Determining the Functional Status of the Corticospinal Tract Within One Week of Stroke
Summary
This protocol is for evaluating corticospinal tract function within 1 week of stroke. It can be used to select and stratify patients in trials of interventions designed to improve upper limb motor recovery and outcomes and in clinical practice for predicting upper limb functional outcomes 3 months after stroke.
Abstract
High interindividual variability in the recovery of upper limb (UL) function after stroke means it is difficult to predict an individual's potential for recovery based on clinical assessments alone. The functional integrity of the corticospinal tract is an important prognostic biomarker for recovery of UL function, particularly for those with severe initial UL impairment. This article presents a protocol for evaluating corticospinal tract function within 1 week of stroke. This protocol can be used to select and stratify patients in trials of interventions designed to improve UL motor recovery and outcomes after stroke. The protocol also forms part of the PREP2 algorithm, which predicts UL function for individual patients 3 months poststroke. The algorithm sequentially combines a UL strength assessment, age, transcranial magnetic stimulation, and stroke severity, within a few days of the stroke. The benefits of using PREP2 in clinical practice are described elsewhere. This article focuses on the use of a UL strength assessment and transcranial magnetic stimulation to evaluate corticospinal tract function.
Introduction
Upper limb function is commonly impaired after stroke, and recovery of UL function is important for regaining independence in daily living activities1. Stroke rehabilitation trials are often aimed at improving UL recovery and outcomes after stroke. The majority of stroke rehabilitation research is conducted with patients at the chronic stage (>6 months poststroke), yet most rehabilitation occurs early after stroke2,3. More research needs to be conducted with patients soon after a stroke to build an evidence base for rehabilitation practice.
One of the greatest challenges when conducting research soon after the stroke is detecting the effects of the intervention against the background of recovery occurring during the initial weeks and months after the stroke. High intersubject variability in clinical presentation and recovery creates noise that can obscure the beneficial effects of interventions. Intervention and control groups are typically balanced on clinical measures of initial neurological impairment. However, these measures are often poor predictors of the patient's potential for subsequent recovery, particularly those with severe initial impairment4,5. This means that groups can be matched for baseline clinical measures and not matched for their recovery potential, which makes it more difficult to ascertain the intervention's effects. Biomarkers can address this challenge by identifying an individual patient's potential for motor recovery, so that groups can be accurately matched and stratified6,7,8. Biomarkers can also be used to select patients who are most likely to respond to the intervention's known or hypothesized mechanisms of action6.
The functional integrity of the corticospinal tract (CST) is a key biomarker that predicts recovery of UL function after stroke5,8,9,10,11,12. The CST conveys descending motor output from the primary motor cortex to the spinal cord and is essential for coordination and fine motor control. Patients with a functional CST after stroke are more likely to regain strength, coordination, and dexterity than patients without. A clinical assessment can be sufficient to confirm that the CST is functional in mildly impaired patients13,14,15. However, patients with more severe initial impairment may or may not have a functional CST, and a neurophysiological assessment using transcranial magnetic stimulation (TMS) is needed9,10,11,16,17.
TMS is a noninvasive and painless technique that can be used to test CST function18. The TMS coil delivers a magnetic stimulus over the primary motor cortex that generates a descending volley in the CST, eliciting a motor-evoked potential (MEP) in the muscles of the contralateral limb19. The presence of a MEP in the paretic arm or hand (MEP+) indicates a functional CST and is associated with greater potential for recovery of UL function. Patients who are MEP- are most likely to have worse UL recovery, with no return of coordinated and dexterous hand function4,6,9,12,16.
Testing all patients with TMS is impractical and unnecessary, as those with mild initial impairment most likely have a functional CST17. Therefore, a hierarchical approach is needed so that TMS is only used for patients with more severe initial impairment. The PREP2 algorithm was developed using a combination of clinical measures and TMS to evaluate CST function and predict likely UL outcome at 3 months poststroke (Figure 1)17. PREP2 starts at day 3 poststroke by testing the strength of shoulder abduction and finger extension in the paretic arm (SAFE score), using Medical Research Council grades. If the sum of these grades is 5 or more out of 10, it is "safe" to assume the patient is MEP+. These patients are expected to have a good or excellent UL outcome by 3 months poststroke, depending on their age17. These patients do not need TMS to determine MEP status, minimizing cost and unnecessary testing for the patient.
Patients with a SAFE score of less than 5 on day 3 poststroke require TMS to determine the functional integrity of their CST. If a MEP can be elicited from the paretic extensor carpi radialis (ECR) or first dorsal interosseus (FDI) muscles, the patient is MEP+ and is expected to recover fine motor control of the hand by 3 months poststroke. Approximately half of patients with a SAFE score less than 5 on day 3 poststroke are MEP+. Importantly, patients can have a SAFE score as low as zero and be MEP+. This illustrates the need for TMS in this subgroup of patients, as clinical assessment alone cannot distinguish between patients with and without a functional CST. Patients who are MEP- have significant CST damage. These patients are expected to have a limited or poor UL functional outcome depending on their overall stroke severity, measured with the National Institute of Health Stroke Scale (NIHSS) (Figure 1)17. These MEP- patients are not expected to regain coordinated and dexterous finger control and can be grouped together for research purposes.
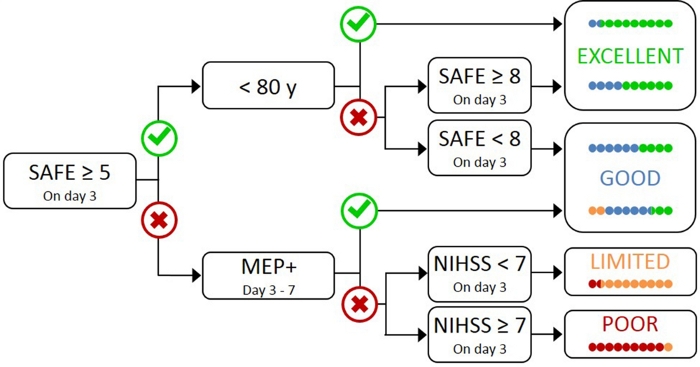
Figure 1: The PREP2 algorithm. SAFE = Shoulder Abduction, Finger Extension score, which is the sum of the Medical Research Council grades for each of these movements out of 5, for a total SAFE score out of 10. MEP+ = Motor Evoked Potentials can be elicited from the paretic extensor carpi radialis (ECR) and/or first dorsal interosseous (FDI) muscles of the paretic UL using transcranial magnetic stimulation. NIHSS = National Institutes of Health Stroke Scale. The algorithm predicts one of four possible UL functional outcomes at 3 months poststroke. Each prediction category is associated with a rehabilitation focus that can be used to tailor UL therapy2. The colored dots represent, proportionally, PREP2 algorithm accuracy. The dots are color-coded based on the outcome category actually achieved 3 months poststroke (Green = Excellent; Blue = Good; Orange = Limited; Red = Poor). Figure reproduced from Stinear et al.17. Please click here to view a larger version of this figure.
In clinical practice, PREP2 predicts one of four outcome categories that can be used to tailor rehabilitation for individual patients and help patients and families to understand what they can expect for their UL recovery. To date, PREP2 is the only externally validated UL prediction tool that combines clinical assessment and biomarker information in a decision tree17. It is also the only UL prediction tool with research on the effects of implementation in clinical practice20,21. PREP2 predictions are accurate for about 75% of patients, too optimistic for 17% and too pessimistic for 8% of patients at 3 months poststroke17. Accuracy is highest for MEP- patients (accurate for 90% of MEP- patients), highlighting the value of using TMS to identify these patients with severe damage to the descending motor pathways17. PREP2 remains correct for around 80% of patients at 2 years poststroke22. This supports the use of PREP2 to predict UL functional motor outcomes at 3 months and longer term. Information about delivering PREP2 predictions and using them in clinical practice is outside the scope of this methods paper, but detailed resources are available online23.
PREP2 provides researchers with a tool to select and stratify patients for clinical trials. This allows patients to be grouped not only according to baseline clinical characteristics, but also their neurobiological potential for UL recovery. Despite the mounting evidence for the use of TMS as a prognostic biomarker for UL recovery, lack of familiarity with TMS protocols in hospital settings with subacute stroke patients may be a barrier to its use in research. Therefore, this protocol aims to demonstrate how to use the SAFE score and TMS to evaluate CST function in patients in a hospital setting early after stroke.
Protocol
All research conducted with human participants must have human ethics approval by the appropriate institutional ethics committee and the study must be conducted in accordance with the declaration of Helsinki.
1. Patient Screening
- Screen all patients for PREP2 suitability within 72 h of stroke onset.
NOTE: Patients are suitable if they have had a unilateral ischemic or hemorrhagic stroke within the last 72 h, have new UL weakness, and are 18 years or older.
2. SAFE Score
NOTE: Ensure that patients with inattention or fatigue are focused on the arm to enable an accurate assessment of strength.
- Position the patient with their back fully supported and upright either in bed or in a chair and their paretic arm by their side with the elbow in extension.
- Demonstrate shoulder abduction. Ask the patient to lift their arm sideways and up towards their ear. Use the Medical Research Council (MRC) grades to score shoulder abduction strength.
NOTE: MRC grades are described as follows: 0 = no palpable muscle activity; 1 = palpable muscle activity but no movement; 2 = limited range of movement without gravity; 3 = full range of motion against gravity but no resistance; 4 = full range of motion against gravity and resistance but weaker than the other side; 5 = normal power. - To score shoulder abduction grades 4 or 5, place your hand over the patient's arm, proximal to the elbow and apply resistance.
NOTE: The patient must be able to achieve full range of motion against resistance to be awarded a score of 4 or higher. - Place the paretic forearm in pronation with the fingers fully flexed and provide support under the wrist.
- Demonstrate finger extension. Ask the patient to straighten their fingers and use the Medical Research Council grades to score finger extension strength.
- To score finger extension grades 4 or 5, apply resistance over the dorsum of the fingers, distal to the metacarpophalangeal joints, throughout the movement.
NOTE: The patient must be able to achieve full extension against resistance to be awarded a score of 4 or higher. - To score fingers with unequal strength, use the majority rule. If three fingers have the same score, use this score. If two fingers have a lower score than the other two fingers, use the lower score.
- Add the MRC grades for shoulder abduction and finger extension together for a SAFE score out of 10. If the patient has a SAFE score of 5 or more on day 3 poststroke they can be assumed to have a functional corticospinal tract and TMS is not required. If the patient has a SAFE score less than 5 on day 3 poststroke, TMS is required to determine their MEP status.
3. Transcranial Magnetic Stimulation (TMS)
- Evaluate patient suitability for TMS.
- Complete a TMS safety checklist with the patient to identify absolute and relative contraindications to TMS24.
NOTE: This information should be gathered through patient and family interview and from the medical records. See representative results section for more detail. - Ask the patient's physician to review the TMS checklist and approve it if appropriate.
- On the day of the TMS test, review the patient's medical status with the clinical team and the patient to ensure there have been no changes since the checklist was signed.
NOTE: Events to consider include fall with head injury, seizure, if the patient has become medically unwell, is hypoglycemic, or has unstable blood pressure. Ensure the patient has taken all prescribed medication prior to the test.
- Complete a TMS safety checklist with the patient to identify absolute and relative contraindications to TMS24.
- Prepare the environment.
- Remove furniture from around the bed. Move the bed away from the wall to allow space for the TMS unit.
- Place the TMS unit at the head of the bed towards the side opposite the paretic limb. Angle the TMS unit so that the person delivering TMS can easily see the screen.
- Test the TMS setup to check it is working appropriately.
NOTE: This protocol uses a single-pulse TMS unit. The electromyography (EMG) signal can be sampled at 2 kHz and filtered with 10 Hz high-pass and 1,000 Hz low-pass filters. The EMG equipment needs to be triggered by the TMS unit such that the EMG trace begins at least 50 ms prior to the TMS stimulus and ends at least 50 ms after the TMS stimulus. - Ensure familiarity with the protocol for summoning emergency assistance in the TMS assessment room in case this is required.
- Prepare the patient.
NOTE: Patients with intravenous (IV) lines, nasogastric feeding, or low concentrations of supplementary oxygen via a nasal cannula can be tested with TMS provided that they are considered medically stable by the treating physician. Supplemental oxygen should continue throughout the TMS session. Suspending and disconnecting nasogastric feeding and non-essential fluids through IV lines will make it easier to conduct the TMS assessment.- Remove any clothing covering the forearms. Remove any items covering the paretic wrist such as a watch or identification bracelet to enable the EMG electrode placement.
- Position the paretic arm on a pillow with the forearm pronated and fully supported from the elbow to the hand.
- Palpate the paretic forearm to locate the muscle belly for the extensor carpi radialis (ECR) muscle. Identify positions for two surface EMG electrodes over the muscle belly, allowing for factors such as the position of IV cannula or dressings.
NOTE: It is essential that at least one electrode is positioned over the muscle belly. This may require discussion with the nursing staff about repositioning dressings before the test if possible. - Clean the skin at each electrode site with an alcohol skin-cleansing wipe. Shave each electrode site to remove any hair. Lightly abrade the electrode sites with an abrasive cream or tape. Take care with patients who have fragile skin and avoid any areas of broken skin.
- Securely apply self-adhesive disposable recording electrodes to each site.
- Locate the electrode sites for the first dorsal interosseus muscle (FDI). One electrode will be placed on the FDI muscle belly and one on the dorsum of the hand.
NOTE: Electrode placement may vary depending on patient factors such as the position of an IV cannula or dressings. - Prepare the skin and apply the self-adhesive recording electrodes as previously described.
- Place the reference electrode strap around the arm just proximal to the elbow. Alternatively, prepare the skin and place a self-adhesive reference electrode over the lateral epicondyle of the humerus.
- Position the patient in bed for the test.
- Lower the bed rails. Move the patient as high up the bed as possible and towards the edge of the bed on the non-paretic side.
NOTE: The patient should only be moved by trained staff. - Put the bed rail back up on the paretic side for safety. Remove the headboard of the bed if possible and remove any unused IV poles attached to the bed that may obstruct the coil position.
- Raise the head of the bed as high as possible. Position pillows behind the patient's back to bring them into an upright sitting position without their head contacting the bed. Do not put a pillow behind the head. If possible, raise the knees to prevent the patient from slipping down the bed during testing.
- Ensure the paretic forearm is in pronation and fully supported by a pillow from the elbow to the wrist. Check for adequate TMS coil access by holding the TMS coil against the patient's head. Make adjustments to the patient's position as required.
- Lower the bed rails. Move the patient as high up the bed as possible and towards the edge of the bed on the non-paretic side.
- Position the patient in a chair or wheelchair for the test (alternative option).
- Ensure the patient is sitting upright and comfortably in the chair. Place a pillow under each arm. Ensure the paretic forearm is pronated and fully supported by the pillow.
- Check the EMG trace: Connect the cables between the patient and the EMG unit. Check to make sure the EMG signal is free from any electrical noise.
- Deliver TMS.
- Two trained staff should be present for the TMS test. Instruct the patient to look straight ahead, keeping their head still and eyes open.
- The person holding the coil should stand next to the patient's head on their non-paretic side and position the center of the coil over the location of the primary motor cortex of the stroke-affected hemisphere. This is approximately 4 cm lateral from the vertex on the interaural line.
NOTE: Another way to identify the starting coil position is to measure approximately a third of the distance from the vertex to the front of the ear. - Orient the coil with the handle pointing backwards, at an approximately 45° angle in the midsagittal plane to produce a posterior-to-anterior current in the underlying tissue.
NOTE: The coil used in this protocol is a flat figure-eight coil, but a branding coil or circular coil can also be used. - Adjust the bed height for the coil holder's comfort. Use a step if necessary. The second person (not the coil holder) is responsible for monitoring patient comfort throughout the TMS session. They may stand at the foot of the bed to monitor the patient and ensure the patient maintains a neutral head position or monitor the patient from the bedside while adjusting the TMS unit controls as necessary.
NOTE: This will depend on the individual TMS setup used. The patient should be monitored for comfort levels, alertness, and any adverse effects such as vasovagal responses to the TMS. - Begin with a stimulus intensity of 30% maximum stimulator output (MSO). Increase intensity in 10% MSO steps with three to five stimuli at each intensity and scalp location.
- Move the coil systematically in 1 cm steps in each direction (anterior, posterior, medial, lateral) to find the optimal location for producing MEPs in the recorded muscles. Small adjustments to the coil rotation may also be necessary.
- Continue increasing the stimulus intensity and moving the coil until the MEPs are consistently observed in one or both muscles or until 100% MSO is reached.
- If 100% MSO is reached with no MEPs observed, use active facilitation to increase corticomotor excitability and the likelihood of eliciting a MEP. Ask the patient to hug a pillow to their chest with both arms, attempting to activate their paretic UL as much as possible. For patients with no distal UL activity, ask them to elevate and retract at the shoulder girdle.
- Classify the MEP status of the patient.
- Classify the patient as MEP+ if MEPs of any amplitude are observed with a consistent latency in response to at least five stimuli. This can be either at rest or during voluntary facilitation. FDI latencies are typically 20–30 ms while ECR latencies are typically 15–25 ms. MEPs do not have to exceed a peak-to-peak amplitude of 50 µV.
- Classify the patient as MEP- if a MEP cannot be elicited at 100% MSO either at rest or while attempting voluntary facilitation.
- Remove electrodes and wipe the skin with an alcohol wipe. The skin may be slightly red but this usually resolves without any treatment.
Representative Results
The SAFE score and TMS can be used to ascertain the functional status of the CST within one week of stroke. Patients who have a SAFE score of at least 5 on day 3, or are MEP+ when tested with TMS, have a functional CST and are expected to regain at least some coordination and dexterity. Patients who are MEP- do not have a functional CST and therefore are likely to be limited to improvements in proximal arm movements and gross movements of the hand. The functional status of the CST can therefore be used to select patients for trials based on their capacity to recover dexterous hand function.
The PREP2 algorithm predicts UL functional outcomes by obtaining the SAFE score and MEP status using this protocol. The PREP2 algorithm has been developed and validated in patients aged 18 years or older, with ischemic or hemorrhagic stroke and new UL weakness, as described in detail elsewhere16,17,20. An important component of the PREP2 algorithm is determining MEP status with TMS for patients with a SAFE score less than 5. Patients must be assessed for suitability for the procedure. This includes completing a safety checklist that is subsequently reviewed and approved by the treating physician. The purpose of the checklist is to identify any contraindications or precautions for using TMS such as the presence of a cardiac pacemaker, seizures, brain surgery, and head injuries. Contraindications and precautions for TMS are well established and previously described in detail24.
A patient is considered to be MEP+ if a MEP is consistently present at an appropriate latency (20–30 ms for FDI, 15–25 ms for ECR) and with any peak-to-peak amplitude. The patient is MEP+ whether a MEP is elicited at rest or while attempting voluntary UL facilitation. The MEP only needs to be present in one muscle for the patient to be considered MEP+. This protocol differs from other protocols that may require a MEP to exceed 50 µV in peak-to-peak amplitude for at least 5 out of 10 traces. These other protocols are designed to establish the patient's rest motor threshold as a basis for further neurophysiological assessment. For prediction of UL recovery, the simple presence or absence of a MEP is a stronger predictor than MEP amplitude and identifying the rest motor threshold is not required8,9,16,25.
Figure 2, Figure 3, and Figure 4 provide examples of EMG recordings from patients tested with TMS within 1 week of stroke.
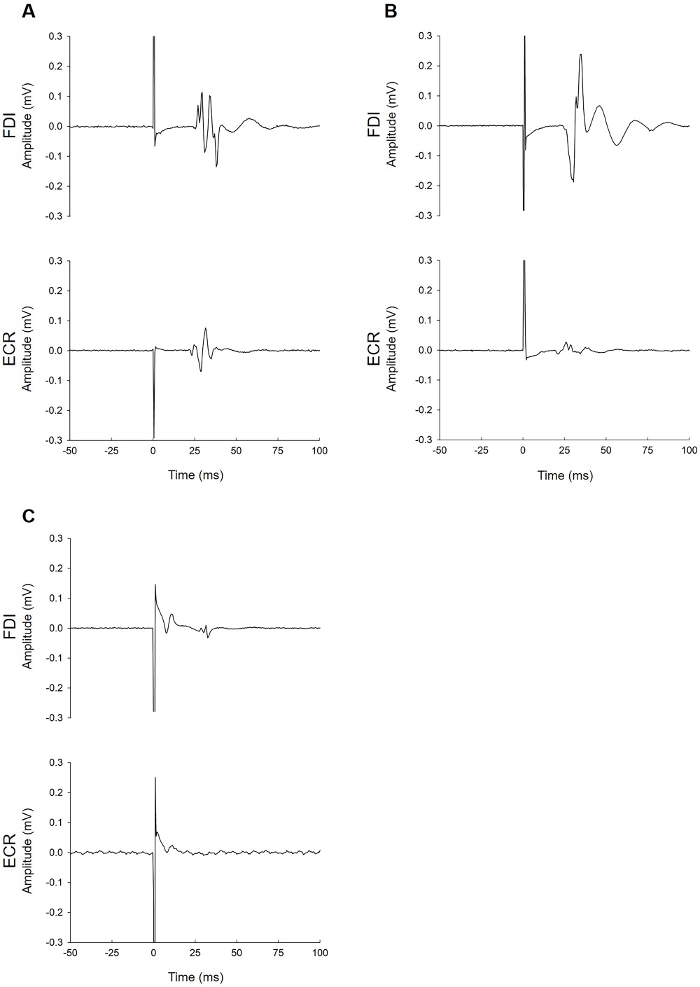
Figure 2: Examples of MEP+ patients. (A) This patient had MEPs in the paretic FDI (top trace) and ECR muscles (bottom trace). The FDI MEP latency (25 ms) was slightly longer than ECR (21 ms), as expected. (B) This patient had MEPs in the FDI and ECR muscles. The ECR MEP amplitude was small (40 µV) but occurred at an appropriate latency. While this patient clearly had a large FDI MEP, they would be considered MEP+ based on the ECR trace alone. (C) This patient had a small MEP in the FDI muscle (40 µV) and no MEP in the ECR muscle. The MEP occurred at an appropriate latency (27.5 ms). This patient can be considered MEP+ because the MEP was observed on at least five traces (see step 3.8.1 in protocol). Please click here to view a larger version of this figure.
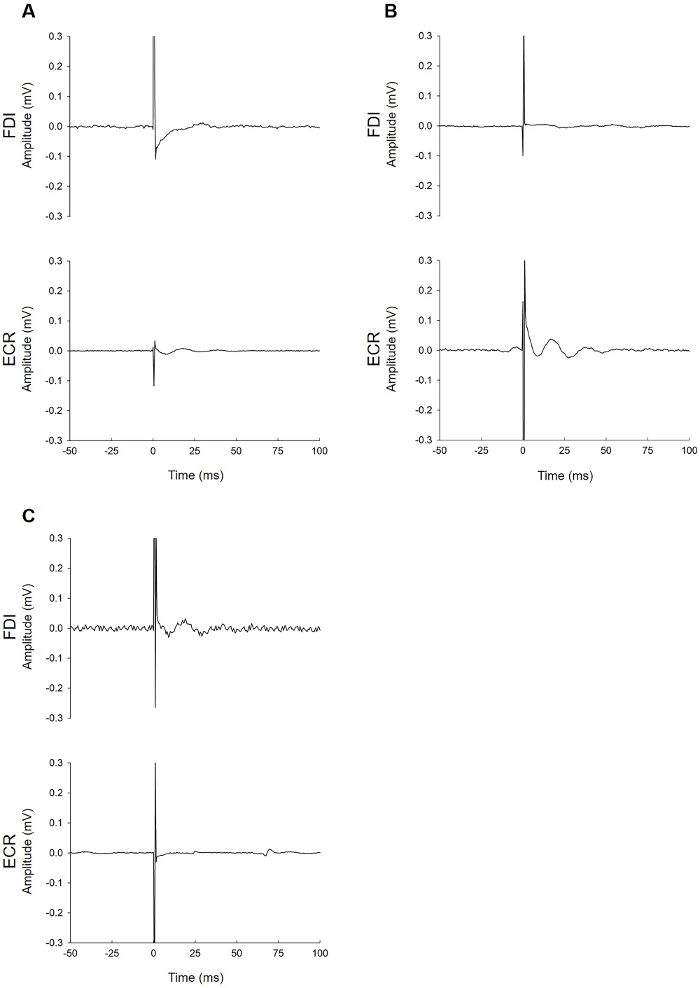
Figure 3: Examples of MEP-patients. These patients failed to demonstrate MEPs at 100% MSO while at rest and attempting active bilateral facilitation to increase the likelihood of eliciting a MEP. The EMG traces do not show muscle activity during facilitation due to severe paresis. (A) This patient had no MEP of any amplitude in either muscle despite all efforts to elicit one. (B) This patient had no MEP in the FDI muscle (top trace). The bottom trace (ECR) contains an elongated tail of the stimulus artifact. When this is present during the latency window for either muscle, identification of a MEP can be difficult. See Figure 4 for advice on troubleshooting EMG noise issues. If the problem cannot be solved, the result of the FDI trace is used, which in this case is MEP-. (C) The fluctuation seen in the ECR muscle EMG trace is not a MEP. This is a motor unit firing sporadically. These can be identified due to their uniform shape and appearance at latencies that do not correspond with the expected latency for ECR. Please click here to view a larger version of this figure.
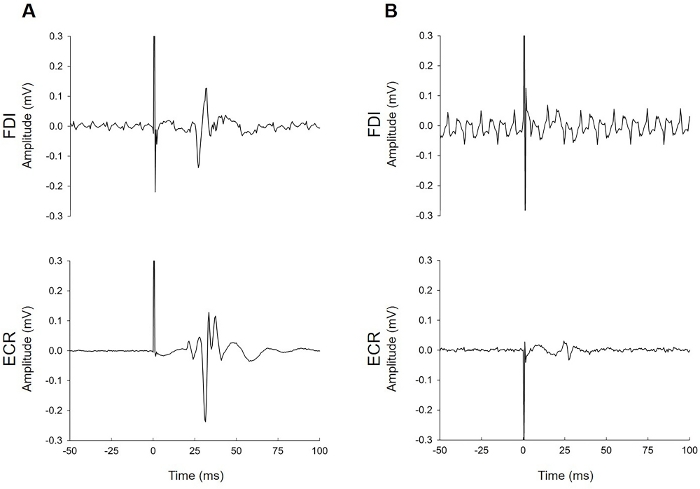
Figure 4: Examples of EMG traces contaminated by electrical noise. (A) This patient had MEPs in both muscles that are clearly identifiable despite the electrical noise in the FDI trace. (B) This patient had MEPs only in the ECR. Noisy signals can be a common problem during EMG recording. The researcher needs to consider whether the noise is environmental (due to issues with electrical noise in the room, or in the EMG setup) or biological (underlying muscle activity from the patient). Troubleshooting suggestions include but are not limited to checking whether skin preparation was adequate, the electrode has lost contact with the skin (this is particularly common with FDI if a patient has perspiration on their hands), issues with the grounding strap or electrode, cables are firmly attached to the patient and the EMG unit, anyone is touching the patient or the TMS trolley during the test, unplugging the bed from its electrical power supply, adjusting lighting (turning off fluorescent lighting), and adjusting the patient position so that they are able to relax with their ULs fully supported on pillows. In both of these traces, the background noise was only present in one muscle. This suggests that noise issues were specific to the setup for that muscle (e.g., a loose cable, poor electrode conduction due to a lack of contact with the skin, or a faulty electrode). Noise that is present in the traces for both muscles indicates issues with the grounding electrode or strap or electrical noise in the environment. Please click here to view a larger version of this figure.
Discussion
CST function evaluated with MEP status is a key prognostic biomarker for UL recovery and outcome after stroke. A total of 95% of patients with a functional CST at 1 week poststroke achieve an Action Research Arm Test (ARAT) score of at least 34 out of 57 by 3 months poststroke17. Conversely, 100% of patients without a functional CST at 1 week poststroke achieve an ARAT score of less than 34 by 3 months poststroke17. Evaluating CST function within a week poststroke may improve patient selection and stratification in trials aimed at improving UL recovery and outcomes after stroke.
The first consideration for the TMS assessment is patient safety. The TMS safety checklist should be reviewed and signed by a physician prior to the TMS assessment. The checklist also needs to be reviewed with the patient on the day of the TMS assessment, to confirm that there have been no changes to their checklist answers. It may be more appropriate to conduct the TMS test in a separate procedure room rather than in the patient's bed-space. In this situation, the skin preparation and electrode placement for surface EMG can take place within the patient's bed-space before transporting to the procedure room for TMS testing. Preparing the patient at the bed-space minimizes the time for the procedure, which may be more tolerable for some patients. If the patient is transported to a procedure room for testing, it is important to ensure all supplemental medical devices (e.g., oxygen therapy, IV lines, catheter, inflatable mattress) are functioning appropriately during and after transportation.
Patient positioning is also an important consideration. A patient who is very fatigued poststroke is likely to be more comfortable if tested in their bed rather than a chair. Testing a patient in the bed can be more challenging, but with careful patient positioning it is possible to position the TMS coil appropriately over the UL representation of the motor cortex with the correct coil orientation. Testing the patient in a chair provides easier access to the head with the TMS coil but may provides greater challenges with patient transfers.
The TMS setup described in this protocol may vary based on the TMS equipment available and patient factors. A flat figure-eight coil was used here but could be substituted with a figure-eight branding coil or circular coil. Similarly, electrode placement may vary depending on the length of the electrode leads, or issues with placement due to skin lesions, IV cannula, and dressings. Typical FDI placement involves one electrode over the FDI muscle belly and one over the lateral aspect of the second metacarpophalangeal joint. This protocol describes a belly-tendon montage for FDI electrode positioning, with the second electrode placed on the dorsum of the hand. Placing the second electrode over the dorsum of the hand is helpful if the patient is perspiring or the electrodes themselves are too large to fit in the standard configuration.
It is essential to complete the TMS assessment accurately, particularly when determining that a patient is MEP-. All efforts need to be made to elicit a MEP if possible, including providing stimulus at up to 100% MSO, ensuring the patient is awake with eyes open during the test, and facilitation of muscle activation in one or both arms. The technique described in this protocol does not use neuronavigation to identify the hotspot for the TMS coil. This removes the need for a magnetic resonance imaging (MRI) scan and reduces the length of the session. However, this also means that movement of the coil while searching for the optimal stimulation location must be systematic and thorough to ensure all efforts have been made to elicit a MEP.
TMS is only required for patients with a SAFE score less than 5. This means TMS is only needed for approximately a third of patients, which reduces cost and improves accessibility. If TMS is unavailable, the accuracy of predictions for patients with a SAFE score less than 5 drops to 55%, even when MRI biomarkers are available17. Research and clinical sites without access to TMS can still complete the first half of the PREP2 algorithm for patients who have a SAFE score of 5 or more. However, this would limit the selection of patients for research trials to those who have mild to moderate UL weakness.
The SAFE score and TMS are useful in clinical practice and provide researchers with a principled method to select and stratify patients for clinical trials based on CST function and the patient's neurobiological capacity for UL recovery.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank Professor Winston Byblow and Harry Jordan for their valuable contribution to this work. This work was funded by the Health Research Council of New Zealand.
Materials
| alcohol/skin cleansing wipes | Reynard | alcohol prep pads | |
| electromyography electrodes | 3M | red dot electrodes | |
| Magstim TMS coil | Magstim | flat figure-8 coil | |
| razors | any | ||
| skin prep tape | 3M | red dot skin prep tape | |
| TMS stimulator | Magstim | Magstim 200 single pulse stimulator |
Referências
- Veerbeek, J. M., Kwakkel, G., van Wegen, E. E., Ket, J. C., Heymans, M. W. Early prediction of outcome of activities of daily living after stroke: a systematic review. Stroke. 42 (5), 1482-1488 (2011).
- Lohse, K. R., Schaefer, S. Y., Raikes, A. C., Boyd, L. A., Lang, C. E. Asking New Questions with Old Data: The Centralized Open-Access Rehabilitation Database for Stroke. Frontiers in Neurology. 7, 153 (2016).
- Stinear, C., Ackerley, S., Byblow, W. Rehabilitation is initiated early after stroke, but most motor rehabilitation trials are not: a systematic review. Stroke. 44 (7), 2039-2045 (2013).
- Stinear, C. M. Prediction of recovery of motor function after stroke. Lancet Neurology. 9 (12), 1228-1232 (2010).
- Byblow, W. D., Stinear, C. M., Barber, P. A., Petoe, M. A., Ackerley, S. J. Proportional recovery after stroke depends on corticomotor integrity. Annals of Neurology. 78 (6), 848-859 (2015).
- Stinear, C. M. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurology. 16 (10), 826-836 (2017).
- Kim, B., Winstein, C. Can Neurological Biomarkers of Brain Impairment Be Used to Predict Poststroke Motor Recovery? A Systematic Review. Neurorehabilitation and Neural Repair. 31 (1), 3-24 (2016).
- Boyd, L. A., et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. International Journal of Stroke. 12 (5), 480-493 (2017).
- Escudero, J. V., Sancho, J., Bautista, D., Escudero, M., Lopez-Trigo, J. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke. 29 (9), 1854-1859 (1998).
- Pennisi, G., et al. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 30 (12), 2666-2670 (1999).
- Rapisarda, G., Bastings, E., de Noordhout, A. M., Pennisi, G., Delwaide, P. J. Can motor recovery in stroke patients be predicted by early transcranial magnetic stimulation?. Stroke. 27 (12), 2191-2196 (1996).
- Bembenek, J. P., Kurczych, K., Karli Nski, M., Czlonkowska, A. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke – a systematic review of the literature. Functional Neurology. 27 (2), 79-84 (2012).
- Smania, N., et al. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke. 38 (3), 1088-1090 (2007).
- Nijland, R. H., van Wegen, E. E., Harmeling-van der Wel, B. C., Kwakkel, G. EPOS Investigators. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke. 41 (4), 745-750 (2010).
- Katrak, P., et al. Predicting upper limb recovery after stroke: the place of early shoulder and hand movement. Archives of Physical Medicine and Rehabilitation. 79 (7), 758-761 (1998).
- Stinear, C. M., Barber, P. A., Petoe, M., Anwar, S., Byblow, W. D. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain. 135 (Pt 8), 2527-2535 (2012).
- Stinear, C. M., et al. PREP2: A biomarker-based algorithm for predicting upper limb function after stroke. Annals of Clinical and Translational Neurology. 4 (11), 811-820 (2017).
- Groppa, S., et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clinical Neurophysiology. 123 (5), 858-882 (2012).
- Barker, A. T., Jalinous, R., Freeston, I. L. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1 (8437), 1106-1107 (1985).
- Stinear, C. M., Byblow, W. D., Ackerley, S. J., Barber, P. A., Smith, M. C. Predicting Recovery Potential for Individual Stroke Patients Increases Rehabilitation Efficiency. Stroke. 48 (4), 1011-1019 (2017).
- Connell, L. A., Smith, M. C., Byblow, W. D., Stinear, C. M. Implementing biomarkers to predict motor recovery after stroke. NeuroRehabilitation. 43 (1), 41-50 (2018).
- Smith, M. C., Ackerley, S. J., Barber, P. A., Byblow, W. D., Stinear, C. M. PREP2 Algorithm Predictions Are Correct at 2 Years Poststroke for Most Patients. Neurorehabilitation and Neural Repair. 33 (8), 635-642 (2019).
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 120 (12), 2008-2039 (2009).
- Talelli, P., Greenwood, R. J., Rothwell, J. C. Arm function after stroke: neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clinical Neurophysiology. 117 (8), 1641-1659 (2006).

