackson ImmunoResearch | 115-545-003 | Used as a secondary antibody for mouse monoclonal antibody |
| DPBS, no calcium, no magnesium | Fisher Scientific | Gibco 14190144 | |
| Dylight 488 conjugated AffiniPure Goat Anti-Moue IgG (H+L) | Jackson ImmunoResearch | 115-485-146 | Used as a secondary antibody for mouse monoclonal antibody |
| Fisherbrand Coverglass for Growth Cover Glasses | Fisherbrand | 12545101 | |
| FLUORO-GEL II with DAPI | Electron Microscopy Sciences | 50-246-93) | Mounting media in step 1.11 |
| FLUORO-GEL with DABCO | Electron Microscopy Sciences | 17985-02 | With DAPI added to 1 µg/mL; mounting media in step 2.2.5 |
| Glycine-HCl | Fisher BioReagents | BP3815 | |
| Glycine-HCl | ACROS Organics | 6000-43-7 | |
| KmUTAG-fl | Kerafast | KmUTAG reagents are available on Kerafast.com | |
| Oneblock Western-CL blocking buffer | Prometheus | 20-313 | |
| PBS, Phosphate Buffered Saline, 10X Solution | Fisher BioReagents | BP3994 | |
| PNT2 cell line | Sigma-Aldrich | 95012613 | Normal prostate epithelium immortalized with SV40. |
| pSPOT1 | (ChromoTek GmbH) | ev-1 | https://www.chromotek.com/fileadmin/user_upload/pdfs/Datasheets/pSpot1_v1.pdf |
| SUMO 6F2 | DSHB | SUMO 6F2 | SUMO 6F2 was deposited to the DSHB by Pelisch, F. / Hay, R.T. (DSHB Hybridoma Product SUMO 6F2) |
| SUMO protease buffer [10x] | 500 mM Tris-HCl, pH 8.0, 2% NP-40, 1.5 M NaCl | ||
| SUMO-2 Antibody 8A2 | DSHB | SUMO-2 8A2 | SUMO-2 8A2 was deposited to the DSHB by Matunis, M. (DSHB Hybridoma Product SUMO-2 8A2) |
| TCEP-HCL | GoldBio | 51805-45-9 | Used as a reducing agent at a concentration of 5mM |
| Triton X-100 | Fisher BioReagents | 9002-93-1 | Used for permeablization at 0.1% in DPBS/PBS(for worms) |
Referências
- Kerscher, O., Felberbaum, R., Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Cell and Developmental Biology. 22, 159-180 (2006).
- Kerscher, O. . SUMOylation. , 1-11 (2016).
- Hay, R. T. SUMO: a history of modification. Molecular Cell. 18 (1), 1-12 (2005).
- Fu, J., et al. Disruption of SUMO-specific protease 2 induces mitochondria mediated neurodegeneration. PLoS Genetics. 10 (10), e1004579-e1004579 (2014).
- Zhang, H., Kuai, X., Ji, Z., Li, Z., Shi, R. Over-expression of small ubiquitin-related modifier-1 and sumoylated p53 in colon cancer. Cell biochemistry and biophysics. 67 (3), 1081-1087 (2013).
- Wang, Q., et al. SUMO-specific protease 1 promotes prostate cancer progression and metastasis. Oncogene. 32 (19), 2493-2498 (2013).
- Karami, S., et al. Novel SUMO-Protease SENP7S Regulates β-catenin Signaling and Mammary Epithelial Cell Transformation. Scientific Reports. 7 (1), 46477 (2017).
- Pelisch, F., et al. A SUMO-Dependent Protein Network Regulates Chromosome Congression during Oocyte Meiosis. Molecular Cell. 65 (1), 66-77 (2017).
- Zhang, X. D., et al. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Molecular Cell. 29 (6), 729-741 (2008).
- Baker, M. Reproducibility crisis: Blame it on the antibodies. Nature News. 521 (7552), 274-276 (2015).
- Wang, Z., Prelich, G. Quality control of a transcriptional regulator by SUMO-targeted degradation. Molecular and Cellular Biology. 29 (7), 1694-1706 (2009).
- Li, S. J., Hochstrasser, M. A new protease required for cell-cycle progression in yeast. Nature. 398 (6724), 246-251 (1999).
- Peek, J., et al. SUMO targeting of a stress-tolerant Ulp1 SUMO protease. PLoS ONE. 13 (1), e0191391 (2018).
- Edgar, L. G. Chapter 13 Blastomere Culture and Analysis. Methods in Cell Biology. 48, 303-321 (1995).
- Pelisch, F., et al. Dynamic SUMO modification regulates mitotic chromosome assembly and cell cycle progression in Caenorhabditis elegans. Nature Communications. 5 (1), 769 (2014).
- Dillingham, M. S., et al. Fluorescent single-stranded DNA binding protein as a probe for sensitive, real-time assays of helicase activity. Biophysical Journal. 95 (7), 3330-3339 (2008).
- Ruckman, J., et al. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. The Journal of Biological Chemistry. 273 (32), 20556-20567 (1998).
- Hughes, D. J., et al. Generation of specific inhibitors of SUMO-1- and SUMO-2/3-mediated protein-protein interactions using Affimer (Adhiron) technology. Science Signaling. 10 (505), eaaj2005 (2017).
- Lopata, A., et al. Affimer proteins for F-actin: novel affinity reagents that label F-actin in live and fixed cells. Scientific Reports. 8 (1), 6572 (2018).
- Gilbreth, R. N., et al. Isoform-specific monobody inhibitors of small ubiquitin-related modifiers engineered using structure-guided library design. Proceedings of the National Academy of Sciences of the United States of America. 108 (19), 7751-7756 (2011).
- Berndt, A., Wilkinson, K. A., Heimann, M. J., Bishop, P., Henley, J. M. In vivo characterization of the properties of SUMO1-specific monobodies. Biochemical Journal. 456 (3), 385-395 (2013).

