Isolation, Culture, Characterization, and Differentiation of Human Muscle Progenitor Cells from the Skeletal Muscle Biopsy Procedure
Summary
We present techniques for isolating, culturing, characterizing, and differentiating human primary muscle progenitor cells (hMPCs) obtained from skeletal muscle biopsy tissue. hMPCs obtained and characterized through these methods can be used to subsequently address research questions related to human myogenesis and skeletal muscle regeneration.
Abstract
The use of primary human tissue and cells is ideal for the investigation of biological and physiological processes such as the skeletal muscle regenerative process. There are recognized challenges to working with human primary adult stem cells, particularly human muscle progenitor cells (hMPCs) derived from skeletal muscle biopsies, including low cell yield from collected tissue and a large degree of donor heterogeneity of growth and death parameters among cultures. While incorporating heterogeneity into experimental design requires a larger sample size to detect significant effects, it also allows us to identify mechanisms that underlie variability in hMPC expansion capacity, and thus allows us to better understand heterogeneity in skeletal muscle regeneration. Novel mechanisms that distinguish the expansion capacity of cultures have the potential to lead to the development of therapies to improve skeletal muscle regeneration.
Introduction
Skeletal muscle is the largest organ system in the human body, accounting for 30−40% of whole body mass1. In addition to its well-recognized role in locomotion, skeletal muscle maintains body temperature and posture, and plays a central role in whole body nutrient homeostasis. Research involving human participants, animals, and cell culture models are all valuable to address questions pertaining to skeletal muscle biology and regeneration. Isolation and culture of human primary muscle progenitor cells (hMPCs) provides a robust model that allows for cell culture techniques and manipulations to be applied to human samples. An advantage of using hMPCs is that they retain the genetic and metabolic phenotype from each donor2,3. Maintenance of the donor phenotype allows researchers to examine inter-individual variation in the myogenic process. For example, we have employed our hMPC characterization method to identify age- and sex-related differences in hMPC population expansion capacity4.
The purpose of this protocol is to detail techniques to isolate, culture, characterize, and differentiate hMPCs from skeletal muscle biopsy tissue. Building on previous work that described hMPCs and identified potential cell surface makers for hMPC isolation5,6, this protocol fills a critical gap in knowledge by linking the isolation to the characterization of hMPCs. Further, the detailed step-by-step instructions included in this protocol make hMPC isolation and characterization accessible to a broad scientific audience, including those with limited prior experience with hMPCs. Our protocol is among the first to describe use of an imaging cytometer to track cell populations. Newly designed imaging cytometers are state-of-the-art, high-throughput, and microplate-based, enabling live cell imaging, cell counting, and multichannel fluorescence analysis of all cells in each well of a culture vessel within minutes. This system allows for rapid quantification of dynamic changes in proliferation and viability of an entire cell population with only minimal disruption to the culture. For example, we are able to perform objective measures of confluence on successive days in vitro to determine growth kinetics of each culture derived from different donors. Many protocols in the literature, particularly those involving the differentiation of MPCs, require cells to reach a defined level of confluence before initiating differentiation or treatment7. Our method allows for objective determination of the confluence of each well in a culture vessel allowing researchers to initiate treatment in an unbiased, non-subjective manner.
In the past, a major limitation of using primary hMPCs was low yields that limit the number of cells available for experiments. We and others have shown the yield of MPCs from skeletal muscle biopsy tissue is 1−15 MPCs per milligram of tissue (Figure 1)8. Because our protocol allows for four passages of the cells prior to purification with fluorescence activated cell sorting (FACS), our cryopreserved hMPC yields, derived from small amounts of biopsy tissue (50−100 mg), are sufficient to address research aims where multiple experiments are required. Our FACS protocol produces a ~80% pure (Pax7 positive) MPC population, thus our protocol is optimized for both yield and purity.
Protocol
This protocol was approved by the Institutional Review Board at Cornell University. All participants were screened for underlying health conditions and gave informed consent.
1. Obtaining Human Muscle Tissue via the Skeletal Muscle Biopsy
- Identify the vastus lateralis muscle via palpation, anatomical landmarks, and active contraction of the muscle.
- Palpate the vastus lateralis by having the participant tighten their quadriceps muscle and find the belly of the muscle, about 1/3 of the way from the patella to the hip, and just ventrolateral to the femur.
- Use a paper measuring tape to mark an area with a surgical marker about 4−6 inches from the top of the patella representing the belly of the muscle.
- Shave any hair near the marked area and then cleanse with a surgical grade antiseptic that is used before medical procedures (For example: 2% chlorhexidine gluconate (CHG)/ 70% isopropyl alcohol (IPA)) .
- Anesthetize the biopsy site with 1% lidocaine.
NOTE: Avoid injecting the lidocaine into the skeletal muscle. - Make a 4−5 mm incision pathway at the marked site.
- After the area is anesthetized, push a Bergstrom needle with trocar through the fascia into the belly of the muscle (approximately 2−3 cm).
- Draw muscle tissue into the Bergstrom needle by withdrawing the cutting trocar 2−3 cm while an assistant applies suction by withdrawing an attached 60 mL syringe plunger.
- Close the cutting chamber.
- Rotate the base of the biopsy needle a quarter turn and repeat the sequence of opening the needle, applying suction, and closing the needle.
NOTE: Each time the needle is closed, a cut of muscle tissue should be obtained inside the Bergstrom needle. During sample collection, the needle will remain in the thigh in the same vertical position while it is rotated longitudinally through 360° to obtain each sample. This sequence can be repeated 3−4 times. - Expel the collected biopsy tissue from the needle into a sterile nonadherent dressing pad.
- Examine the tissue and remove any visible adipose and fibrotic tissues using sterile scalpel blades or tweezers.
- Place a portion (50−100 mg) of tissue into a sterile tube containing CO2-independent nutrient medium (e.g., Hibernate A).
NOTE: The weight of the tissue is determined as follows. First place the capped tube containing the nutrient medium on a scale and tare. Next place the tissue into the tube, recap the tube, and then weigh the tube containing the tissue in the nutrient medium. - Store the tube containing the tissue and nutrient medium at 4 °C until processing.
NOTE: Biopsy tissue should be processed within 48 h of collection.
2. Isolating Human Muscle Progenitor Cells from Biopsy Tissue
- Mince biopsy tissue.
- Transfer the muscle tissue from the nutrient medium into a sterile Petri dish in a biological safety cabinet or similar sterile workplace.
- Use sterile scalpel blades to mince tissue into ~1 mm3 pieces.
- Wash minced biopsy tissue to remove residual debris.
- Transfer the muscle tissue into a 15 mL conical tube containing 10 mL of phosphate-buffered saline (PBS) and allow the tissue to settle via gravity.
- Aspirate the supernatant being careful not to disturb the muscle tissue.
- Add 10 mL of PBS to the washed tissue and allow the muscle tissue to resettle via gravity.
- Aspirate the supernatant being careful not to disturb the muscle tissue.
- Add 10 mL of low glucose Dulbecco's modified Eagle medium (DMEM) and allow the tissue to resettle via gravity.
- Aspirate the supernatant being careful not to disturb the muscle tissue.
- Incubate the tissue in digest media.
- Resuspend the muscle tissue in 3 mL of digest media (2 mg/mL collagenase D in low glucose DMEM).
NOTE: Collagenase D stock solutions can be prepared at 20 mg/mL in low glucose DMEM and stored at -80 °C, then diluted 1:10 in low glucose DMEM at time of use. - Incubate the tube containing the muscle tissue in a water bath at 37 °C for 30 min.
- Triturate the muscle tissue suspension every 10 min using a 10 mL serological pipette or a wide bore pipette.
- Add 83 µL of additional digest media and 24 µL of dispase solution (0.76 mM calcium acetate, 1.39 mM sodium acetate, 13.91 mg/mL dispase II in low glucose DMEM). Return the suspension to the 37 °C water bath.
- Triturate the muscle tissue suspension every 5−10 min with a wide bore pipet tip.
- Continue trituration until a uniform slurry is achieved, and for no longer than 1.5 h to prevent over digestion.
NOTE: Sufficient mincing of the tissue and the enzymatic activity of collagenase and dispase can impact sample digestion. A uniform slurry should be able to pass through a normal pipette tip with minimal occlusion after 1.5 h. If a uniform slurry is not achieved after 1.5 h, double check that the tissue mincing is sufficient, and tissue is minced to the appropriate size (step 2.1.2). Additionally, the activity of the enzymes should be tested as lot to lot variation in enzymatic activity does occur.
- Resuspend the muscle tissue in 3 mL of digest media (2 mg/mL collagenase D in low glucose DMEM).
- Pellet cells and freeze in recovery media.
- Add 6 mL of growth media (GM; 79% Ham's F12, 20% fetal bovine serum, 1% penicillin/streptomycin, 5 ng/mL basic fibroblast growth factor [bFGF], pH 7.4, passed through a 0.22 µm filter) to the muscle slurry.
- Pipette the suspension through a 70 µm cell strainer into a 50 mL conical tube. Rinse the strainer with an additional 4 mL of GM.
NOTE: Sample can be transferred back into a 15 mL conical tube for ease of visualizing the pellet. A larger wash volume can be used if subsequent cell yields are low. - Centrifuge at 300 x g for 5 min at room temperature (RT).
- Aspirate the supernatant being sure not to disrupt the pellet. Flick the tube to resuspend the pellet in the remaining media. If cells are not going to be cultured immediately proceed to step 2.4.5 for instructions on long-term storage, otherwise proceed to step 3.1.6.
NOTE: If immediate culture is desired, GM in cell culture plates should be pre-incubated (steps 3.1.1 and 3.1.2) before biopsy processing begins. - Add 1.5 mL of recovery cell culture freezing media (Table of Materials) to the recovered pellet.
- Transfer the slurry into a cryovial. Place the cryovial in an isopropanol-controlled rate chiller overnight at -80 °C. Store cryovial at -80 °C until ready for culture.
NOTE: Cryovials containing slurry can be kept in liquid nitrogen for long term storage (greater than 1 month). Using an isopropanol-controlled rate chiller allows cells to be reproducibly frozen at a rate of 1 °C/ min resulting in optimal cell recovery rates as evidenced by viability after freeze and increased likelihood of attachment to cell culture vessels9. Alternatively, a beaker containing RT isopropanol, placed in a -80 °C freezer may be used.
3. Culturing Human Muscle Progenitor Cells
- Thaw the muscle slurry.
- Prior to thawing and seeding hMPCs, pre-equilibrate cell culture plates in GM. Add 250 µL of GM to 4 wells of a collagen-coated (collagen I), 24-well cell culture plate. Fill the remaining wells with 500 µL of sterile base media (i.e., F12) to prevent evaporation during long-term culture.
NOTE: For biopsies between 50 g and 100 g, four wells of a 24-well plate are appropriate. If the biopsy tissue is less than 50 g, two wells may be more appropriate. Residual debris and low cell number prevents the use of a cell counting technique at this step; therefore, the exact seeding density is not determined and varies slightly from biopsy to biopsy. - Incubate cell culture vessels with GM at 37 °C in 5% CO2 for 1 h prior to seeding.
- Remove cryovials from the -80 °C freezer and place in a 37 °C water bath; the liquid in the tube should be completely submerged in water.
- When thawing is almost complete (~5 min), transfer the cryovial to a sterile laminar flow hood. Transfer the muscle slurry out of the cryovial tube and add to a 15 mL conical tube containing 6 mL of pre-warmed GM at a rate of 1 mL/min.
- Centrifuge the muscle slurry at 300 x g for 5 min at RT.
NOTE: A swinging bucket centrifuge can make it easier to visualize small pellets at this step but is not essential. - Aspirate the supernatant being sure not to disrupt the pellet. Flick the tube to resuspend the pellet in the remaining media and resuspend in 1 mL of GM.
- Transfer 250 µL of the resuspended pellet (now considered an hMPC suspension) to each of the 4 wells of the pre-incubated 24-well cell culture plate.
- Prior to thawing and seeding hMPCs, pre-equilibrate cell culture plates in GM. Add 250 µL of GM to 4 wells of a collagen-coated (collagen I), 24-well cell culture plate. Fill the remaining wells with 500 µL of sterile base media (i.e., F12) to prevent evaporation during long-term culture.
- Culture and passage hMPCs.
- Maintain hMPC cultures in GM at 37 °C in 5% CO2.
NOTE: Stocks of GM without the bFGF (i.e., F12, antibiotics, FBS) are prepared in batches and kept at 4 °C for up to 2 weeks. bFGF is added to GM on the day of use. Media containing bFGF can be stored for 48 h at 4 °C. - Twenty-four hours after isolation, aspirate the GM, gently wash the culture vessel 2x with pre-warmed GM, and add fresh GM.
NOTE: 24 h should provide hMPCs adequate time to attach; no hMPCs should be removed after washing. This step will also remove remaining debris that was generated during the cell harvest, making the vessel more amenable to accurate confluence scanning using an imaging cytometer (section 5 below). After this initial media change, GM is changed every 48 h. - Passage hMPCs when 70% confluence is achieved or when they have remained on the same culture dish for 10 days, whichever occurs first.
NOTE: 70% confluence is approximately 55,000 cells/cm2. - To passage, add 250 µL of pre-warmed trypsin to each well of a 24-well cell culture plate and incubate for ~5 min.
- Tap the cell culture vessel on a firm surface to detach hMPCs. A light microscope can be used to verify that the hMPCs have detached.
- Transfer the trypsin/hMPC suspensions to 5 mL of GM in a 15 mL conical tube.
- Centrifuge the hMPC suspension at 300 x g for 5 min at RT.
- Remove hMPC suspensions from the centrifuge and place on ice in the sterile laminar flow hood.
NOTE: Keeping hMPCs on ice during passing results in less cell aggregation. - Aspirate supernatant from hMPCs and gently resuspend pellet in 1 mL of GM.
- Count cells using a hemocytometer or an automated cell counter.
NOTE: A 1:5 dilution of cell suspension to cell counting buffer is generally appropriate. - Seed hMPCs onto collagen-coated culture dishes containing pre-warmed GM at a density of 3,500 cells per cm2. A combination of 10 cm plates and 24-well plates is ideal. The 24-well plates can be scanned daily on an imaging cytometer to monitor growth.
- Follow the same procedure (starting at step 3.2.4.) for subsequent passages.
NOTE: Cell health and purity can be monitored across passages using a marker of cellular senescence (e.g., β-galactosidase) and immunostaining for Pax7.
- Maintain hMPC cultures in GM at 37 °C in 5% CO2.
- Cryopreserve the hMPCs.
- Cryopreserve excess cells for later use to make the culture volume more manageable.
- For cryopreservation, follow the trypsinization procedure described in steps 3.2.4.-3.2.9.
- While the cell suspension is being centrifuged, prepare a mixture of 20% (e.g., 200 µL) dimethyl sulfoxide (DMSO) and 80% (e.g., 800 µL) GM. Pipette up and down 20x to ensure adequate mixing. Leave the mixture to rest on ice.
- Based on cell count, dilute the hMPC suspension to 2 x 106/mL in GM.
- Combine the hMPC suspension and the 20% sterile DMSO/80% GM mixture 50/50. The final cryopreservation media is a combination of DMSO (10%) and GM (90%) containing 1 x 106 hMPCs/mL.
- Place 1 mL of the hMPC suspension prepared in step 3.3.5 into as many cryovials as the initial cell count allows. Then place aliquoted hMPCs in an isopropanol-controlled rate chiller in a -80 °C freezer overnight.
NOTE: At this step, typically between 5 and 15 million cells are obtained, 30−60% of which are identified as hMPCs by FACS.
4. Isolating Pax7+ Human Muscle Progenitor Cells via Flow Cytometry
- To prepare hMPCs from live cultures (step 3.2.11), trypsinize enough culture vessels for 1 x 106−2 x 106 total cells. Prepare cell suspensions as described in steps 3.2.4−3.2.9.
- Prepare hMPCs from cryopreserved cultures (step 2.4.6 or 3.3.6).
- Select 1−2 tubes containing ~1 x 106 passage four hMPCs for each donor.
NOTE: Using passage four hMPCs allows for adequate cell yield while still maintaining proliferative potential until passage six (Figure 2). - Rapidly thaw cells by removing them from the -80 °C freezer and placing them in a 37 °C water bath with the liquid in the tube completely submerged.
- When thawing is almost complete (~5 min), transfer hMPCs to a sterile laminar flow hood and transfer the contents of the cell suspension into 6 mL of pre-warmed GM in a 15 mL conical tube at a rate of 1 mL/min.
- Prepare cell suspensions as described in steps 3.2.7−3.2.9.
- Resuspend each pellet in 3 mL of FACS buffer (500 mL PBS, 12.5 mL normal goat serum, 2 mL 0.5 M EDTA, pH 7.4, passed through a 0.22 µm filter).
- Centrifuge the cell suspension at 300 x g for 5 min at RT.
- Aspirate supernatant from hMPCs and gently resuspend pellet in 250 µL of FACS buffer; place on ice.
- Place 100 µL of cells in a 1.7 mL microcentrifuge tube and leave on ice to be used as live/dead controls (step 4.5.1).
NOTE: The 100 ul of cells to be used for live/dead controls can come from taking a small volume (~20 ul) of resuspended cells from each donor to be sorted or from banked cells.
NOTE: It is important to not take more than 20 ul of resuspended cells from a single donor. Bring volume up to 100 ul with FACS buffer if < 100 ul of cells is available.
- Select 1−2 tubes containing ~1 x 106 passage four hMPCs for each donor.
- Stain hMPCs for cell surface markers.
- Prepare the following antibody cocktail (amounts shown are for each culture, scale-up appropriately): 5 µL CD56 (neural cell adhesion molecule [NCAM]; PE-Cy7-conjugated) and 5 µL CD29 (β1-integrin; AlexFluor488-conjugated). The final antibody dilution is 1:50.
- Add 10 µL of the antibody cocktail to each hMPC suspension and incubate on ice for 30 min in the dark.
- Add 10 mL of FACS buffer to each suspension/antibody cocktail mixture.
- Centrifuge each tube at 300 x g for 5 min at RT.
- Resuspend the pellet in 300 µL of FACS buffer and transfer to a 5 mL round bottom polystyrene tube.
- Keep the suspension on ice and protected from light.
- Prepare the compensation beads.
NOTE: Positive controls for each antibody and a negative control (unstained beads) should be prepared using compensation beads. Compensation bead controls should be run on the flow cytometer prior to the samples to be sorted. These controls allow for determination of how much the fluorochrome from each antibody can be detected in the other's channel so that compensation can be applied.- Vortex compensation beads.
- In three 1.7 mL microcentrifuge tubes, add one drop (50 µL) of compensation beads.
- Add 1 µL of each antibody to the appropriate tube, or none to the negative control.
- Incubate in the dark for 15 min.
- Add 1.5 mL of FACS buffer to each tube and vortex.
- Centrifuge each tube at 300 x g in a benchtop centrifuge for 5 min at RT.
- Aspirate supernatant and resuspend the pellet in 200 µL of FACS buffer.
NOTE: The pellet may be very difficult to visualize; care should be taken when aspirating the supernatant. - Place the suspension in a 5 mL round bottom polystyrene tube and then keep the tube on ice and protected from light.
- Prepare live and dead controls.
- Divide the 100 µL of hMPCs from step 4.2.8 into two 1.7 mL microcentrifuge tubes (one for the live control and one for the dead control).
- Place the dead control tube in a heating block pre-warmed to 70 °C for >10 min. During this time, keep the live control on ice.
- Centrifuge each tube at 300 x g in a benchtop centrifuge for 5 min at RT.
- Aspirate supernatant and resuspend the pellet in 150 µL of FACS buffer.
- Combine the live/dead controls into a single 5 mL round bottom polystyrene tube.
- Perform viability staining.
- Add 1 µL of 7-aminoactinomycin D (7-AAD) to all tubes containing cells to be sorted and the live/dead control.
- Add GM to collagen-coated 24-well (250 µL/well) and 10 cm (8 mL/well) plates and allow to equilibrate in a cell culture incubator at 37 °C in 5% CO2.
- Sort hMPCs via flow cytometry.
- Sort hMPCs on a flow cytometer with 100 µm nozzle at a pressure of 20 psi. Determine live hMPCS based on side and forward scatter as well as 7-AAD negativity (sorting parameters and a representative sort can be seen in Figure 3).
- Cells positive for PE-Cy7 (780/60 nm) and AlexFluor488 (530/30 nm) represent CD56+/CD29+ cells and thus are Pax7 positive cells (hMPCs). Sort double positive cells into collection tubes containing a 300 µL cushion of FACS buffer.
NOTE: The purity of sorted cultures can be determined by immunostaining for Pax7 followed by downstream flow cytometry analysis (Figure 4). - Discard all other cells.
- Transfer the sorted cells to a 15 mL conical tube.
- Spin the hMPC suspension at 300 x g for 5 min at RT.
- Carefully aspirate the supernatant and resuspend in 1 mL of GM.
- Count cells using a hemocytometer or an automated cell counter.
NOTE: While the number of cells may be inferred as the number of events that are positively sorted, this count has been found to overestimate the number of cells by 10−40% compared to traditional cell counting methods. - Seed the hMPCs as outlined in step 3.2.11.
NOTE: Verification of the purity of hMPC population may be determined by immunostaining for Pax7 and analysis via flow cytometry.
5. Characterizing Human Muscle Progenitor Cell (hMPC) Cultures Using an Imaging Cytometer
- Use the imaging cytometer (Table of Materials) to perform confluence scans.
- Select Create New Scan and then enter/select the Plate Category, Plate Profile and Plate ID. The imaging cytometer can accommodate 96, 24, and 6 well plates.
- Under Application, select Confluence > Confluence 1.
- Select a well to view using the navigator on the right of the screen.
- Find a plane of focus where the cells appear white compared to the background and select register manual or allow the imaging cytometer to select the plane of focus by selecting register auto.
NOTE: For the 96 well plates suggested in the Table of Materials, an approximate position is 4.3 units. - Use the mouse to highlight all wells that need to be scanned. The entire well area will automatically be scanned. Select Start Scan.
- Select analysis settings optimal for plate and cell type.
NOTE: For hMPCs, the following settings are optimal: intensity = 6, saturated intensity = 0, diameter = 8, minimum thickness = 3, min cluster size = 500, and precision = high. - Select Start Analysis.
NOTE: Visually inspect images to ensure that the user and imaging cytometer agree regarding the perimeter of cells. If a large discrepancy exists, rescan the plate using a different plane of focus or different analysis settings. Confluence scanning can be used to compare growth between cultures, time the administration of a treatment, or to monitor cells and decide when to initiate differentiation. If differentiation is desired, details are provided in section 6.
- Use the imaging cytometer to count cells.
- Prepare a staining solution containing 12 mL of Ham's F12, 6 µL of Hoechst 33342 and 24 µL of propidium iodide.
- Aspirate media and replace with staining solution, incubate at 37 °C for 15 min.
- Aspirate staining solution and replace with Ham's F12.
NOTE: It is important to use a media with low levels of phenol red to obtain clear images. Alternatively, PBS may be used. - Load the plate onto the imaging cytometer and under Application, select Cell Viability > Live + Dead + Total. The live channel will be a bright field image, the dead channel will be the propidium Iodide, and the total channel will be the Hoechst 33342.
- Set the Live channel to bright field and click Register Auto under Focus Setup.
- Under the Total channel, set the channel to blue. Use Find focus to bring the nuclei into focus or use the up and down arrows to manually set the focus. Click Set offset to select the focus.
- Under the Dead channel, set the channel to red. Use Find focus to bring the dead cells into focus or use the up and down arrows to manually set the focus. Click Set offset to select the focus.
- Select Start Scan to start the scan.
- Select analysis parameters that are appropriate for the plate and cell type. Select Start Analysis to start the analysis. After the analysis is complete, visually verify that the analysis is appropriately counting cells (e.g., for the total channel check that every nucleus is being recognized as a nucleus and that the imaging cytometer is not counting any spots in the background as nuclei). If the scan and analysis are satisfactory, return the plate to the incubator, otherwise rescan or modify analysis parameters.
6. Differentiating Human Muscle Progenitor Cells (hMPCs) to Myotubes
- Determine the hMPC confluence using the protocol in section 5.1. To differentiate hMPCs into myotubes, it is ideal for cells to be 80−85% confluent as determined by the confluence function on the imaging cytometer (see section 5). If hMPCs from more than one unique donor are used, confluence scanning allows cells from different donors to begin differentiation at the time they reach confluence.
NOTE: At 80% confluence, there are approximately 66,000 cells/cm2. - Wash cells 2x with differentiation media (97% Ham's F12, 2% equine serum, 1% penicillin/streptomycin, pH 7.4, passed through a 0.22 µm filter).
- Maintain cells in differentiation media for as long as the experiment dictates while switching media daily.
- Directly assess myotube formation by immunostaining for embryonic myosin heavy chain, which is typically detectable after 7−10 days of differentiation.
NOTE: The time required to form myotubes may differ slightly depending on donor and number of cells when differentiation was initiated; therefore, it is recommended that cultures from each donor be monitored individually.
Representative Results
Representative flow cytometry results of hMPC isolation from human muscle tissue can be viewed in Figure 1. hMPCs can be identified by first gating events based on side scatter and forward scatter to eliminate dead cells or debris, followed by selecting only cells which are negative for 7-AAD and therefore are viable. Selection of cells positive for both the cell surface markers CD56 and CD29 represents the hMPC population. A biopsy of 60 mg only provides approximately 75−250 hMPCs.
A representative confluence scan is shown in Figure 2A. The green outlines in Figure 2B were generated by the imaging cytometer and show how the imaging cytometer determined confluence based on the analysis settings selected. In both images shown, the confluence determined by the imaging cytometer agrees with the confluence visually determined by the user. However, these images also highlight that the imaging cytometer is not perfect. For example, the red arrow highlights an imperfection in the plate which is being counted as cells. If a large number of these imperfection are present on the plate, the resulting confluence will not accurately represent the confluence of the cells. Figure 2C shows a representation of all three channels used to count cells (brightfield, blue and red) and the merged image. Figure 2D shows heterogeneity between donors. Discovering and accurately characterizing this heterogeneity is a key application of the imaging cytometer. Figure 2E shows that confluence measurements and nuclei counts from unique donors are highly correlated.
Figure 3 provides a guideline for how to identify hMPCs based on the FACS procedure described in this protocol. Gating based on forward and side scatter allows for separation of viable cells from debris (Figure 3A). A comparison of forward scatter by height to forward scatter by area was used to distinguish events representing single cells (the boxed area in Figure 3B). Viable cells are marked by a lack of incorporation of the viability stain 7-AAD (Figure 3C). Finally, cells positive for both CD56 and CD29 are identified as hMPCs (Figure 3D). To validate the FACS procedure described in this protocol the selection of Pax7 positive cells can be determined by immunostaining cells with a Pax7-specific antibody and measuring expression on a flow cytometer. Figure 4 compares the number of hMPCs as determine by Pax7 immunostaining (Figure 4A) vs. the FACS procedure (Figure 4B) detailed in this protocol from the same population of cells. Figure 5 uses Pax7 immunostaining and analysis via flow cytometry to show the enrichment of Pax7 expressing cells in the total population after FACS (Figure 5A compared to Figure 5B) as well as the maintenance of the number of Pax7 expressing cells after passaging (Figure 5B compared to Figure 5C).
hMPCs can be differentiated to form myotubes by following section 6. To determine whether isolated hMPCs maintain myogenic capacity cells can be immunostained with an antibody specific for embryonic myosin heavy chain and visualized using fluorescent microscopy. Representative images of embryonic myosin heavy chain positive myotubes can be viewed in Figure 6.
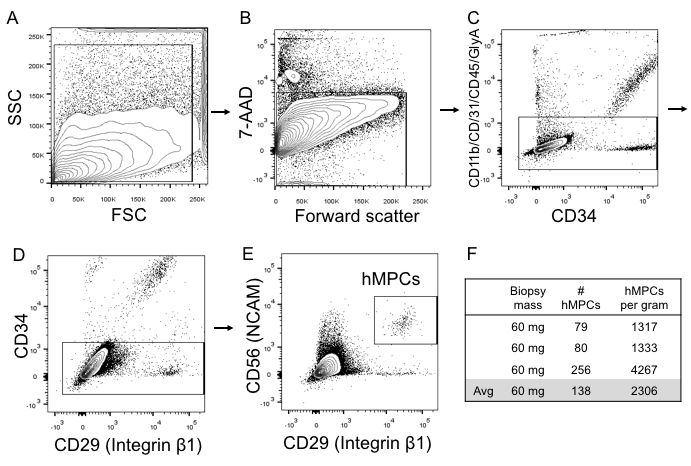
Figure 1: Representative flow cytometry images for hMPCs sorted directly out of muscle biopsy tissue. (A) Side scatter area (y-axis) vs. forward scatter area (x-axis) gating strategy used to identify all cells in a donor sample. (B) 7-AAD viability stain (y-axis) vs. forward scatter (x-axis) to identify live cells. (C) Negative selection sorting marker profile (-CD11b, -CD31, -CD45, -GlyA [y-axis]) vs. selection marker CD34. (D) Negative selection marker CD34 (y-axis) vs. the positive selection marker CD29. (E)Positive selection marker CD56 (y-axis) vs. the positive selection marker CD29 (x-axis). (F)Yields from three different skeletal muscle biopsies. FSC = forward scatter; SSC = side scatter; hMPCs, human muscle progenitor cells.
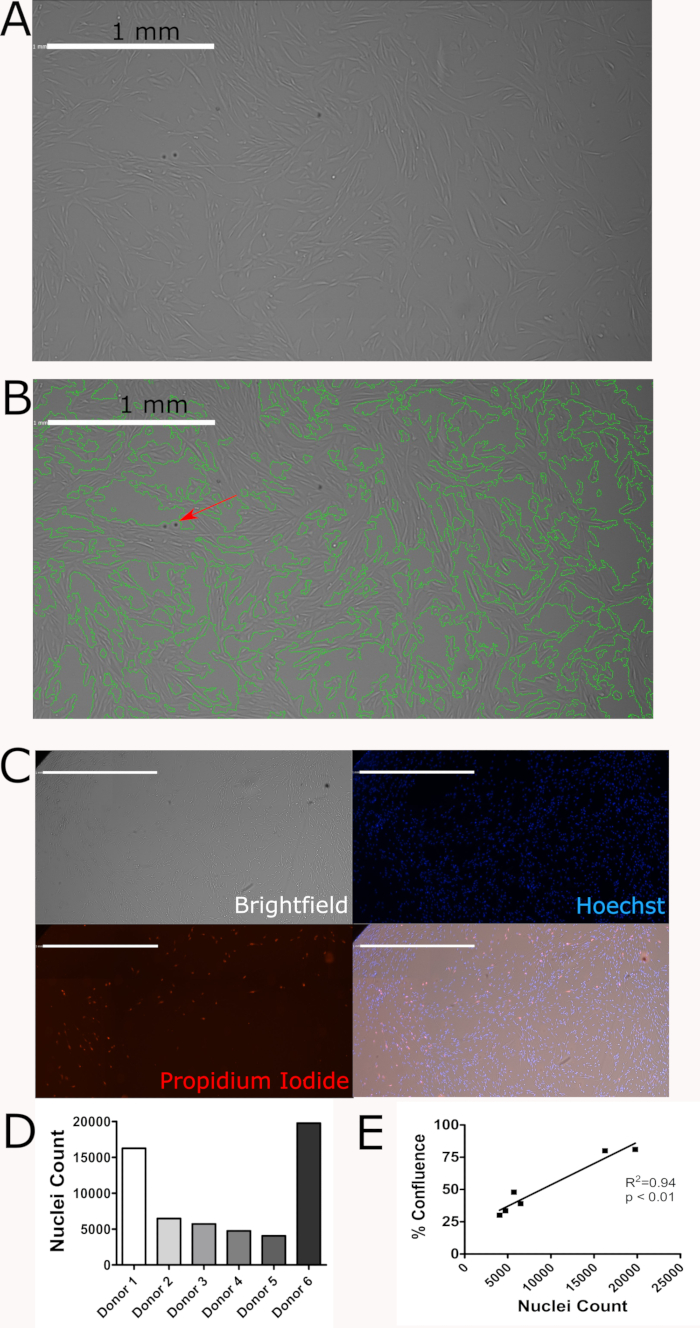
Figure 2: Proliferative potential of hMPCs derived from human donors is maintained after 6 passages. (A)Representative confluence scan. (B) Representative confluence scan with green outlines showing how the imaging cytometer determined confluence. The red arrow shows an imperfection on the plate. (C) Representative brightfield, Hoechst 33342 (blue), and propidium Iodide (red) staining and a merged image of these three channels. Scale bars = 1mm. (D) Nuclei counts from 6 unique donors highlights hMPC heterogeneity. (E) Confluence and nuclei count are highly correlated. Please click here to view a larger version of this figure.
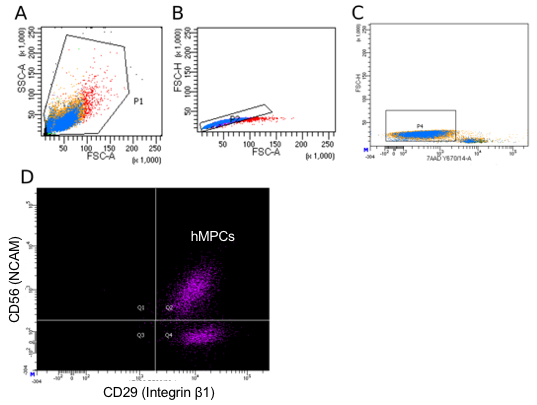
Figure 3: Representative flow cytometry sorting parameters for hMPC isolation. (A) Side scatter area (y-axis) vs. forward scatter area (x-axis) gating strategy used to identify all cells in a donor sample. (B) Forward scatter height (y-axis) vs. forward scatter area (x-axis) to disqualify doublets from the sorting population. (C) Forward scatter height (y-axis) vs. 7-AAD incorporation to identify viable cells. (D) PE-Cy7 (CD56) vs. AF488 (CD29) staining with Q2 representing double positive cells (hMPCs). Color represents the density of events.
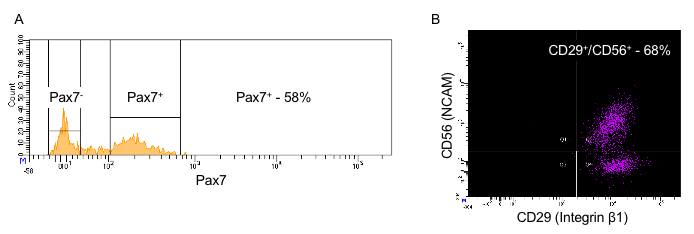
Figure 4: Comparison of CD29/CD56 positivity vs. Pax7 positivity in passage 4 hMPCs from the same donor. (A) Count (y-axis) vs. Pax7 expression (x-axis). (B)Positive selection marker CD56 (y-axis) vs. positive selection marker CD29 (x-axis). NCAM = neural cell adhesion molecule; hMPC = human muscle progenitor cell.
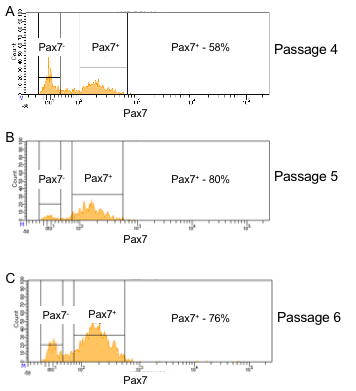
Figure 5: Pax7 positivity is maintained in hMPCs derived from the same donor over multiple passages. (A) Pax7 expression (x-axis) of cells which had been passaged 4 times prior to FACS sorting. (B) Pax7 expression (x-axis) of hMPCs 1 passage after FACS sorting for CD29 and CD56 positivity (5 total passages). (C) Pax7 expression (x-axis) of hMPCs 2 passages after FACS sorting for CD29 and CD56 positivity (6 total passages).
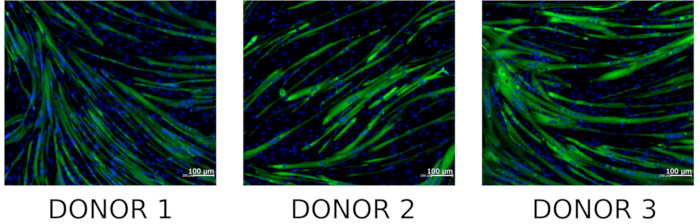
Figure 6: Staining of hMPC derived myotubes for embryonic myosin heavy chain. Representative microscopic images of differentiated hMPCs co-stained with DNA stain (Hoechst 33342, blue) and embryonic myosin heavy chain (green) (n = 3). Please click here to view a larger version of this figure.
Discussion
Primary hMPCs are an important research model used to understand skeletal muscle biology and the regenerative process. Additionally, hMPCs have the potential to be used for therapy. However, there are recognized challenges in using primary hMPCs for both research and therapy, including the limited understanding of cells derived from humans10. There is also a large degree of variation in the expansion capacity among donor cultures, which limits the potential for use of hMPCs and can affect research results11. For example, it has recently been demonstrated that hMPCs isolated using the method described in this protocol produced cells that varied in expansion capacity and this was driven by transcriptional profile that did not align with sex or age11.
Here, we present an efficient and high yield method for isolating hMPCs in sufficient quantities for a number of downstream applications including differentiation into myotubes, thereby modeling the myogenic process in vitro. Our method relies on FACS to isolate CD56+/CD29+ cells, which have previously been identified as representing an enriched Pax7 expressing population12. Other equally valid cell sorting strategies have also been described5,6.
The most important aspect of our hMPC isolation and culture method is the careful monitoring of individual cultures. The donor-based heterogeneity includes number of hMPCs obtained from each biopsy, their time to initiate proliferation in vitro, and their population expansion rate. Therefore, if differences in myotube formation after a treatment is the question of interest individual, hMPC cultures should be switched to treatment based on a confluence level determined a priori and assessed objectively using the imaging cytometer.
Our method to isolate and culture hMPCs has been optimized to obtain yields of cells in the millions for in vitro experiments from relatively little starting biopsy tissue (50−100 mg). The major benefit of this approach is that multiple assays and experiments can be performed on hMPCs from the same donor, allowing for maintenance of donor phenotype across experiments while introducing little inter-experiment variability. Our method differs from recently published methods that focus on extracting the purest population of Pax7 expressing cells directly out of biopsy tissue where the focus is xenotransplantation8. The challenge with these previous methods however, is that they require a starting tissue weight of greater than one gram. Therefore, they are not practical for research involving human skeletal muscle biopsies. One limitation of our method is that the potential for xenotransplantation has not been assessed. However, this type of procedure was not one of the original intended downstream applications. Future directions of this method may include assessing the engraftment capacity of hMPCs, after undergoing our isolation procedure, to determine whether there is potential for use of this procedure in MPC transplantation studies.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank the Cornell University, Biotechnology Resource Center Imaging Facility for their help with fluorescence activated cell sorting. We also thank Molly Gheller for her help with participant recruitment and Erica Bender for conducting the skeletal muscle biopsies. Finally, we thank the participants for their time and participation in the study.This work was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG058630 (to B.D.C. and A.E.T.), by a Glenn Foundation for Medical Research and American Federation for Aging Research Grant for Junior Faculty (to B.D.C.), and by the President's Council for Cornell Women (to A.E.T.).
Materials
| 0.25% Trypsin, 2.21 mM EDTA | Corning | 25-053-Cl | Trypsin used for removing adherent hMPCs from cell culture vessels |
| 10 cm cell culture plate | VWR | 664160 | Plates used for culturing hMPCs |
| 15 mL Falcon tube | Falcon | 352196 | 15 mL conical tubes used throughout the hMPC isolation and culturing protocols |
| 24 well cell culture plate | Grenier Bio-One | 662 160 | Plates used for culturing hMPCs |
| 7-AAD Viability Staining Solution | eBioscience | 00-6993-50 | Viability stain for identifying living cells during FACS sorting |
| Alexa Fluor 488 anti-human CD29, Clone: TS2/16 | BioLegend | 303016 | Conjugated antibody for FACS |
| Black 96-well cell culture plate | Grenier Bio-One | 655079 | 96-well cell culture plate ideal for fluorescent imaging using the Celigo S |
| Celigo S | Nexcelcom Bioscience | Imaging cytometer used to track hMPC cultures | |
| Cell Strainer | VWR | 352350 | Cell strainer to eliminate large pieces of debris during muscle biopsy processing |
| Collagen Type I (Rat Tail) | Corning | 354236 | Collagen for coating cell culture plates |
| Collagenase D | Roche | 11 088 882 001 | Used for degradation of collagen and other connective tissue in the skeletal muscle biopsy tissue |
| Dimethyl Sulfoxide | VWR | WN182 | Used for cryopreservation of hMPCs |
| Dispase II | Sigma Life Sciences | D4693 | A protease used for enzymatic digestion of skeletal muscle biopsy tissue |
| Dulbecco's Modified Eagle Medium Low Glucose powder | Gibco | 31600-034 | Low glucose DMEM for muscle biopsy processing |
| Dulbecco's Phosphate Buffered Saline | Gibco | 21600-010 | PBS for muscle biopsy processing |
| EDTA Disodium Salt Dihydrate | J.T. Baker | 4040-01 | Required for FACS buffer |
| Fetal Bovine Serum | VWR | 89510-186 | Fetal bovine serum used for hMPC growth media |
| Ham's F12 | Gibco | 21700-026 | Base media for hMPCs |
| Heat Inactivated Equine Serum | Gibco | 26-050-070 | Horse serum used to make hMPC differentiation media |
| Hemocytometer | iNCyto | DHC-N0105 | Used to count cells |
| Hibernate A | Gibco | A1247501 | Media for preserving skeletal muscle biopsy tissue |
| Hoechst 33342, trihydrochloride, trihydrate | Life Technologies | H21492 | DNA stain for identifying all cells using the Celigo S |
| Isopropanol | Fisher Scientific | A416P-4 | Used for controlled rate freezing of hMPCs |
| Moxi buffer | Orflo | MXA006 | Buffer for automated cell counter |
| Moxi Cassettes | Orflo | MXC002 | Cassesttes for automated cell counter |
| Moxi z Mini Automated Cell Counter | Orflo | Automated cell counter | |
| Mr. Frosty Freezing Container | Thermo Fisher Scientific | 5100-0001 | Commerically available controlled rate cell freezing container |
| Normal Goat Serum (10%) | Thermo Fisher Scientific | 50062Z | Goat serum used in FACS buffer |
| PE-Cy7 Mouse Anti-human CD56 , Clone: B159 | BD Pharmingen | 557747 | Conjugated antibody for FACS |
| Penicillin/Streptomycin 100X Solution | Corning | 30-002-CI | Antibiotics added to culture media |
| Propidium iodide | Thermo Fisher Scientific | P3566 | DNA stain for identifying dead cells using the Celigo S |
| Recombinant Human basic fibroblast growth factor | Promega | G5071 | Supplement in hMPC growth media to prevent spontaneous differentiation |
| Recovery Cell Culture Freezing Medium | Gibco | 12648-010 | Media used to cryoperseve muscle biopsy slurries |
| Sodium Bicarbonate | Fisher Scientific | S233-3 | Added to Ham's F12 |
| Sterile Round Bottom 5 mL tubes | VWR | 60818-565 | Tubes used for FACS |
| UltraComp eBeads | eBioscience | 01-2222-42 | Compensation beads fort calibrating flow FACS settings |
Referências
- Janssen, I., Heymsfield, S. B., Wang, Z., Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. Journal of Applied Physiology. 89 (1), 81-88 (2000).
- D’Souza, D. M., et al. Decreased satellite cell number and function in humans and mice with type 1 diabetes is the result of altered notch signaling. Diabetes. 65 (10), 3053-3061 (2016).
- Scheele, C., et al. Satellite cells derived from Obese humans with type 2 diabetes and differentiated into Myocytes in vitro exhibit abnormal response to IL-6. PLoS One. 7 (6), e39657 (2012).
- Riddle, E. S., Bender, E. L., Thalacker-Mercer, A. E. Expansion capacity of human muscle progenitor cells differs by age, sex, and metabolic fuel preference. American Journal of Physiology: Cell Physiology. 315 (5), C643-C652 (2018).
- Charville, G. W., et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Reports. 5 (4), 621-632 (2015).
- Alexander, M. S., et al. CD82 Is a Marker for Prospective Isolation of Human Muscle Satellite Cells and Is Linked to Muscular Dystrophies. Cell Stem Cell. 19 (6), 800-807 (2016).
- Thalacker-Mercer, A., et al. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiological Genomics. 45 (12), 499-507 (2013).
- Garcia, S. M., et al. High-Yield Purification, Preservation, and Serial Transplantation of Human Satellite Cells. Stem Cell Reports. 10 (3), 1160-1174 (2018).
- Yokoyama, W. M., Thompson, M. L., Ehrhardt, R. O. Cryopreservation and thawing of cells. Current Protocols in Immunology. , (2012).
- Bareja, A., Billin, A. N. Satellite cell therapy – from mice to men. Skeletal Muscle. 3 (1), 2 (2013).
- Riddle, E. S., Bender, E. L., Thalacker-Mercer, A. Transcript profile distinguishes variability in human myogenic progenitor cell expansion capacity. Physiological Genomics. 50 (10), 817-827 (2018).
- Xu, X., et al. Human Satellite Cell Transplantation and Regeneration from Diverse Skeletal Muscles. Stem Cell Reports. 5 (3), 419-434 (2015).

