Isolation, Transfection, and Culture of Primary Human Monocytes
Summary
Presented here is an optimized protocol for isolating, culturing, transfecting, and differentiating human primary monocytes from HIV-infected individuals and healthy controls.
Abstract
Human immunodeficiency virus (HIV) remains a major health concern despite the introduction of combined antiretroviral therapy (cART) in the mid-1990s. While antiretroviral therapy efficiently lowers systemic viral load and restores normal CD4+ T cell counts, it does not reconstitute a completely functional immune system. A dysfunctional immune system in HIV-infected individuals undergoing cART may be characterized by immune activation, early aging of immune cells, or persistent inflammation. These conditions, along with comorbid factors associated with HIV infection, add complexity to the disease, which cannot be easily reproduced in cellular and animal models. To investigate the molecular events underlying immune dysfunction in these patients, a system to culture and manipulate human primary monocytes in vitro is presented here. Specifically, the protocol allows for the culture and transfection of primary CD14+ monocytes obtained from HIV-infected individuals undergoing cART as well as from HIV-negative controls. The method involves isolation, culture, and transfection of monocytes and monocyte-derived macrophages. While commercially available kits and reagents are employed, the protocol provides important tips and optimized conditions for successful adherence and transfection of monocytes with miRNA mimics and inhibitors as well as with siRNAs.
Introduction
Human immunodeficiency virus-1 (HIV-1) infection causes severe immune dysfunction, which can lead to opportunistic infections and acquired immunodeficiency syndrome (AIDS). Although HIV-infected patients undergoing cART are characterized by low viral loads and normal CD4+ T cell counts, functioning of the immune system can be compromised in these individuals, leading to a dysfunctional immune response that has been linked to an increased risk of developing cancer1. The mechanisms of immune dysfunction in HIV patients on cART remain largely unknown. Therefore, characterizing patient-derived immune cells and investigating their biology and function is a critical component of current HIV research.
Monocytes and macrophages are key regulators of immune responses and play fundamental roles in HIV infection2,3,4,5. Heterogeneous and plastic in nature, macrophages can be broadly classified into classically activated (M1) or alternatively activated (M2). While this general classification is necessary when setting up experimental conditions, the polarization status of macrophages may be reversed by a variety of cytokines6,7,8,9. Although several studies have investigated the effects of HIV infection on monocytes and dendritic cells, molecular details of monocyte-mediated responses are largely unknown6,7,10,11,12,13,14,15,16,17,18,19. Among the factors involved in immune cell regulation and function, microRNAs (miRNAs), short non-coding RNAs that post-transcriptionally regulate gene expression, have been shown to play an important role in the context of major cellular pathways (i.e., growth, differentiation, development, and apoptosis)20. These molecules have been described as important regulators of transcription factors essential for dictating the functional polarization of macrophages21. The potential role of miRNAs in monocytes from HIV-infected individuals undergoing cART has been investigated, but progress in the field requires much more work22,23,24,25,26. This paper discusses an optimized method to transfect miRNAs and siRNAs into primary human monocytes from HIV-infected patients and controls.
This protocol relies on commercially available reagents and kits, as continuity in the technical procedure helps eliminate unnecessary experimental variables when working with clinical samples. Nonetheless, the method provides important tips (i.e., the number of cells plated or brief incubation with serum-free media to promote the adherence of cells to the plate). Additionally, the polarization conditions used in this protocol are derived from published work27,28,29.
Protocol
All methods described below have been approved by the Louisiana State University Health Sciences Center New Orleans Institutional Review Board. All blood was collected after obtaining informed consent.
NOTE: The entire procedure is performed under sterile conditions in a biosafety level 2 (BSL2) facility so that caution is used to handle biological materials. In particular, each step is performed using sterile techniques under a biosafety cabinet. After each step involving blood, blood products, cells, or cell product pipetting, it is important to rinse all plastic material (i.e., serological pipettes, pipette tips, and tubes) with 10% bleach from a waste container inside the hood prior to proper disposal.
1. Isolation of primary human monocytes by immunomagnetic negative selection
- Collect 40 mL of fresh, human whole blood (from either an HIV+ patient or healthy control) in four 10 mL ethylenediaminetetraacetic acid (EDTA) vacuum tubes (10 mL of blood per tube). Using sterile techniques under a biosafety cabinet, transfer all 40 mL of blood into one 50 mL conical propylene tube.
- Following the manufacturer's protocol for the selected human monocyte isolation kit (Table of Materials), add 2 mL of monocyte isolation cocktail, provided in the kit, to the tube of blood. Vortex magnetic beads, also provided in the kit, for 30 s, and add 2 mL to the tube of blood.
- If less than 40 mL of blood is available, scale down the reagents added. To mix the solution, pipette up and down with a plastic 25 mL serological pipette and incubate for 5 min at room temperature (RT).
- Separate the blood mixture equally into four 50 mL tubes and add 30 mL of sterile phosphate-buffered saline (PBS) containing 1 mM EDTA to each tube. Mix by pipetting up and down with a plastic 25 mL serological pipette.
- Place the tubes in magnet holders for 10 min to remove the antibody-conjugated magnetic beads. Use four magnet holders simultaneously, one for each tube, to allow consistent incubation and isolation times for each blood sample.
- Draw up the contents from the center of each tube, using a pipette, while they are still in the magnet holders. Be careful not to draw up red blood cells (no more than 10% of the 10 mL starting volume), and place the contents into one of four new 50 mL tubes.
- Add 500 µL of vortexed magnetic beads to each 50 mL tube. Pipette up and down with a 25 mL pipette and incubate at RT for 5 min. Then, place the tubes into magnet holders for 5 min.
- Carefully transfer the contents from the center of each tube while still in magnet holders into one of four new 50 mL tubes. Directly place each new 50 mL tube in the magnet holders for 5 min.
- Carefully transfer contents from the center of each tube into one of four new 50 mL tubes. Spin all new 50 mL tubes at 300 x g for 5 min. Aspirate the supernatant and resuspend all four cell pellets in a total of 10 mL of sterile PBS.
- Count cells by trypan blue exclusion using a hemocytometer.
NOTE: 8-20 x 106 cells are generally obtained from 40 mL of whole blood.
2. Culturing of primary human monocytes
- Using a 37 °C water bath, warm serum-free RPMI 1640 media supplemented with 1% penicillin-streptomycin (pen/strep), and (while continuing to use sterile techniques under a biosafety cabinet) resuspend the isolated monocytes in this media at a concentration of 1 x 106 cells/mL.
- Add 1 mL of resuspended cells to each well of a 6 well plate or into a 35 mm dish (the final number of cells should be 1 x 106 cells/plate), and place in a 37 °C incubator with 5% CO2. Wait 0.5-1.0 h for cells to adhere.
- Using a 37 °C water bath, warm heat-inactivated (HI) fetal bovine serum (FBS). Add 100 µL (10% final concentration) of FBS to each plate. Add growth factors to the cells to promote macrophage differentiation.
- Prime macrophages for an M1-like phenotype by adding 25 ng/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) to media. Prime macrophages for an M2-like phenotype by adding 50 ng/mL of macrophage colony-stimulating factor (M-CSF) to media28.
NOTE: Both GM-CSF and M-CSF allow monocyte differentiation to a general macrophage phenotype (M0) while priming cells for M1 or M2, respectively7,28,30.
- Prime macrophages for an M1-like phenotype by adding 25 ng/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) to media. Prime macrophages for an M2-like phenotype by adding 50 ng/mL of macrophage colony-stimulating factor (M-CSF) to media28.
3. Transfecting primary human monocytes in culture
- Transfect monocytes with miRNA mimics or inhibitors or siRNA using a kit containing a polymer-based transfection reagent (Table of Materials).
- Following the manufacturer's protocol for the transfection kit (and continuing the use of sterile techniques under a biosafety cabinet), first dilute the selected miRNA mimics/inhibitors or siRNAs in the buffer to a final concentration of 1.83 µM. Prepare 10 µL of diluted mimic/inhibitor per transfection of 1 x 106 cells.
- Prepare the transfection reagent by adding 1 µL of the provided polymer to a fresh 1.5 mL microcentrifuge tube, followed immediately by adding 90 µL of provided buffer (for a total of 91 µL of reagent per transfection). Vortex for 3-5 s.
- Pipette 90 µL of transfection solution into the tube containing 10 µL of diluted miRNA mimic/inhibitor or siRNA. Mix by gentle pipetting and incubate for 15 min at RT.
- Add 100 µL of transfection complex to one well (or dish) of 1 x 106 plated monocytes. Incubate cells for 4 h at 37 °C, then replace the medium with 3 mL of complete media (RPMI 1640 supplemented with 1% penicillin-streptomycin and 10% heat-inactivated FBS) containing either GM-CSF or M-CSF.
4. M1/M2 differentiation and activation
- Monocytes immediately begin differentiating to broad M0 macrophages upon plating in culture. On the third day after plating, continue the using sterile techniques under a biosafety cabinet and replace media with 3 mL of fresh RMPI 1640 media (supplemented with 1% penicillin-streptomycin, 10% heat-inactivated FBS, and either 25 ng/mL GM-CSF to promote M1-like polarization or 50 ng/mL M-CSF to promote M2-like polarization). Culture the cells in these conditions for a total of 6 days from initial plating in an incubator at 37 °C, 5% CO2.
- To advance polarization of primed cells to the M1-macrophage phenotype, activate cells on day 6 of incubation by replacing cell media with new media containing 5% heat-inactivated FBS, 1% pen/strep, 100 ng/mL E. coli-derived lipopolysaccharide (LPS), and 20 ng/mL interferon gamma (IFN-γ).
- To advance polarization of primed cells to the M2-macrophage phenotype, activate cells on day 6 of incubation by replacing cell media with new media containing 5% heat-inactivated FBS, 1% pen/strep, 10 ng/mL M-CSF, and 20 ng/mL interleukin 4 (IL-4).
- After 24 h, harvest the cells for RNA, protein, or flow cytometry analyses.
- When cells are ready for collection, wash cells in the dish 2x with PBS (at RT for RNA extraction or chilled on ice for protein extraction). Because the differentiated macrophages are now firmly attached to the plates, lyse the cells in plates directly to obtain material for RNA and protein analyses.
- For collection of material for flow cytometry, add PBS containing 2 mM EDTA to the dish, incubate the cells for 10 min at 37 °C, gently scrape the cells from the dish, and collect the contents in a 1.5 mL microcentrifuge tube before proceeding with standard protocols.
5. Flow cytometry
- Rinse cells in PBS to remove the culturing medium. Spin down 100,000 cells (per each flow cytometry condition) and resuspend the pellet in 100 µL of PBS containing 2 µL of HuFcR binding inhibitor. Incubate at RT for 15 min.
- Add 50 µL of staining buffer and the desired antibodies (here, CD80, CD83, CD163, and CD209 were used) in the recommended amounts.
- Mix gently and incubate at 4 °C in the dark for 30 min.
- Wash 2x with PBS and resuspend the stained cells in 150 µL of PBS before running the sample on a cytofluorimeter.
Representative Results
Using the procedure described, primary human monocytes from HIV-infected individuals and healthy donors were isolated. All data presented here were obtained from HIV+ subjects undergoing cART with low (<20 copies/mL) or undetectable viral loads and normal CD4+ T cell counts. Immediately after isolation, cells were stained, and flow cytometry was performed to confirm the purity of cell populations. Results showed that >97% of cells stained positive for CD14 (data not shown). For polarization of macrophages, a published protocol was used28. Primary human monocytes were cultured in the presence of GM-CSF or M-CSF for 6 days. On the sixth day, cells were activated towards either M1 or M2 macrophages. Twenty-four hours post-activation, cells were harvested and stained for flow cytometry analysis of macrophage cell markers.
Figure 1 shows representative histograms of M1-activated control- (Figure 1A) and patient-derived (Figure 1B) cells with increased levels of CD80 and CD83 and decreased levels of CD163 when compared to non-activated T0 cells, as well as M2-activated cells with increased levels of CD163 and CD209. Panel C in shows expression of CD80, CD83, CD163, and CD209 in M1 and M2 polarized cells. The graph represents the average data obtained from three control- and three HIV-derived sets of cells. As expected, expression levels of CD80 and CD83 increased in M1 compared to M2 polarized cells, while CD209 and CD163 were more highly expressed in M2 compared to M1 polarized cells. Interestingly, CD80 and CD83 appeared to be more highly expressed in control-derived cells compared to HIV-derived cells. However, potential differences in the ability to polarize and/or expression levels of polarization markers in HIV-derived cells compared to controls requires further investigation. Although GM-CSF, M-CSF, LPS, and IFN-γ were chosen as treatments, other combinations of growth factors, cytokines, or stimulators may be used with this protocol28.
After preliminary experiments showed that the isolation procedure was successful, freshly collected monocytes were plated in 6 well plates and transfected with a scrambled, near-infrared-labeled miRNA to determine transfection efficiency. Cells were imaged 24 h post-transfection by fluorescent confocal microscopy. With this method, >90% efficiency of transfection was achieved, as determined by flow cytometry (Figure 2A) and confocal microscopy (Figure 2B).
Next, viability of the cells after transfection was determined. Figure 3 shows the viability of cells, determined using a colorimetric assay as an average of cells derived from two patients (Figure 3A) and two controls (Figure 3B) transfected at day 1 (Figure 3A) or day 4 (Figure 3B) and harvested at day 7. For this experiment, 50,000 cells were plated on a 96 multi-well plate and transfected following the protocol. In general, transfection did not significantly reduce the viability of cells, regardless of the stage of maturation of the cells and the transfection conditions (i.e., mock, scrambled siRNA/miRNA, or siRNA/miRNA). It should be noted that Figure 3 represents data obtained from patient-derived cells (panel A) and control-derived cells (panel B). This was necessary, since not enough cells to perform the full experiment (both viability and western blot for day 1 and day 4 transfections, in six different conditions per transfection) with a single sample were able to be obtained. Nevertheless, the figure provides representative results obtainable with either control- or patient-derived cells.
Then, the effectiveness of siRNA transfection on target mRNA was assessed by evaluating protein expression. Results in Figure 4 show effective downregulation of EIF4EBP1, a translational regulator highly abundant in these cells, upon transfection with a specific siRNA at both day 1 (Figure 4A) and day 4 (Figure 4C). The same regulator also maintained expression under the various control conditions (i.e., untransfected, mock, scrambled siRNA, and EIF4EBP1 siRNA without transfection reagent: siR*). Quantification of western blot experiments for day 1 and day 4 transfection are shown in Figure 4B and Figure 4D, respectively. Additionally, expression levels of miR-146a-5p following transfection at day 1 or day 4 by RT-qPCR of HIV-derived cells were determined (Figure 4E). Cells transfected with miRNA mimic showed a 48- to 72-fold increase in miRNA expression over untransfected cells, while all transfection controls show no appreciable changes.
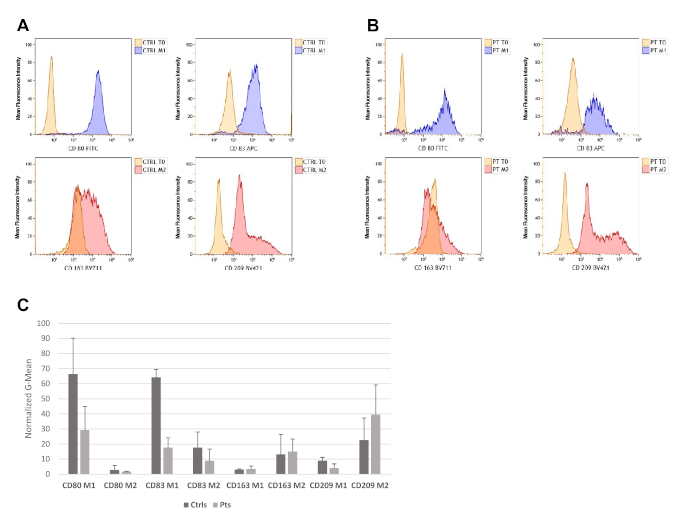
Figure 1: Monocyte-derived macrophages are successfully polarized and activated towards M1 or M2 macrophage phenotypes. Flow cytometry analysis results of cells derived from one healthy control (A) and one HIV-derived cell sample (B) show levels of CD80, CD83, CD163, and CD209. The experiment was repeated with two additional controls and two additional HIV-positive samples with similar results. Cell population of interest was gated on the basis of forward and side scatter parameters, followed by doublet discrimination. (C) Bar graph showing CD80, CD83, CD163, and CD209 in M1 and M2 polarized cells. The graph represents the average data and standard deviations obtained from three control- (Ctrl) and three HIV-derived (Pts) sets of cells. Please click here to view a larger version of this figure.
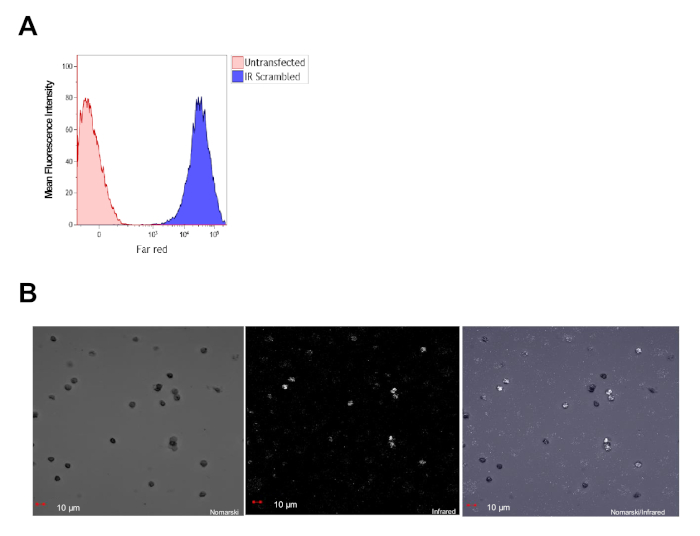
Figure 2: Primary human CD14+ cells are efficiently transfected with miRNAs. (A) Flow cytometry analysis of primary monocytes derived from healthy controls, 24 h post-transfection, showing >90% transfection efficiency. (B) Representative confocal image taken 24 h post-transfection shows that all cells in the field express the miRNA conjugated with a near-infrared dye (in white). Please click here to view a larger version of this figure.
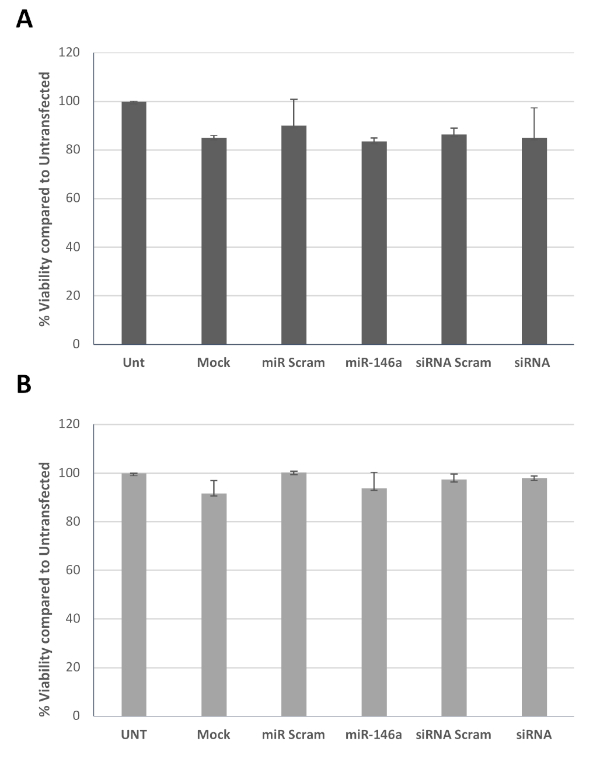
Figure 3: Viability of transfected cells. Graph bars represent average cell viability of two HIV+ patients (A) and two healthy controls (B) determined using a colorimetric assay, after transfection with miR-146a-5p or siRNA to EIF4EBP1 (siRNA) and the appropriate controls at day 1 (A) or day 4 (B), all tested at day 7. Please click here to view a larger version of this figure.
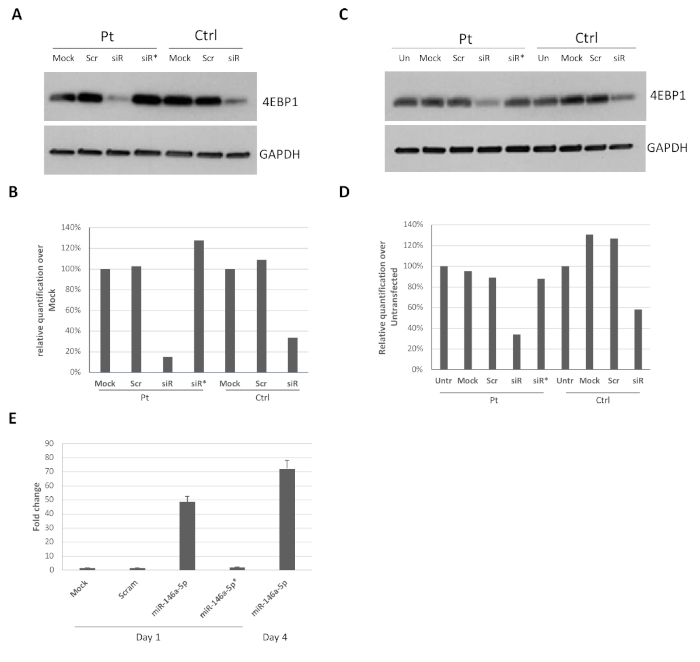
Figure 4: Efficient downregulation of protein levels upon siRNA/miRNA transfection post-isolation of CD14+ monocytes. Control and HIV-derived monocytes (1 x 106 cells) were transfected at day 1 (A) or day 4 (C) post-isolation using siRNA against EIF4EBP1 mRNA. Panels (B) and (D) represent quantification of EIF4EBP1 expression compared to GAPDH and is expressed as the percentage over mock for patient (Pt) or control (Ctrl) (A) or untransfected for patient or control (B). (E) Representative bar graph of two experiments showing miR-146a-5p expression in HIV-derived cells transfected at day 4 (bars 1-4) or day 1 (right bar) and harvested at day 7. The fold change is calculated over the untransfected sample (siR = siRNA). siR* or miR-145a-5p* indicate incubation of the cells with siRNA or miR-146a-5p without the transfection reagent. The experiment was repeated 2x with control-derived cells and produced essentially the same results (data not shown). Please click here to view a larger version of this figure.
Discussion
The presented protocol demonstrates the use of primary cells from HIV-infected subjects as a model for studying monocytes and macrophages. HIV+ patients undergoing cART live with infection for multiple years and can also have other co-infections related a compromised immune system. To study immunomodulation in the presence of HIV chronic infection, cells were harvested from patients directly. As miRNAs have been shown to play major roles in cell development and differentiation, the protocol focuses on the ability to manipulate miRNA expression in these primary cells (Figure 2, Figure 3, Figure 4). Using the same procedure, this protocol also works very well for siRNAs (Figure 3, Figure 4). Due to the potential phagocytic activity of mature macrophages, in addition to mock and scramble siRNA controls, a control is used (indicated as siR* or miR* in Figure 3 and Figure 4), in which the transfection reagent is omitted. Data confirm that the transfection reagent is required for proper siRNA/miRNA delivery into the cells, as without the reagent, cells do not spontaneously uptake the miRNA or siRNA, even when they are already differentiated into macrophages (day 4 after plating and polarization, Figure 3 and Figure 4).
While using commercially available kits for isolation and transfection of human primary CD14+ monocytes, there are key steps optimized to make the procedure reproducible and successful. Specifically, 1) the number of cells plated is critical for their survival and differentiation, and 1 x 106 cells/35 mm dish was found to work best. 2) Freshly isolated CD14+ cells do not uniformly attach to the culture dish if seeded in the presence of FBS. As a consequence, when replacing the medium 4 h after transfection, all unattached cells will be removed, making plate-to-plate conditions highly variable. 3) It was found that replacing the medium 4 h following transfection, reduces toxicity due to transfecting reagents while not affecting the efficiency of transfection. 4) Transfection conditions were optimized to require less siRNA or miRNA (15 nM) than concentrations recommended by the manufacturer (25 nM). 5) Due to the highly adherent nature of the cells, adding lysis buffer directly to the plate greatly improves the concentration of proteins or RNA harvested. However, if it is necessary to remove cells from the plate (i.e., for flow cytometry analysis), it is best to use PBS with EDTA and gently scrape the cells. This method reduces the number of collected cells by approximately 20%-30%, so it is important to plan experiments accordingly to obtain sufficient cell numbers for further analysis.
When followed correctly, this procedure demonstrates the obtaining of highly pure CD14+ population of monocytes, transfection of siRNAs and small RNAs such as miRNAs, culture conditions, and differentiation into M1 or M2 macrophages. This method may be applied to study complex diseases or infections other than HIV.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank the HIV Clinical/Tumor Biorepository Core for providing patient samples and the Cellular Immunology Metabolism Core for providing flow cytometry analysis. This project was funded by NIH P20GM121288 and P30GM114732.
Materials
| 0.5M EDTA | Invitrogen | AM9260G | |
| BD Vacutainer Plastic Blood Collection Tubes with K2EDTA | BD Biosciences | 366643 | |
| Brilliant Stain Buffer | BD Horizon | 563794 | Flow cytometry |
| CD14 PerCP | Invitrogen | 46-0149-42 | Flow cytometry- conjugated antibody |
| CD163 BV711 | BD Horizon | 563889 | Flow cytometry- conjugated antibody |
| CD209 BV421 | BD Horizon | 564127 | Flow cytometry- conjugated antibody |
| CD80 FITC | BD Horizon | 557226 | Flow cytometry- conjugated antibody |
| CD83 APC | BD Horizon | 551073 | Flow cytometry- conjugated antibody |
| Easy 50 EasySep Magnet | StemCell Technologies | 18002 | |
| Easy Sep Direct Human Monocyte Isolation Kit | StemCell Technologies | 19669 | |
| EIF4EBP1 mAb | Cell Signaling | 9644 | Monoclonal antibody for Western blot |
| EIF4EBP1 siRNA | Santa Cruz | sc-29594 | |
| Fetal Bovin Serum Defined Heat Inactivated | Hyclone | SH30070.03HI | |
| Gallios Flow Cytometer | Beckman Coulter | B43618 | |
| GAPDH mAb | Santa Cruz | SC-47724 | Monoclonal antibody for Western blot |
| HuFcR Binding Inhibitor | eBiosciences | 14-9161-73 | Flow cytometry- blocking buffer |
| Kaluza Analysis Software | Beckman Coulter | B16406 | Software to analyze flow cytometry data |
| Lipopolysaccharides from Escherichia coli O55:B5 | Sigma | L4524 | |
| miRCURY LNA microRNA Mimic hsa-miR-146a-5p | Qiagen | YM00472124 | |
| MISSION miRNA Negative Control | Sigma | HMC0002 | Scrambled miRNA conjugated with a near infrared dye |
| Nunc 35mm Cell Culture Dish | Thermo Scientific | 150318 | |
| PBS | Gibco | 20012027 | |
| Penicillin-Streptomycin | Gibco | 15140122 | |
| Recombinant Human GM-CSF | R&D Systems | 215-GM-050 | |
| Recombinant Human IFN-γ | R&D Systems | 285-IF-100 | |
| Recombinant Human IL-4 | R&D Systems | 204-IL-010 | |
| Recombinant Human M-CSF | R&D Systems | 216-MC-025 | |
| RPMI 1640 with L-Glutamine | Corning | 10040CVMP | |
| Scrambled Control siRNA | Santa Cruz | sc-37007 | |
| Viromer Blue Transfection Reagent Kit | Lipocalyx | VB-01LB-01 | |
| WST-1 Cell Proliferation Reagent | Roche | 5015944001 | Colorimetric assay to assess cell viability |
Referências
- Slim, J., Saling, C. F. A Review of Management of Inflammation in the HIV Population. Biomedical Research International. 2016, 3420638 (2016).
- Herskovitz, J., Gendelman, H. E. HIV and the Macrophage: From Cell Reservoirs to Drug Delivery to Viral Eradication. Journal of Neuroimmune Pharmacology. 14 (1), 52-67 (2019).
- Machado Andrade, V., Stevenson, M. Host and Viral Factors Influencing Interplay between the Macrophage and HIV-1. Journal of Neuroimmune Pharmacology. 14 (1), 33-43 (2019).
- Merino, K. M., Allers, C., Didier, E. S., Kuroda, M. J. Role of Monocyte/Macrophages during HIV/SIV Infection in Adult and Pediatric Acquired Immune Deficiency Syndrome. Frontiers in Immunology. 8, 1693 (2017).
- Wacleche, V. S., Tremblay, C. L., Routy, J. P., Ancuta, P. The Biology of Monocytes and Dendritic Cells: Contribution to HIV Pathogenesis. Viruses. 10 (2), (2018).
- Davis, M. J., et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio. 4 (3), e00264 (2013).
- Raggi, F., et al. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Frontiers in Immunology. 8, 1097 (2017).
- Van Overmeire, E., et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Pesquisa do Câncer. 76 (1), 35-42 (2016).
- Vogel, D. Y., et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 219 (9), 695-703 (2014).
- Almeida, M., Cordero, M., Almeida, J., Orfao, A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 19 (3), 261-271 (2005).
- Ciesek, S., et al. Impaired TRAIL-dependent cytotoxicity of CD1c-positive dendritic cells in chronic hepatitis C virus infection. Journal of Viral Hepatitis. 15 (3), 200-211 (2008).
- Granelli-Piperno, A., Golebiowska, A., Trumpfheller, C., Siegal, F. P., Steinman, R. M. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proceedings of the National Academy of Sciences of the United States of America. 101 (20), 7669-7674 (2004).
- Hearps, A. C., et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 26 (7), 843-853 (2012).
- Heggelund, L., et al. Stimulation of toll-like receptor 2 in mononuclear cells from HIV-infected patients induces chemokine responses: possible pathogenic consequences. Clinical and Experimental Immunology. 138 (1), 116-121 (2004).
- Hernandez, J. C., et al. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS Research and Human Retroviruses. 27 (10), 1099-1109 (2011).
- Hernandez, J. C., Latz, E., Urcuqui-Inchima, S. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology. 57 (1), 36-42 (2014).
- Low, H. Z., et al. TLR8 regulation of LILRA3 in monocytes is abrogated in human immunodeficiency virus infection and correlates to CD4 counts and virus loads. Retrovirology. 13, 15 (2016).
- Sachdeva, M., Sharma, A., Arora, S. K. Functional Impairment of Myeloid Dendritic Cells during Advanced Stage of HIV-1 Infection: Role of Factors Regulating Cytokine Signaling. PLoS ONE. 10 (10), e0140852 (2015).
- Sachdeva, M., Sharma, A., Arora, S. K. Increased expression of negative regulators of cytokine signaling during chronic HIV disease cause functionally exhausted state of dendritic cells. Cytokine. 91, 118-123 (2017).
- Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116 (2), 281-297 (2004).
- Li, H., Jiang, T., Li, M. Q., Zheng, X. L., Zhao, G. J. Transcriptional Regulation of Macrophages Polarization by MicroRNAs. Frontiers in Immunology. 9, 1175 (2018).
- Hu, X., et al. Genome-Wide Analyses of MicroRNA Profiling in Interleukin-27 Treated Monocyte-Derived Human Dendritic Cells Using Deep Sequencing: A Pilot Study. International Journal of Molecular Sciences. 18 (5), (2017).
- Huang, J., et al. MicroRNA miR-126-5p Enhances the Inflammatory Responses of Monocytes to Lipopolysaccharide Stimulation by Suppressing Cylindromatosis in Chronic HIV-1 Infection. Journal of Virology. 91 (10), (2017).
- Lodge, R., et al. Host MicroRNAs-221 and -222 Inhibit HIV-1 Entry in Macrophages by Targeting the CD4 Viral Receptor. Cell Reports. 21 (1), 141-153 (2017).
- Ma, L., Shen, C. J., Cohen, E. A., Xiong, S. D., Wang, J. H. miRNA-1236 inhibits HIV-1 infection of monocytes by repressing translation of cellular factor VprBP. PLoS ONE. 9 (6), e99535 (2014).
- Riess, M., et al. Interferons Induce Expression of SAMHD1 in Monocytes through Down-regulation of miR-181a and miR-30a. Journal of Biological Chemistry. 292 (1), 264-277 (2017).
- Buchacher, T., Ohradanova-Repic, A., Stockinger, H., Fischer, M. B., Weber, V. M2 Polarization of Human Macrophages Favors Survival of the Intracellular Pathogen Chlamydia pneumoniae. PLoS ONE. 10 (11), e0143593 (2015).
- Jaguin, M., Houlbert, N., Fardel, O., Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cellular Immunology. 281 (1), 51-61 (2013).
- Lacey, D. C., et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. Journal of Immunology. 188 (11), 5752-5765 (2012).
- Tarique, A. A., et al. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. American Journal of Respiratory Cell and Molecular Biology. 53 (5), 676-688 (2015).

