An Implantable System For Chronic In Vivo Electromyography
Summary
Presented here is a protocol for the manufacturing of an implantable system for in vivo chronological recording of evoked and spontaneous electromyographic potentials. The system is applied to the investigation of reinnervation of laryngeal muscles following nerve injury.
Abstract
Electromyography (EMG) measures the muscle response to electrical stimulation or spontaneous activity of motor units and plays an important role in assessing neuromuscular function. Chronic recording of EMG activity reflecting a muscle’s reinnervation status after nerve injury has been limited, due to the invasive nature of traditional EMG recording techniques. In this regard, an implantable system is designed for long-term, in vivo EMG recording and nerve stimulation. It has been applied and tested in a study on reinnervation of laryngeal muscles. This system consists of 1) two bipolar electrode nerve cuffs and leads for stimulating each of two nerves: the recurrent laryngeal nerve (RLN) and internal branch of the superior laryngeal nerve (SLN); 2) two EMG recording electrodes and leads for each of the two laryngeal muscles: posterior cricoarytenoid (PCA) muscle and thyroarytenoid-lateral cricoarytenoid (TA-LCA) muscle complex; and 3) a skin receptacle interfacing all implanted lead terminals to an external recording preamplifier and stimulator using a connection cable. The wire leads are Teflon-coated, multi-filament, type 316 stainless steel. They are coiled and can stretch during body movement of the awake animal to prevent lead breakage and electrode migration. This system is implanted during an aseptic surgery. Afterwards, baseline EMG recordings are performed before the RLN is transected in the second surgery to study muscle reinnervation. Throughout the study, multiple physiological sessions are conducted in the anesthetized animal to obtain evoked and spontaneous EMG activity that reflects the reinnervation status of laryngeal muscles. The system is compact, free of infection over the course of the study, and highly durable. This implantable system can provide a reliable platform for research in which long-term recording or nerve stimulation is required in an anesthetized or freely moving animal.
Introduction
EMG recording is a useful technique for measuring electrical activity produced by a skeletal muscle when activated by electrical stimulation of its nerve or spontaneous firing of its motor units. Monitoring EMG signals can be used for assessment of neuromuscular transmission and muscle biomechanics1. EMG recording also plays an important role in characterizing the quality and magnitude of muscle reinnervation following nerve injury2,3,4,5. However, multiple EMG recordings over the entire period of reinnervation cannot be achieved by an invasive approach. Therefore, implantable devices have been designed and developed for repeated, chronic stimulation and recording in neuromuscular systems6,7,8,9,10,11,12,13. The aim of this paper is to describe a protocol for the manufacturing and implantation of a stable system for obtaining reliable chronological EMG data from the larynx.
This system is applied here to the study of laryngeal muscle reinnervation. A brief overview of the larynx is provided for orientation (Figure 1). A precise coordination between sensory and motor components is essential for proper muscular movement during respiration, voicing, and airway protection. The PCA muscle, located in the posterior larynx, is the sole abductor of the vocal fold. This muscle is spontaneously activated during inspiration to increase glottal area for inhalation. The TA-LCA complex is the major adductor of the vocal fold. Activation of this muscle complex along with another adductor (i.e., the interarytenoid muscle) medialize the fold for vibration and sound production and close the fold for airway protection during swallowing.
Additionally, motor neuron fibers innervate both abductor and adductor muscles in the RLN. The abductor and adductor muscles can be distinguished based on motor unit composition14,15. The PCA muscle exhibits increased firing during hypercapnic and/or hypoxic conditions16 due to the presence of inspiratory motor units. In contrast, reflex glottic closure (RGC) motor units, which close the glottis reflexively through activation of sensory receptors within the laryngeal mucosa, is present in the TA-LCA muscle complex. The internal branch of the superior laryngeal nerve (SLN) carries the afferent fibers of sensory receptors in the larynx17. Although voicing is primarily an adductor function, both abductor and adductor motor units are involved in this highly evolved laryngeal behavior.

Figure 1: Anatomy of the larynx. The components of this implantable system are also displayed. SLN = superior laryngeal nerve; RLN = recurrent laryngeal nerve; PCA = posterior cricoarytenoid muscle; TA-LCA = thyroarytenoid–lateral cricoarytenoid muscle complex; DBS = deep brain stimulation. This figure has been reproduced with permission from Wiley27. Please click here to view a larger version of this figure.
Injury to the RLN can result in vocal fold paralysis (VFP), which compromises both abducting and adducting functions due to laryngeal muscle denervation14,18,19. Subsequently, regeneration of RLN nerve fibers and reinnervation of muscles commonly occurs. However, reinnervation is a random process and results in misdirected, inappropriate muscle reconnection in most cases. This is referred to as synkinesis, in which spontaneous activation of abductor and adductor antagonists is faulty and produces ineffective or even paradoxical movement of the vocal folds14,19,20,21. With synkinesis, the critical function that is lost is vocal fold abduction, resulting in inadequate ventilation. Although there are ongoing attempts to treat laryngeal synkinesis by either 1) blocking glottic closure with Botox22,23 or 2) electrically stimulating the glottic opening with an implantable pacemaker24,25, there is no clinical intervention that reliably prevents synkinesis26. However, there is evidence that electrical conditioning of the PCA muscle during reinnervation at a low frequency promotes appropriate neuromuscular reconnection and minimizes synkinesis from happening. Studies are currently being conducted to elucidate the underlying mechanisms2.
The focus of this paper is to describe a simple and inexpensive implantable system for chronic nerve stimulation and EMG recording. This system can be used to investigate the effects of low frequency electrical conditioning of the PCA muscle on the specificity of its subsequent reinnervation. EMG signals obtained by this system can reflect the quality and quantity of laryngeal muscle reinnervation over time.
Protocol
This study has been approved by the institutional animal care and use committee (IACUC) of Vanderbilt University and was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, Maryland). This system includes five implantable components and one external cable.
1. Two Bipolar RLN Stimulus Electrode Cuffs, Each with Pair of Coiled Lead Wires and Terminal Pins
- Use Teflon-coated, multi-filament, type 316 stainless steel wire (with insulated diameter of 0.0078” or 0.198 mm) for each cuff lead wire. Cut a 70 cm length of wire and coil it into a 12 cm long spring using a coiling device or procure prefabricated coiled leads. If necessary, stretch the spring to increase its length for each implant site. Leave the ends of the coiled leads straight at 3 mm and 10 mm lengths and deinsulate them.
- Solder a gold-plated copper female pin onto the 3 mm end of the coiled lead.
- To prepare the nerve cuff, cut a 5 mm segment of silicone tube (OD = 0.156”, ID = 0.094”; or OD = 3.96 mm, ID = 2.39 mm) from a roll of the tubing.
- To insert a lead into the tube, use a 25 G hypodermic needle to pierce through the tubing wall 1.5 mm from the end and off-center close to the inner wall. Backfill the 10 mm end of the lead into the tip of the needle. Withdraw the needle to deposit the deinsulated portion into the tube. Bend back the bare wire end outside the tube and twist onto the lead at its point of entry into the tube.
NOTE: Use an operating microscope to perform these steps. A probe can be placed into the tube to curve the wire against the inner wall. The goal is to position the bare portion of the wire so that stimuli can be delivered to the nerve without risking mechanical damage to the nerve. - Insert the second lead 1.5 mm from the opposite end of the tube using the same procedure. Align the point of entry to that of the first lead. Pierce the wall with the needle so that the bare portion of the wire is deposited near the inner wall opposite to the first lead.
NOTE: Looking down the tube, the two stimulus electrodes should form a 45° “V” shape, which will straddle the nerve once in place and assure current delivery through the nerve from anode to cathode. - Make an S-shaped slit in the tube wall opposite the electrode points of entry using a pair of curved scissors.
NOTE: The spiral lips of the cuff can then be opened to situate the nerve inside between the electrodes during surgery. - Insert a length of 6-0 monofilament, nonabsorbable suture into the cuff wall at each end using a curved microsurgical needle for eventual securement of the cuff around the nerve.
- Apply medical grade type-A silicone gel to reinsulate all exposed bare wire outside the cuff.
2. Two bipolar SLN Stimulus Electrode Cuffs, Each with Pair of Coiled Lead Wires and Terminal Pins
- Assemble the SLN stimulus electrode cuff in the same way as the RLN stimulus electrode cuff. However, use the smaller diameter (OD = 0.125”, ID = 0.062”; or OD = 3.18 mm, ID = 1.57 mm) tube, because the nerve is smaller in diameter.
3. Two PCA muscle EMG recording electrodes, each with Coiled Lead Wire and Terminal Pin
- Assemble a coiled lead for the PCA muscle electrode as done in step 1.1.
- Solder a female pin onto the lead as done in step 1.2.
- Insert the 10 mm end of the PCA muscle lead into the tip of a deep brain stimulation (DBS) electrode using the same strategy for needle-lead insertion into a cuff (step 1.4). Bend the end of the lead to form a hook and clip it to provide a total of 5 mm recording length.
NOTE: In this application, the PCA muscle and its reinnervating nerve terminals are exposed to electrical conditioning. Stimuli are generated by an implantable pulse generator (IPG) and delivered to the laryngeal muscle through a DBS electrode (Figure 1, inset). This system is adapted from therapeutic brain stimulation (e.g., Parkinson’s disease). The DBS electrode will be inserted into a submuscular pocket and anchored in place. If technology for electrical conditioning of the muscle is not required, the PCA EMG electrode can be directly inserted into the muscle and anchored by its hook.
4. Two TA-LCA Muscle Complex EMG Recording Electrodes, Each with Coiled Lead Wire and Terminal Pin
- Assemble a coiled lead for the TA-LCA muscle electrode as done in step 1.1.
- Solder a female pin onto the lead as done in step 1.2.
- Excise a 5 mm x 10 mm rectangular piece of knitted polyester graft. Make a hole in the center of the mesh with a 20 G hypodermic needle. Introduce the 10 mm end of the lead into the hole with an additional 3 mm of coil protruding beyond the hole. Affix the lead to the mesh using 6-0 monofilament, nonabsorbable suture.
NOTE: This piece of mesh will be used to anchor the electrode lead to the thyroid cartilage overlying the muscle complex. - Bend the end of the lead to form a hook and clip it to provide a total of 5 mm recording length.
5. Skin Receptacle for Interfacing Connections Between Electrodes and External Equipment
- Utilize a single row female pin stripe connector to make the receptacle. Cut two pieces (each 17.5 mm in length) from the strip, each containing eight pin holes. First, roughen the external surfaces of each piece with sandpaper, then glue them together with phenol in a fume hood to make a double-row connector. Place the connector in 60–80 °C water in a fume hood for 30 min to allow for glue hardening.
NOTE: This double-row assembly format will provide convenience in the assignment of pinholes for left- vs. right-side electrodes. - Cut a 25.6 mm length piece from the strip to make the connector’s faceplate (the portion that will protrude outside the implant site for skin anchoring). Cut a 5.4 mm x 17.4 mm rectangular hole in the middle of the faceplate with a scalpel.
- Place the double-row connector inside the rectangular hole of the faceplate until it is flushed with the faceplate surface without protrusion. If the connector does not fit into the rectangular hole of the faceplate, the hole can be slightly enlarged with a file. Since the connector holes are not symmetrical, insert the connector edge with the larger diameter holes into the faceplate.
NOTE: As a result, a female pin inserted into the opposite edge of the connector with the smaller diameter hole will snap and lock into place. - Use phenol to glue the connector and the faceplate together. Place the assembly in 60–80 °C water in a fume hood for 30 min to allow for glue hardening.
- Drill a 1.3 mm hole at each corner of the faceplate and on each side of the faceplate halfway from the ends for a total of six holes.
NOTE: These holes will be used to suture the final skin receptacle at the implant site. - Cut a 15 mm length tube of knitted polyester graft to surround the assembly below the faceplate, making the assembly biocompatible. To fix the tube to the assembly, use a hypodermic needle to thread stainless steel wires through the wall at three equally spaced positions (each 3.8 mm apart) along its length.
- Place equally spaced notches in each corner of the connector to anchor the wires against the assembly surface. Twist the ends of each wire with a pair of pliers to cinch the tube to the assembly to form a skirt.
- Make a permanent mark on the polyester patch at one end of the receptacle.
NOTE: Use this mark for orientation to identify the rostral end of the receptacle during implant surgery. In the rostral to caudal direction, the following pin electrode assignment for each of the two rows (left side and right side) should be as follows: PCA EMG, TA-LCA EMG, empty hole, empty hole, RLN anode, RLN cathode, SLN anode, and SLN cathode.
6. External Connection Cable to Recording Pre-amplifier and Stimulator
NOTE: A cable is used for making connections between the implanted skin receptacle and external equipment during nerve stimulation-EMG recording sessions (sections 8 and 10). It is composed of 12 insulated wires terminating with male pins to insert into female pins in the skin receptacle. This cable consists of two parts: an EMG recording plug and nerve stimulation wires. A recording plug is necessary to isolate low voltage EMG signals from higher voltage stimulus artifacts radiating from stimulus pins. For the same reason, two holes in each row of the skin receptacle are left unoccupied to separate recording pins from stimulation pins.
- To make the EMG recording plug, use a male strip connector (same length and width, but one-half the height of a female connector). Cut it into two pieces, each containing only two holes. Affix the two pieces using phenol adhesive using the same approach to make the double-row connector in the skin receptacle (step 5.1). Take the four EMG recording wires in the cable and insert their terminal male pins into each of the four holes until they lock in place with the tips protruding beyond the strip edge.
- Use bone cement to seal the top of the plug to insulate wire-pin junctions.
- Use the remaining eight wires in the cable terminating in male pins to make individual connections to the nerve stimulation cuffs via their female pins.
7. First Implant Surgery
- Obtain a 1–2 year-old, 20–25 kg canine of either sex from a licensed farm. Acclimate the animal before aseptic implant surgery. Autoclave all equipment before surgery. Withhold food for 10–12 h before the surgery.
- Prepare the animal for surgery.
- Shave the animal’s head and neck and clean the skin with alcohol and betadine scrub solution. Anesthetize the animal by intravenous injection of 2–4 mg/kg tiletamine and zolazepam combination, followed by 3% isoflurane in oxygen through intubation.
- Place the animal on an operating table with a heating pad in supine position and surgically drape the animal. Monitor animal’s heart rate, respiratory rate, body temperature, and oxygen saturation at least every 15 min throughout the surgery to ensure physiological stability at a moderate plane of anesthesia.
- Make a midline neck incision from the thyroid notch to manubrium. Dissect the trachea free from the esophagus and expose the inferior border of the cricoid cartilage.
- Position the stimulus cuff onto each of the bilateral SLNs and RLNs. Close the lips of each cuff using the enclosed sutures.
- Make a cartilage window with a biopsy punch (4 mm in diameter) at the anterior surface of the thyroid cartilage on each side. Expose the lateral aspects of both TA-LCA muscle complexes. Insert the EMG recording electrodes into the TA-LCA muscle complexes using a 23 G needle by inserting the barb into the tip of the needle. Suture the electrode polyester patch onto cartilage.
- Place the DBS electrode along with its companion hook-wire EMG recording electrode underneath the PCA muscle on each side. Use an endoscope to confirm that stimulation produces vocal fold abduction for each channel. Anchor the DBS electrodes to the cricoid cartilage by 4-0 nonabsorbable sutures.
- Insert all the wire leads of the nerve stimulation-EMG recording electrodes into the receptacle via their female pins. Press the pins into holes with an insertion tool fashioned from a hemostat. Seal the inferior surface of the receptacle to insulate lead-pin junctions using bone cement.
- After the cement hardens, place the receptacle at the rostral end of the midline incision through the skin and suture it to subcutaneous tissues via its polyester skirt. Attach the skin edge to the receptacle by sutures passing through the holes in the faceplate.
NOTE: One jaw of the hemostat has an end slit leading to a counter-sink hole. The lead wire can be positioned through the slit into the hole and the countersink placed against the head of the pin. The second jaw is placed on the opposite side of the receptacle. Squeezing the hemostat presses the pin into its respective receptacle hole.
- After the cement hardens, place the receptacle at the rostral end of the midline incision through the skin and suture it to subcutaneous tissues via its polyester skirt. Attach the skin edge to the receptacle by sutures passing through the holes in the faceplate.
- Make an incision on the left neck to expose the trapezius muscle. Perform dissection to make a submuscular pocket for placement of the implantable pulse generator. Tunnel each DBS lead subcutaneously to the neck incision for insertion into the IPG.
- Close all surgical wounds with sutures. Monitor the animal closely until full recovery from the surgery.
- Provide postoperative analgesics (e.g., buprenorphine: 0.01–0.02 mg/kg) routinely for up to 48 h. Administer antibiotics (e.g., cefpodoxime: 10 mg/kg) orally to the animal for at least 3 days. House the animal singly thereafter for throughout the study, and restrict exercise for a period of 10 days to allow normal wound healing and stabilization of the implanted device.
NOTE: The skin receptacle should be cleaned daily with tissue-compatible antiseptic solution. In addition, dummy male pins should be inserted into the female pins of the skin receptacle routinely except during the EMG recording sessions. This maneuver will avoid the accumulation of debris in the receptacle, allow effective connections to be made with the external cable, and prevent infection.
8. Nerve Stimulation-EMG Recording Sessions at Baseline
NOTE: Perform these sessions 2x–3x after implant surgery (section 7) and before nerve transection surgery (section 9) to obtain baseline EMG signals when the bilateral RLNs are intact. Apply the following protocol during a standard nerve stimulation-EMG recording session (sections 8 and 10).
- Withhold food before the procedure for 10–12 h. Anesthetize the animal with tiletamine and zolazepam combination (initial loading dose 2–4 mg/kg by intravenous injection, then maintain with 0.4 mg/kg per hour via an i.v. line). Place the animal on a heating pad in supine position and maintain the animal in a moderate plane of anesthesia. Monitor the animal’s vitals during the procedure as described in step 7.2.
- Insert a zero-degree rigid endoscope with an attached CCD video camera through a laryngoscope to visualize vocal fold motion at the level of the glottis.
- Interface the external cable that connects to the lab stimulator and EMG preamplifiers to the skin receptacle via its plug and pins. Connect the outputs from the preamplifiers to a data acquisition device and/or an oscilloscope to display, record, and measure EMG signals.
- Deliver stimuli (single square-wave pulses, 0.1–0.5 ms duration, 0.5–2.0 mA amplitude) to the left and right RLNs, respectively, to record evoked EMG responses from bilateral TA-LCA complexes and PCA muscles under each condition.
- Deliver stimuli (single square-wave pulses, 0.1–0.5 ms duration, 0.5–2.0 mA amplitude) to the left and right SLNs, respectively, to record evoked EMG responses from bilateral TA-LCA complexes and PCA muscles under each condition.
- Deliver CO2 mixed with room air through the mouth of the animal to induce hypercapnia and increase the animal’s respiratory effort. Limit exposure to 1 min, during which the maximum inspiratory motor unit recruitment will occur. Record spontaneous EMG activities of TA-LCA complexes and PCA muscles under this hypercapnic condition.
- Monitor the animal until full recovery from anesthesia and return the animal to the facility.
9. Second Surgery for Nerve Transection and Anastomosis
- Perform the second surgery 10–14 days after the first surgery. Withhold food for 10–12 h before surgery.
- Anesthetize the animal, drape and monitor vitals intraoperatively using the technique described in step 7.2.
- Remove the sutures and reopen the midline incision by blunt dissection whenever possible. Avoid damage to the previous implantation during the dissection. Expose the bilateral RLNs through dissection. Isolate, transect and anastomose each nerve with 7-0 monofilament, nonabsorbable sutures to induce bilateral laryngeal paralysis.
- Irrigate the neck incision with sterile saline and gentamycin antibiotic. Close the muscular and subcutaneous tissues using 3-0 absorbable sutures. Close skin with 3-0 nonabsorbable monofilament sutures.
- Closely monitor the animal until full recovery from surgery.
- Provide analgesics (e.g., buprenorphine: 0.01–0.02 mg/kg) routinely for up to 48 h postoperatively. Give antibiotics (e.g., cefpodoxime: 10 mg/kg) orally to the animal for at least 3 days. Restrict the animal from exercise for a period of 10 days to allow normal wound healing.
10. Nerve Stimulation-EMG Recording Sessions Following Bilateral RLN Injuries
- Perform these sessions 1x per week during the first 3 months, then biweekly thereafter. Follow the protocol described in section 8 for these sessions.
Representative Results
Examples of the components are shown in Figure 2. From left to right in Figure 2A are the nerve stimulus cuff, TA-LCA recording electrode, PCA recording electrode, and skin interface receptacle, respectively. The relative size of these components can be appreciated. The skin receptacle (Figure 2B) has two rows of holes into which the female pins at the end of each coiled wire (Figure 2D) are inserted. They are inserted opposite the faceplate (arrow) during the implantation surgery. The receptacle has a polyester skirt (Figure 2C) attached to its connector sidewalls. This skirt is designed to anchor the receptacle in position by connective tissue infiltration. Each Teflon-coated stainless-steel EMG lead (Figure 2E) is deinsulated (5 mm) at the tip to form a hook-shaped electrode for muscle recording. The stimulation cuff has two electrodes threaded against the inner cuff wall. They are separated by a distance of 2 mm (Figure 2F) and form a “V” shape (Figure 2G) to ensure current delivery across the nerve.
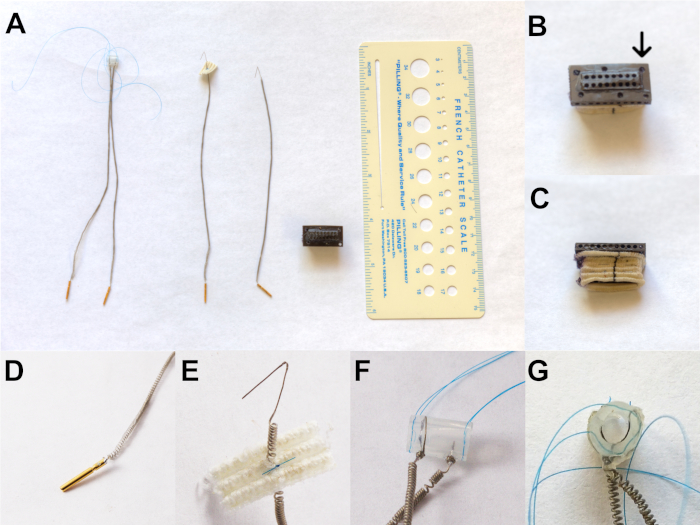
Figure 2: Components of the implant system. (A) From left to right is the nerve stimulus cuff, TA-LCA recording electrode, PCA recording electrode, and skin interface receptacle, respectively. (B) The skin receptacle showing two rows of holes. (C) The receptacle showing a polyester skirt attached to its connector sidewalls. (D) Coiled wire containing female pins to be inserted into B. (E) Teflon-coated stainless-steel EMG lead is deinsulated (5 mm) at the tip to form a hook-shaped electrode for muscle recording. (F) The stimulation cuff has two electrodes threaded against the inner cuff wall, which are separated by 2 mm. (G) “V” shape formation of electrodes to ensure current delivery across the nerve. This figure has been modified with permission27. Please click here to view a larger version of this figure.
Figure 3 shows the implanted skin receptacle and how the cable from external equipment is interfaced to the receptacles. It should be noted that dummy male pins (not shown) are inserted into the female pins of the receptacle to keep them free of debris between recording sessions.
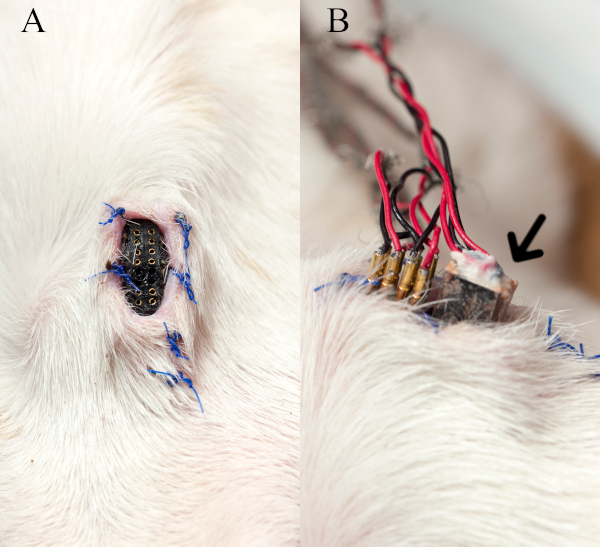
Figure 3: Skin receptacle and interface cable. (A) The implanted skin receptacle on the anterior neck without dummy male pins is shown. (B) The image depicts how the stimulus pins and EMG recording plug (arrow) of the cable from external equipment is interfaced to the receptacle during a nerve stimulation-EMG recording session. This figure has been modified with permission27. Please click here to view a larger version of this figure.
Figure 4 shows an EMG recording from one of the baseline sessions with the RLNs intact.
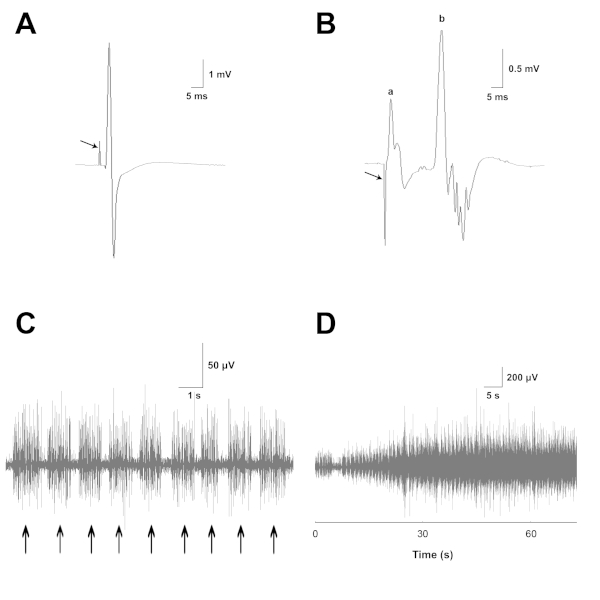
Figure 4: EMG recordings from laryngeal muscles with normal innervation. (A) Example recording from the PCA muscle where RLN stimulation produces a stimulus artifact (arrow) followed by a large evoked EMG potential. (B) Example recording of the TA-LCA muscle complex, in which SLN stimulation produces a stimulus artifact (arrow). Represented here is (a) a short latency monosynaptic muscle response and (b) a longer latency polysynaptic RGC response. (C) Bursts (arrows) of spontaneous EMG activity recorded from the PCA muscle during normal inspirations. (D) Increase of inspiratory EMG activity over the course of CO2 delivery. This figure has been modified with permission27. Please click here to view a larger version of this figure.
In a recording from the PCA muscle (Figure 4A), RLN stimulation produces a stimulus artifact (arrow) followed by a large evoked EMG potential. The maximum RLN-evoked responses provide a good index of the overall magnitude of normal innervation as well as the level of reinnervation following subsequent neurorrhaphy, irrespective of motor unit type. This is true because the RLN contains nerve fibers of both inspiratory and reflex glottic closure (RGC) motor units. RLN stimulation recruits both types of units. Evoked EMG motor unit activity is rectified and integrated over a 20 ms time period to obtain a quantitative measure of muscle innervation.
In a recording from the TA-LCA muscle complex (Figure 4B), SLN stimulation produces a stimulus artifact (arrow). This artifact is followed by a short-latency monosynaptic muscle response (a) and longer-latency polysynaptic RGC response (b). The potential (a) is a direct response from the cricothyroid muscle, because this muscle is innervated by the nearby external branch of the SLN. Stray activation of this branch commonly occurs during nerve cuff stimulation of the internal branch to activate the RGC response. The cricothyroid potential is recorded by the TA-LCA electrode, as this muscle is located near the complex. Previous studies have shown that the cricothyroid potential evoked by internal branch stimulation can be selectively abolished by sectioning the external branch of the SLN (Zealear, unpublished observations). The maximum SLN-evoked EMG responses reflect the magnitude of natural innervation of the TA-LCA complex through its RGC sensory-motor pathway. Prior to RLN neurorrhaphy, there is no RGC innervation of the PCA muscle, so no SLN potential should be detected from this muscle. Following nerve transection and repair, SLN-evoked potentials reflect the amount of correct RGC reinnervation of the TA-LCA complex and incorrect RGC reinnervation of the PCA muscle. RGC activity is quantified by rectification and integration over a 20 ms time period to capture the entire RGC waveform.
In (Figure 4C), bursts (arrows) of spontaneous EMG activity are recorded from the PCA muscle during normal inspirations. This inspiratory EMG activity increases over the course of CO2 delivery, as shown in (Figure 4D) at a slower sweep speed. Spontaneous PCA EMG activity provides a good estimate of the magnitude of normal innervation of this muscle by its original inspiratory motoneurons. There is no inspiratory innervation of the TA-LCA complex, so no inspiratory potentials should be detected from these muscles. This is because only inspiratory motor units are involved in abducting the vocal fold at maximal inspiratory effort in the anesthetized animal. Following nerve transection and repair, spontaneous inspiratory potentials reflect the magnitude of correct reinnervation of the PCA muscle and magnitude of incorrect reinnervation of the TA-LCA complex. Recordings of inspiratory EMG activity are amplified, rectified, and integrated over an 8 s time period.
Discussion
This paper describes the steps required in the manufacturing of a novel, economical, and implantable system for stimulation of laryngeal nerves and recording of EMG responses from laryngeal muscles over a long term. The protocol is uncomplicated and can produce an implant that is compact enough to be utilized in an animal as small as a rat. There are several critical steps that should be emphasized. First, lead wires should be coiled carefully and uniformly to prevent lead de-insulation, kinking or breakage. If a coiling machine is not available, prefabricated coiled leads can be obtained commercially. Second, the strategy of inserting lead wires into a silicone tube to form a “V” that straddles the nerve is critical to promote current delivery through the nerve inside the cuff. If both leads are placed on the same side of the tube, shunting of current between electrodes can occur. It is also important that the leads are positioned against the tube inner wall to avoid the possibility of slice injury to the nerve.
Third, during the implantation surgery, laryngeal nerves should be dissected carefully to prevent damage. At the later stage of implantation, when inserting pins into the receptacle, force should be applied to the pin in alignment to its hole to prevent sudden bending of the head of the pin. Subsequently, bone cement should be distributed thoroughly on the receptacle bottom for complete insulation and prevention of crosstalk between channels. Finally, prevention of infection is critical to ensure integrity of the implant system over time. It can be achieved by a combination of several maneuvers: addition of a skirt to the receptacle, administration of antibiotics, daily cleaning of the wound and receptacle with tissue-compatible antiseptic solution, and placement of dummy male pins into the female pins of the receptacle to keep them clean of debris between sessions.
The protocol has been proven successful in this dog laryngeal model. However, some modifications or alternative strategies may be considered for other applications. For example, the deinsulated sensing tips of the PCA and TA-LCA EMG electrodes are anchored in the muscles by an external means-either the polyester graft or the DBS electrode. In an application in which external anchoring is not needed or performed, the barb of the electrode alone can serve as the anchor. In such a case, Teflon-coated, stainless steel, monofilament wire may be preferable to multifilament wire in view of its greater tensile strength, providing a barb that is more stable in tissue. However, it should be noted that multifilament wires may be less prone to breakage. An alternative strategy to fabrication and assembly of the skin receptacle is to 3D-print using biocompatible polymers (e.g., MED610 by Stratasys). This may simplify the manufacturing process.
Following implantation surgery and recovery of the animal, physiological sessions are conducted with the RLNs still intact to obtain baseline data. During a session, absence of EMG signals from a laryngeal muscle may occur following RLN stimulation. In order to troubleshoot the cause (Table 1), it should first be determined whether vocal fold movement is present. If it is present, this means that the nerve is effectively activated by the cuff, but there is a problem with the EMG lead. In this situation, users should further look at the EMG stimulus artifact. If the EMG artifact is absent, there is likely a discontinuity in the EMG input to the preamplifier. Sixty-cycle noise will also be present and large in amplitude. If the artifact is large, shunting from a stimulus pin to the recording pin may be responsible for saturating the channel preamplifier and obliterating the EMG response. If the artifact is normal, then the EMG lead has likely dislocated from the muscle and cannot detect its activity. On the other hand, if the vocal fold movement is absent, then the nerve is not being activated. If the artifact is absent, there may be a discontinuity in the stimulation circuit, preventing nerve activation. If the artifact appears normal, the nerve may have been injured during implant surgery or the cuff may have migrated off the nerve. A similar strategy can be applied to troubleshoot the cause of absent EMG signals during SLN stimulation.
| Stimulated nerve | Target muscle(s) | Ipsilateral vocal fold movement | Stimulus artifact | Causes |
| RLN | PCA and/or TA-LCA | Yes | Absent (60-cycle noise present) | Discontinuity in the EMG input to preamplifier (e.g. lead, pin, cable); |
| Large | Cross-talk between stim and recording pins at the receptacle | |||
| Normal | Dislocation of EMG electrode | |||
| No | Absent | Discontinuity in stimulation circuit | ||
| Normal | 1. RLN injury; 2. Cuff dislocation | |||
| SLN | TA-LCA | Yes | Absent (60-cycle noise present) | Discontinuity in the EMG input to preamplifier (e.g. lead, pin, cable); |
| Large | Cross-talk between stim and recording pins at the receptacle | |||
| Normal | Dislocation of EMG electrode | |||
| No | Absent | Discontinuity in stimulation circuit | ||
| Normal | 1. SLN or RLN injury; 2. Cuff dislocation |
Table 1: Troubleshooting guide.
It should be mentioned that there are two minor limitations in the current application of this technology. First, sudden bending of the female pin during insertion into the receptacle has occurred in several instances. Fortunately, the pins can be straightened and inserted into their holes successfully. If pin damage is irreparable, the lead and its entire component need to be replaced. Therefore, backup components should be readily available before surgery. Second, the time required to complete the surgical implantation is long (~10 h). The long duration partially reflects the large number of stimulation and recoding components required for this study: four nerves, four muscles, a receptacle, and an IPG. If fewer components are required using this technology, the implantation time should be significantly reduced (e.g., the rat tongue model28).
This technological approach introduces several features that have advantage over existing methods. The coiling of lead wires is the most novel and important feature of this system. Coiled leads are not commonly available for non-commercial animal experimentation despite the many benefits they provide. A coiled lead can be expanded to the desired length during implantation. Further, it will stretch in the awake, moving animal to prevent dislocation of the electrode tip or wire breakage after implantation. This feature ensures longevity of the implant and stable nerve stimulation and muscle recording over the long term. Furthermore, adding a tissue compatible skirt around the receptacle prevents exposure of the wound to this foreign body and promotes normal fibrosis and wound healing in the absence of infection. Previous studies without this skirt resulted in early infection and premature termination of the experiment. Lastly, this implant system is compact and multi-channeled, allowing effective data acquisition from numerous neuromuscular structures in animal models of various size.
This technical approach has been adapted and successfully translated to a rat model. This study was designed to investigate the effect of electrical conditioning in preventing tongue muscle atrophy and dysfunction in the aging rat. The hypoglossal nerves were implanted with the cuff electrodes for conditioning and the tongue implanted with the EMG recording electrodes28. This technology can also be utilized in other research applications. As an extension of the current protocol in the canine larynx, the effects of electrical conditioning on promoting selective reinnervation are currently being studied in rabbit facial muscles. This study may provide a foundation for the prevention of facial synkinesis in patients with Bell’s palsy, a common and debilitating medical condition. A final potential use of this technology is to stimulate and record from awake, freely moving animals. At present, such data has been obtained via external cable from awake, unrestrained rats28. In the future, this economical system may also be combined with remote recording-stimulation technology (e.g., telemetry) to activate or probe neuromuscular systems wirelessly.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Hongmei Wu for her contribution to animal care and data collection throughout the study. We thank Amy Nunnally, Jamie Adcock, and Phil Williams for their help with sterile surgeries. The expertise and dedication of the staff of the Vanderbilt University Animal Care Facility was invaluable. This research was supported by NIH grant U01DC016033.
Materials
| 20 G x 1" Gauge hypodermic needle | BD | 305175 | |
| 23 G x 1" Gauge hypodermic needle | BD | 305145 | |
| 25 G x 1" Gauge hypodermic needle | BD | 305125 | |
| 3-0 absorbable sutures, COATED VICRYL | Ethicon | J219H | |
| 3-0 monofilament, nonabsorbable sutures, Prolene | Ethicon | 8684G | |
| 4-0 monofilament, nonabsorbable sutures, Prolene | Ethicon | 8871H | |
| 6-0 monofilament, nonabsorbable taper needle suture, Prolene | Ethicon | 8805 | |
| 7-0 monofilament, nonabsorbable sutures, Prolene | Ethicon | M8735 | |
| Adhesive silicone solvent-Hexamethydisiloxane 98% | ACROS | code 194790100 | for dilution of modical adhesive silicone |
| Bone cement | Zimmer | 1102-16 | 20g powder 10ml liquid |
| Buprenorphine (Buprenex, ampules of 1ml) | Reckitt Benckiser Healthcare (UK) Ltd | 12496-0757-1 | |
| CCD video camera attached to the endoscope | Sony | MCC500MD | |
| Cefpodoxime (Simplicef 100mg tablets) | Zoetis | 5228 | |
| Data acquisition device , PowerLab 16/35 | ADInstruments, Inc | 5761-E | |
| Deep-brain stimulation (DBS) electrodes | Abbott | 6172ANS | |
| Digital oscilloscope | Tektronix | DPO71304SX | |
| Implantable pulse generator (IPG), Infinity | Abbott | 6660ANS | |
| Knitted polyester graft | Meadox Medical Inc | 92220 | 20mm in diameter |
| Medical Grade Polyethylene Micro Tubing | Amazon.com | BB31695-PE/13-10 | OD 0.156", ID 0.094" |
| Metal female pin | Allied Electronics & Automation | 220-S02-100 | |
| Metal male pin | CDM electronics | 220-p02-1 | |
| Prefabricated coiled leads | Medical innovations Inc. | ||
| Silastic Laboratory Tubing | Cole-Parmer | 2415569 | OD 0.125", ID 0.062" |
| Silastic Medical Adhesive Silicone | Dow corning | Type A, 2 oz | |
| Stainless steel monofilament wire | The Harris Products Group | type 316 | 0.008" (coated), 0.005" (bare) |
| Sterile Disposable Biopsy Punch (4mm) | Sklar Instruments | 96-1146 | |
| Strip connector | CDM electronics | 2.6 x 11.6 x 101.5 mm | single row, round, through hole |
| Teflon-coated multi-filament stainless steel wire | Medwire | Part 316, ss7/44T | |
| Tiletamine and Zolazepam combination, Telazol – 5mL | Zoetis | 004866 | |
| Tissue-compatible antiseptic solution, Nolvasan – 1 Gal. | Zoetis | 540561 | |
| Zero-degree rigid endoscope | Karl Storz | 8712AA |
Referências
- Electromyography. Wikipedia, The Free Encyclopedia Available from: https://en.wikipedia.org/wiki/Electromyography (2019)
- Zealear, D. L., et al. Stimulation of denervated muscle promotes selective reinnervation, prevents synkinesis, and restores function. The Laryngoscope. 124 (5), 180-187 (2014).
- Gaweł, M. Electrodiagnostics: MUNE and MUNIX as methods of estimating the number of motor units – biomarkers in lower motor neurone disease. Neurologia i neurochirurgia polska. 53 (4), 251-257 (2019).
- Foerster, G., Mueller, A. H. Laryngeal EMG: Preferential damage of the posterior cricoarytenoid muscle branches especially in iatrogenic recurrent laryngeal nerve lesions. Laryngoscope. 128 (5), 1152-1156 (2018).
- Lin, R. J., Smith, L. J., Munin, M. C., Sridharan, S., Rosen, C. A. Innervation status in chronic vocal fold paralysis and implications for laryngeal reinnervation. Laryngoscope. 128 (7), 1628-1633 (2018).
- Koh, T. J., Leonard, T. R. An implantable electrical interface for in vivo studies of the neuromuscular system. Journal of Neuroscience Methods. 70 (1), 27-32 (1996).
- Grimonprez, A., et al. A Preclinical Study of Laryngeal Motor-Evoked Potentials as a Marker Vagus Nerve Activation. International Journal of Neural Systems. 25 (8), 1550034 (2015).
- Haidar, Y. M., et al. Selective recurrent laryngeal nerve stimulation using a penetrating electrode array in the feline model. The Laryngoscope. 128 (7), 1606-1614 (2018).
- Kneisz, L., Unger, E., Lanmüller, H., Mayr, W. In Vitro Testing of an Implantable Wireless Telemetry System for Long-Term Electromyography Recordings in Large Animals. Artificial Organs. 39 (10), 897-902 (2015).
- Inzelberg, L., Rand, D., Steinberg, S., David-Pur, M., Hanein, Y. A Wearable High-Resolution Facial Electromyography for Long Term Recordings in Freely Behaving Humans. Scientific Reports. 8 (1), (2018).
- Horn, K. M., Pong, M., Batni, S. R., Levy, S. M., Gibson, A. R. Functional specialization within the cat red nucleus. Journal of Neurophysiology. 87 (1), 469-477 (2002).
- Larson, C. R., Kistler, M. K. The relationship of periaqueductal gray neurons to vocalization and laryngeal EMG in the behaving monkey. Experimental Brain Research. 63 (3), 596-606 (1986).
- Zealear, D., Larson, C. A Microelectrode Study of Laryngeal Motoneurons in the Nucleus Ambiguus of the Awake Vocalizing Monkey. Vocal Fold Physiology Volume. 2, 229-238 (1988).
- Zealear, D. L., Billante, C. R. Neurophysiology of vocal fold paralysis. Otolaryngologic Clinics of North America. 37 (1), 1-23 (2004).
- Zealear, D. L., et al. Electrical Stimulation of a Denervated Muscle Promotes Selective Reinnervation by Native Over Foreign Motoneurons. Journal of Neurophysiology. 87 (4), 2195-2199 (2002).
- Insalaco, G., Kuna, S. T., Cibella, F., Villeponteaux, R. D. Thyroarytenoid muscle activity during hypoxia, hypercapnia, and voluntary hyperventilation in humans. Journal of Applied Physiology. 69 (1), 268-273 (1990).
- Ludlow, C. L., Van Pelt, F., Koda, J. Characteristics of Late Responses to Superior Laryngeal Nerve Stimulation in Humans. Annals of Otology, Rhinology & Laryngology. 101 (2), 127-134 (1992).
- Li, Y., et al. Comparison of Ventilation and Voice Outcomes between Unilateral Laryngeal Pacing and Unilateral Cordotomy for the Treatment of Bilateral Vocal Fold Paralysis. ORL. 75 (2), 68-73 (2013).
- Mueller, A. H. Laryngeal pacing for bilateral vocal fold immobility. Current Opinion in Otolaryngology & Head and Neck Surgery. 19 (6), 439-443 (2011).
- Crumley, R. L. Laryngeal Synkinesis Revisited. Annals of Otology, Rhinology & Laryngology. 109 (4), 365-371 (2000).
- Hydman, J., Mattsson, P. Collateral reinnervation by the superior laryngeal nerve after recurrent laryngeal nerve injury. Muscle & Nerve. 38 (4), 1280-1289 (2008).
- Marie, J. P., Navarre, I., Lerosey, Y., Magnier, P., Dehesdin, D., Andrieu Guitrancourt, J. Bilateral laryngeal movement disorder and synkinesia: value of botulism toxin. Apropos of a case. Rev Laryngol Otol Rhinol (Bord). 119 (4), 261-264 (1998).
- Zealear, D. L., Billante, C. R., Sant’anna, G. D., Courey, M. S., Netterville, J. L. Electrically stimulated glottal opening combined with adductor muscle botox blockade restores both ventilation and voice in a patient with bilateral laryngeal paralysis. Annals of Otology, Rhinology and Laryngology. 111 (6), 500-506 (2002).
- Zealear, D. L., et al. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 113 (7), 1149-1156 (2003).
- Mueller, A. H., et al. Laryngeal pacing via an implantable stimulator for the rehabilitation of subjects suffering from bilateral vocal fold paralysis: A prospective first-in-human study. Laryngoscope. 126 (8), 1810-1816 (2016).
- Li, Y., Garrett, G., Zealear, D. Current Treatment Options for Bilateral Vocal Fold Paralysis: A State-of-the-Art Review. Clinical and Experimental Otorhinolaryngology. 10 (3), 203-212 (2017).
- Li, Y., Huang, S., Zealear, D. An implantable system for In Vivo chronic electromyographic study in the larynx. Muscle & Nerve. 55 (5), 706-714 (2017).
- Connor, N. P., et al. Tongue muscle plasticity following hypoglossal nerve stimulation in aged rats. Muscle & Nerve. 47 (2), 230-240 (2013).

