Analysis of Non-Human Primate Pancreatic Islet Oxygen Consumption
Summary
This protocol demonstrates the accurate and reproducible measurement of oxygen consumption in non-human primate pancreatic islets. The islet loading techniques and coating of the microplate provide a framework for efficient measurement of respiration in other types of cultured spheroids.
Abstract
The measurement of oxygen consumption in spheroid clusters of cells, such as ex vivo pancreatic islets, has historically been challenging. We demonstrate the measurement of islet oxygen consumption using a 96-well microplate designed for the measurement of oxygen consumption in spheroids. In this assay, spheroid microplates are coated with a cell and tissue adhesive on the day prior to the assay. We utilize a small volume of adhesive solution to encourage islet adherence to only the bottom of the well. On the day of the assay, 15 islets are loaded directly into the base of each well using a technique that ensures optimal positioning of islets and accurate measurement of oxygen consumption. Various aspects of mitochondrial respiration are probed pharmacologically in non-human primate islets, including ATP-dependent respiration, maximal respiration, and proton leak. This method allows for consistent, reproducible results using only a small number of islets per well. It can theoretically be applied to any cultured spheroids of similar size.
Introduction
In order to maintain normal blood glucose levels, the pancreatic β cell must sense elevations in glucose and secrete insulin accordingly. The coupling of insulin secretion with glucose levels is directly linked to glucose metabolism and the production of ATP through mitochondrial oxidative phosphorylation. Thus, mitochondria play a critical role in stimulus-secretion coupling1. Assessing β-cell mitochondrial function can reveal defects that lead to impaired insulin secretion. The secretion of glucagon by pancreatic α cells is also closely tied to mitochondrial function2. Although immortalized islet cell lines have proven useful for some types of assays, the physiology of these cells does not accurately recapitulate whole islet function, as illustrated by the potentiation of insulin secretion by glucagon3,4 and the inhibition of glucagon secretion by insulin/somatostatin5,6 in intact islets. This demonstrates the need for measuring oxygen consumption using whole, intact islets.
Techniques for the measurement of islet cell respirometry have evolved over time, from the use of oxygen-sensitive fluorescent dyes7 to solid-state sensors that directly measure oxygen consumption8. Initially designed for monolayer, adherent cells, commonly used cell culture plate systems have proven to be ineffective for pancreatic islets. As islets do not naturally adhere to the wells, they are prone to being pushed to the periphery of the culture well resulting in inaccurate measurement of oxygen consumption9. To combat this problem, specialized 24-well plates with a central depression that could contain islets were developed9. However, the 24-well plate system was limited by the large number of islets required (50-80 per well) and the number of conditions that could be tested simultaneously10. The recent development of 96-well microplates designed specifically for extracellular flux analysis in spheroids has overcome these barriers, enabling the measurement of islet respirometry with 20 or fewer islets per well10.
Here, we demonstrate the use of this system to measure oxygen consumption in islets from the Japanese macaque (Macaca fuscata), an animal model with similar islet biology to humans11,12. In this protocol, 15 macaque islets are analyzed per well. In our hands, 15 islets per well produced higher baseline oxygen consumption than fewer islets, with robust activation and repression of respiration in response to pharmacologic manipulation. We highlight the steps to prepare for the assay, an effective method for consistent loading of islets at the center of each well, and common challenges when performing this assay.
Protocol
1. Preparation of Microplate and Sensor Cartridge on the Day Prior to Running the Assay
Islets were isolated from three year old Japanese macaques as previously described13. This method is very similar to that used to isolate human islets from cadaver donors, but differs from mice, in which pancreata are often inflated with collagenase solution while the animal is under sedation and prior to organ removal. Islet retrieval was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Oregon National Primate Research Center (ONPRC) and Oregon Health and Science University and were approved by the ONPRC IACUC. The ONPRC abides by the Animal Welfare Act and Regulations enforced by the United States Department of Agriculture (USDA) and the Public Health Service Policy on Humane Care and Use of Laboratory Animals in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
- Preparation of Spheroid Microplate
- Prepare 3 mL of a 0.1M solution of sodium bicarbonate. Filter sterilize the solution. We utilized a 0.45 µm filter (Table of Materials).
- In a cell culture hood, add 200 µL of cell and tissue adhesive (Table of Materials) to 2.8 mL of 0.1M sodium bicarbonate. Then, add 20 µL of this solution to the bottom of each well of the 96-well spheroid microplate (Table of Materials). Ensure that air bubbles are removed from the pipet tip and only the bottom of the plate is covered. Coating of the microplate prevents islets from moving up the sides of the wells when drugs are added and mixed into the well during the assay.
NOTE: It is only necessary to coat as many wells as will be used for islets when the assay is run. If only a few wells will be used, the cell adhesive solution can be scaled down appropriately. The cell adhesive used in the assay is a formulated protein solution extracted from the marine mussel. Alternatively, microplate wells can be coated with 20 µL of 100 µg/mL poly-D-lysine. - Incubate the plate in a 37 °C, non-CO2 incubator for one hour.
- In a sterile environment, aspirate cell adhesive solution from the plate. Wash each well two times with 400 µL of 37 °C sterile water using a multichannel pipet. Allow to air dry.
- After 30-40 minutes, cover the plate and store overnight at 4 °C.
- Hydration of the Sensor Cartridge
- In a sterile environment, open the sensor cartridge package (Table of Materials) and remove the contents. Place the sensor cartridge upside down next to the utility plate.
- Fill each well of the utility plate with 200 µL sterile water and lower the sensor cartridge back into the utility plate. Place assembled sensor cartridge and utility plate in a 37 °C, non-CO2 incubator overnight.
- Preparation of Calibrant
- Aliquot 25 mL of calibrant (Table of Materials) into a 50 mL conical tube. Place tube in a 37 °C, non-CO2 incubator overnight.
2. Protocol for Media Preparation, Loading of Islets, and Loading of Sensor Cartridge on the Day of Assay
- Preparation of media
- To 48.5 mL of base media (minimal DMEM, see Table of Materials), add 500 µL each of 1 mM sodium pyruvate and 2 mM glutamine. Additionally, add 496 µL of 200 mg/ml glucose (final concentration of glucose in the media is 5.5 mM).
NOTE: Baseline glucose concentration was kept constant for these experiments. However, glucose can also be used to stimulate respiration in addition to the pharmacologic manipulations described blow10. - Confirm that the pH is 7.4 +/- 0.1, adjusting if necessary.
- To 48.5 mL of base media (minimal DMEM, see Table of Materials), add 500 µL each of 1 mM sodium pyruvate and 2 mM glutamine. Additionally, add 496 µL of 200 mg/ml glucose (final concentration of glucose in the media is 5.5 mM).
- Preparation of sensor cartridge
- Take sensor cartridge out of incubator, remove sensor cartridge lid, and dump out water from wells. Place 200 mL of pre-warmed calibrant into each well and replace sensor cartridge lid.
- Place sensor cartridge back in incubator for about 1 hour (until needed).
- Preparation of drugs for mitochondrial stress assay
- Open the stress test kit (Table of Materials) and remove the contents. Make up stock and working solutions for Oligomycin, Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), and Rotenone/Antimycin A (AA) as follows.
- Oligomycin: add 630 µL of assembled media to tube to make 100 µM stock. Then, dilute to 45 µM with media to obtain port concentration (final well concentration after injection will be 4.5 µM)
- FCCP: add 720 µL of assembled media to tube to make 100 µM stock. Dilute to 10 µM with media to obtain port concentration (final well concentration will be 1 µM)
NOTE: BSA and/or FBS should not be added to media, as this can affect the action of mitochondrial uncouplers14. - Rotenone/AA: add 540 µL of assembled media to tube to make 50 µM stock. Dilute to 25 µM with media to obtain port concentration (final well concentration will be 2.5 µM)
NOTE: The above concentrations have produced consistent results in our hands. Additional FCCP led to no further increase in respiration. However, drug concentrations may need to be adjusted depending on islet size, and especially with islets from different species or non-islet spheroids. For reference, the average diameter of a non-human primate islet is about 150 µm15.
- Open the stress test kit (Table of Materials) and remove the contents. Make up stock and working solutions for Oligomycin, Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), and Rotenone/Antimycin A (AA) as follows.
- Preparation of spheroid plate and transfer of islets
- Hand-pick pancreatic islets into a 60 mm x 15 mm cell culture dish containing assembled media to obtain near 100% purity.
NOTE: Islets should appear rounded, with a clearly defined periphery, and appear denser than exocrine tissue contaminants. Avoid islets that appear damaged or frayed. In this experiment, islet size was not directly measured, although we attempted to pick islets of approximately equivalent size for each well and sample. - Load each well of spheroid microplate with 175 µL of assembled media using a multichannel pipette.
- Using a P20 pipette set to 15 µL, aspirate 15 islets from cell culture dish. Islets should be visible in the pipette tip to the naked eye.
- To transfer islets to spheroid microplate, lower pipette tip to the bottom of the well, barely lift up, and slowly pipette out a very small volume (~5 µL). Confirm that all islets have left the pipette tip. Each well should receive 15 islets. Occasionally check spheroid microplate under the microscope to verify that islets are at the bottom of each well rather than stuck to the sides.
NOTE: Avoid loading the corner wells with islets. Oxygen and pH flux out of the plastic can occur during the assay, and this is exacerbated in the four corner wells. - Once all islets have been transferred to the spheroid microplate, incubate the microplate in a 37 °C, non-CO2 incubator while completing the steps below.
- Hand-pick pancreatic islets into a 60 mm x 15 mm cell culture dish containing assembled media to obtain near 100% purity.
- Loading the sensor cartridge and running the assay
- Remove sensor cartridge from incubator. Ports A-C for each well must be loaded with either drug or media. Wells containing islets and the four corner wells should be loaded with drug. Non-corner wells without islets should be loaded with media. Port D can be left empty. Load port A (top left of each sensor) with 20 µL of oligomycin or media. Load port B (top right) with 22 µL of FCCP or media. Load port C (bottom left) with 25 µL of Rotenone/AA or media.
- Program an extracellular flux analyzer assay for a 30 minute baseline respiration period (5 cycles of 3 minute mix, 3 minute measure), 42 minute oligomycin measurement period (7 cycles of 3 minute mix, 3 minute measure), 30 minutes FCCP (5 cycles of 3 minute mix, 3 minute measure), and 30 minutes Rotenone/AA (5 cycles of 3 minute mix, 3 minute measure).
- Run the assay and follow instructions on screen for calibrating the sensor and inserting the microplate.
Representative Results
To load islets into microplate, 15 islets should be aspirated in 15 µL of media, as shown in Figure 1A. Islets will naturally settle toward the bottom of the pipet tip within a few seconds. Then, the pipet tip is lowered to the bottom of the well. The tip is very slightly lifted, and a small volume (about 5 µL) is pipetted out along with the islets. This technique results in consistent placement of islet at the bottom of the microplate well (Figure 1B) allowing for accurate oxygen consumption measurements.
Figure 2 shows representative results by individual well for oxygen consumption throughout the assay. This particular experiment demonstrates what can happen when wells are loaded poorly, with many islets stuck up on the sides of the well rather than at the bottom of the well. This can be caused by pipetting too much media into the well after the islets have already been pipetted out of the tip. The flow of additional media pushes the islets out of the bottom of the well. When this happens, the baseline level of oxygen consumption will be very low, and this well will show little to no response to FCCP. The bolded line in Figure 2 demonstrates this phenomenon.
Figure 3 shows a different experiment in which most wells did show significant baseline respiration and response to drugs. However, two wells (with bolded lines) showed no response to rotenone/AA. This suggests that the drug was not properly released into the well. In this case, as with cases of no basal oxygen consumption, these wells can be excluded from further analysis.
Figure 4 shows the results of a successful assay. Here we show an example of summary data from a separate experiment in which wells were properly loaded with islets and correctly injected with drugs. ATP-dependent mitochondrial oxygen consumption was effectively inhibited by oligomycin, maximal respiration-well above basal levels-was induced by FCCP, and mitochondrial respiration was completely shut down-below oligomycin levels-by inhibition of the electron transport chain with rotenone/AA.
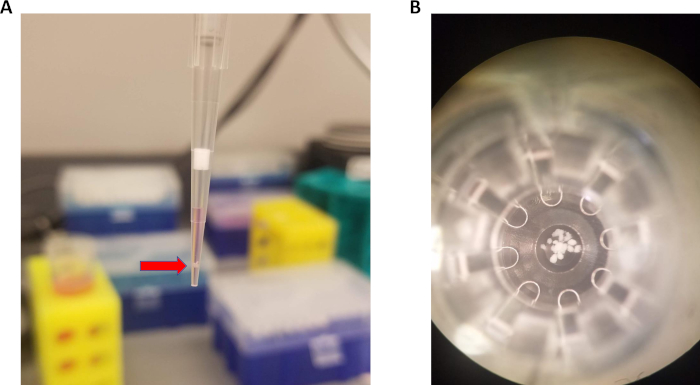
Figure 1: Pipetting technique for loading islets into microplate. (A) Approximately 15 islets (red arrow) pipetted with 15 µL of media. (B) Islets centered at the bottom of the spheroid microplate. Please click here to view a larger version of this figure.
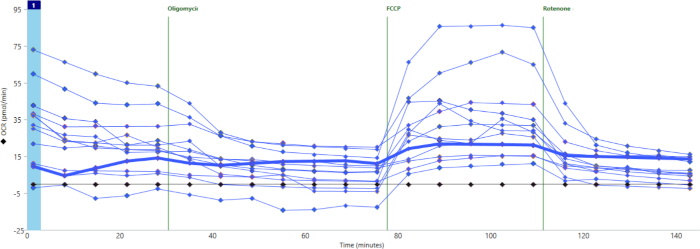
Figure 2: Well-to-well variability due to difference in islet loading. Wells loaded improperly (bold blue line) show little to no basal oxygen consumption and minimal response to cell stress test drugs. Please click here to view a larger version of this figure.
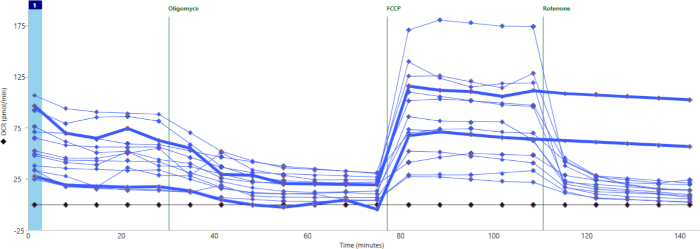
Figure 3: Failure of drug injection. Two wells (bold blue lines) were not injected with rotenone/AA and show no significant decrease in oxygen consumption. Please click here to view a larger version of this figure.
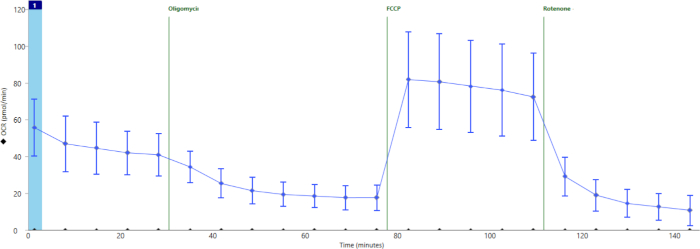
Figure 4: Averaged data across all wells in an experiment after exclusion of poorly loaded or injected wells. Average oxygen consumption throughout the cell stress test assay in a separate experiment including 16 technical replicates. Error bars show standard deviation for n = 16 wells. Please click here to view a larger version of this figure.
Discussion
The study of islet oxygen consumption has previously been hampered by the spherical shape of islets, their lack of adherence to culture surfaces, and the number of islets required per well. In this protocol, we highlight the efficacy of the 96-well spheroid microplate for measuring islet oxygen consumption on a small number of islets and demonstrate a technique for handling and loading islets which is technically feasible and produces consistent results.
In order for islets to adhere to the bottom of the microplate and be unperturbed during mixing steps throughout the assay, the 96-well microplate is coated with a cell adhesive solution the day before the assay. While this is generally beneficial, if islets are not loaded properly they are liable to stick to the side of the well rather than the bottom, thus affecting or precluding accurate measurement. To combat this, we recommend coating wells with a very small volume of adhesive solution-only 20 µL. Additionally, the pipetting technique we demonstrate ensures that islets are loaded to the bottom of the well and not pushed up the sides by the flow of additional media.
The 96-well spheroid microplate has overcome previous limitations of measuring islet oxygen consumption, including the high number of islets required and the number of conditions that can be tested simultaneously. Indeed, a recent study10 demonstrated the use of this system with a single human islet per well. However, measurement of oxygen consumption using single islets resulted in very low basal measurements of oxygen consumption, which were significantly above background in only the largest islets (diameter above 290 µm). NHP islets tend to be smaller than human islets, with an average diameter of only 150 µm15. In our hands, 15 NHP islets per well showed higher baseline and a more responsive profile during the cell stress test than fewer islets. We also tested five and ten islets per well, but found that basal oxygen consumption and signal to noise were significantly reduced.
In addition to the ability to use a low number of islets per well, the 96-well microplate allows for the testing of multiple different conditions at once. This is beneficial in cases where islets are treated with different compounds or genetically manipulated. However, when comparing groups of human or non-human primate islets that come from different sources (e.g., diabetic versus non-diabetic islets), samples are often received on different days. Thus, in these cases it is not possible to take full advantage of this system.
In order to quantify various aspects of mitochondrial oxygen consumption, we manipulated different aspects of respiration pharmacologically. Drug concentrations used were based on a pervious study in human islets10. The dose of FCCP was optimized to induce maximal mitochondrial respiration in our hands. Specifically, the concentration of oligomycin was 4.5 µM, FCCP was 1 µM, and rotenone/AA was 2.5 µM. However, these concentrations likely need to be calibrated for different applications of this protocol.
The data produced can be either analyzed directly or normalized using a number of methods. In this experiment, equal numbers of islets of similar sizes were used for each sample, and data was not normalized by cell number or islet size, which can be difficult to directly quantify. An alternative method of normalization is by total cellular protein, which involves lysing cells after the assay is performed and quantifying protein levels. Data can also be normalized to basal respiration levels. This may be informative in certain cases, such as the quantification of reserve capacity as a percentage of basal levels. However, with these normalization methods and others, information can be lost during normalization as previously described16.
The system described here provides a unique tool to better understand islet physiology. Indeed, the importance of oxygen consumption in islet health is illustrated by the observation that islet oxygen consumption rates are closely tied with islet health and the probability of successful islet transplantation17. This system provides an excellent platform for consistent quantification of islet oxygen consumption which can, in theory, also be applied to any similarly-sized cultured spheroids.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the Vanderbilt High Throughput Screening Core for the use of their facilities, Agilent Biotechnologies, Dr. Paul Kievit (Oregon Health and Science University) for non-human primate islet isolations, and Eric Donahue (Vanderbilt University) for assistance with Figure 1. J.M.E. was supported by NIGMS of the National Institutes of Health under award number T32GM007347. M.G. was supported by the NIH/NIDDK (R24DK090964-06) and the Department of Veterans Affairs (BX003744).
Materials
| Cell culture dish, 60 mm X 15 mm style | Corning | 430166 | |
| Cell-Tak Cell and Tissue Adhesive | Corning | 354240 | |
| Conical tube, 50 mL | Falcon | 352070 | |
| Dextrose anhydrous | Fisher Scientific | BP350-1 | For glucose solution, 200 mg/ml, sterile filetered |
| Disposable reservoirs (sterile), 25 ML | Vistalab | 3054-1033 | for loading multichannel pipet |
| EZFlow Sterile 0.45 μm PES Syringe Filter, 13 mm | Foxx Life Sciences | 371-3115-OEM | |
| L-glutamine | Gibco | 25030-081 | 200 mM (100x) |
| Multichannel pipette tips | ThermoFisher Scientific | 94410810 | |
| Multichannel pipette, 15-1250 μL | ThermoFisher Scientific | 4672100BT | Recommended |
| P20, P200, and P1000 pipettes | Eppendorf | 2231000602 | |
| pH Probe | Hanna Instruments | HI2210-01 | |
| Pipette tips, 20 μL, 200 μL, 1000 μL | Olympus | 24-404, 24-412, 24-430 | |
| Seahorse XF Base Media | Agilent | 103334-100 | |
| Seahorse XF Cell Mito Stress Test Kit | Agilent | 103015-100 | Includes Oligomycin, FCCP, and Rotenone/Antimycin A |
| Seahorse XFe96 Analyzer | Agilent | S7800B | Including prep station with 37 °C non-CO2 incubator |
| Seahorse XFe96 Spheroid Fluxpak Mini | Agilent | 102905-100 | Includes sensor cartridge, spheroid microplate, and calibrant |
| Sodium bicarbonate | Fisher Scientific | BP328-500 | |
| Sodium pyruvate | Gibco | 11360-070 | 100 mM (100x) |
| Stereo Microscope | Olympus | SZX9 | |
| Syringe (sterile), 5 mL | BD | 309603 | For sterile filtration |
| Water (sterile) | Sigma | W3500-500mL |
Referências
- Mulder, H. Transcribing β-cell mitochondria in health and disease. Molecular Metabolism. 6 (9), 1040-1051 (2017).
- Maechler, P., Wollheim, C. B. Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. The Journal of Physiology. 529 (Pt 1), 49-56 (2000).
- Curry, D. L. Glucagon Potentiation of Insulin Secretion by the Perfused Rat Pancreas. Diabetes. 19 (6), 420 (1970).
- Song, G., Pacini, G., Ahrén, B., D’Argenio, D. Z. Glucagon Increases Insulin Levels by Stimulating Insulin Secretion Without Effect on Insulin Clearance in Mice. Peptides. 88, 74-79 (2017).
- Vergari, E., et al. Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nature Communications. 10 (1), 139 (2019).
- Watts, M., Ha, J., Kimchi, O., Sherman, A. Paracrine regulation of glucagon secretion: the β/α/δ model. American Journal of Physiology–Endocrinology and Metabolism. 310 (8), E597-E611 (2016).
- Sweet, I. R., et al. Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Technology & Therapeutics. 4 (5), 661-672 (2002).
- . Agilent Seahorse XF Instruments Overview and Selection Guide Available from: https://www.agilent.com/en/products/cell-analysis/seahorse-xf-instruments-selection-guide (2019)
- Wikstrom, J. D., et al. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS One. 7 (5), e33023 (2012).
- Taddeo, E. P., et al. Individual islet respirometry reveals functional diversity within the islet population of mice and human donors. Molecular Metabolism. 16, 150-159 (2018).
- Conrad, E., et al. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. American Journal of Physiology–Endocrinology and Metabolism. 310 (1), E91-E102 (2016).
- Steiner, D. J., Kim, A., Miller, K., Hara, M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2 (3), 135-145 (2010).
- Elsakr, J. M., et al. Maternal Western-style diet affects offspring islet composition and function in a non-human primate model of maternal over-nutrition. Molecular Metabolism. , (2019).
- Soutar, M. P. M., et al. FBS/BSA media concentration determines CCCP’s ability to depolarize mitochondria and activate PINK1-PRKN mitophagy. Autophagy. , 1-10 (2019).
- Hirshberg, B., et al. Pancreatic Islet Transplantation Using the Nonhuman Primate (Rhesus) Model Predicts That the Portal Vein Is Superior to the Celiac Artery as the Islet Infusion Site. Diabetes. 51 (7), 2135-2140 (2002).
- Divakaruni, A. S., Paradyse, A., Ferrick, D. A., Murphy, A. N., Jastroch, M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods in Enzymology. 547, 309-354 (2014).
- Papas, K. K., et al. Islet Oxygen Consumption Rate (OCR) Dose Predicts Insulin Independence in Clinical Islet Autotransplantation. PLoS One. 10 (8), e0134428 (2015).

