Ubiquitin Chain Analysis by Parallel Reaction Monitoring
Summary
This method describes the assessment of global changes in ubiquitin chain topology. The assessment is performed by the application of a mass spectrometry-based targeted proteomics approach.
Abstract
Assessment of the global profile of ubiquitin chain topologies within a proteome is of interest to answer a wide range of biological questions. The protocol outlined here takes advantage of the di-glycine (-GG) modification left after the tryptic digestion of ubiquitin incorporated in a chain. By quantifying these topology-characteristic peptides the relative abundance of each ubiquitin chain topology can be determined. The steps required to quantify these peptides by a parallel reaction monitoring experiment are reported taking into consideration the stabilization of ubiquitin chains. Preparation of heavy controls, cell lysis, and digestion are described along with the appropriate mass spectrometer setup and data analysis workflow. An example data set with perturbations in ubiquitin topology is presented, accompanied by examples of how optimization of the protocol can affect results. By following the steps outlined, a user will be able to perform a global assessment of the ubiquitin topology landscape within their biological context.
Introduction
The close regulation of protein function and stability is of paramount importance, as they are major drivers of phenotypic control of biology. The function of a protein is constructed from two components: its intrinsic polypeptide sequence and any posttranslational modifications (PTMs). Various chemical PTMs have been identified including glycosylation, phosphorylation, acetylation, and methylation1. In 1975, Goldstein et al.2 identified a small protein and named it ubiquitin due to its ubiquitous nature. Ubiquitin was found to be important in protein degradation3. However, since then it has been established that the function of ubiquitin as a signaling molecule extends far beyond the regulation of protein stability. Ubiquitin signaling is involved in a wide range of other biological functions, such as protein stabilization, autophagy, cell cycle control, and protein trafficking4.
While other PTMs are generally binary (i.e., the protein is modified or left unmodified) for a given site, ubiquitin can modify a protein both as a monomer or as a polymeric chain, with the attached ubiquitin itself being ubiquitinated. Further, this polyubiquitination chain can develop in several topologies with the ubiquitination of the previous ubiquitin attaching to one of eight linkage sites5,6. Ubiquitin is transferred by a multistep enzymatic process (Figure 1) where the C-terminus of ubiquitin is linked to one of its seven lysine residues (K06, K11, K27, K29, K33, K48, or K63) or the N-terminal of the previous ubiquitin (referred to as the M1 or linear ubiquitination)5,6. This chain topology is key to the fate of the protein under modification. For example, the modification with a K48 or K11 linked chain leads to the degradation of the modified protein at the proteasome, while a linear chain is necessary for the activation of NF-kB signaling. Thus, the relative distribution of these chain topologies is relevant to a wide variety of biological questions.
The use of mass spectrometry (MS) is of particular benefit to ubiquitin chain topology analysis as it is not reliant on antibody-based or affinity-based interactions7,8, many of which have limited specificity and do not differentiate between the various chain types. A different detection possibility is using genetically modified ubiquitin mutants. Here, a specific lysine is exchanged for arginine, which cannot support the modification by ubiquitin. The lack of ubiquitin chain formation on the substrate protein is then interpreted as evidence for a specific topology9.
MS-based identification of ubiquitinated proteins is based on the fact that the C-terminus of ubiquitin contains an arginine residue in position 74 that is recognized by trypsin during the proteolytic preparation of the samples for MS analysis, separating the C-terminal double glycine. This GlyGly (-GG) remains attached to the ε-amino group of the lysine residue of the substrate protein. For ubiquitin topology analysis, the modification occurs on one of the seven lysine residues of ubiquitin. This creates a set of seven key peptides that carry a lysine residue that is modified by GG, specific for each of the topologies (Figure 2). For example, with K06 topology, the lysine at position 6 on the amino acid sequence will be protected from tryptic digestion with a -GG modification of 114 Da added to this lysine.
Identification of specific predetermined peptides by MS is referred to as targeted proteomics, or more specifically, targeted peptide acquisition10. Two methods have been developed depending on the performance of the mass spectrometer being used. These are selected reaction monitoring (SRM), also called multiple reaction monitoring (MRM), and parallel reaction monitoring (PRM). SRM involves the selection of transitions consisting of the precursor m/z and the product ion m/z. Conversely, PRM requires only the precursor m/z. Post selection, a full survey scan of the product ions is performed. This has the advantage that no selection of appropriate product ions for quantitation on the specific mass spectrometer is necessary prior to the measurement11. Both SRM and PRM can, depending on the instrument, be scheduled. Scheduling is the practice of assigning a time window during which a particular ion will be included for analysis, as peptides are eluting at defined retention times from the chromatographic system. Reducing the number of ions being interrogated at any given time increases the frequency of interrogation of those ions scheduled at that time, thus improving data accuracy.
In general, the application of targeted proteomics for ubiquitin topology is the same as any other targeted proteomics experiment. However, two key differences are important: First, the stability of ubiquitin chains must be considered. There are multiple potent deubiquitinating enzymes (DUBs) that rapidly degrade chains upon cell lysis. The ubiquitin-specific proteases fall into two categories, the thioesterases and metalloproteases. Most of the ubiquitin-hydrolases are thioesterases and carry a cysteine residue in their active center. By alkylating this cysteine residue, they can be inactivated. As such, the use of ubiquitin stabilization buffers containing alkylating agents, like N-ethylmaleimide (NEM), and highly denaturing chemicals, and keeping samples chilled is vital to a successful analysis. Second, unlike other targeted experiments, the peptide choice is fixed. In a typical targeted experiment, a proteotypic peptide can be selected for good chromatographic and ionization performance. For some topology-characteristic peptides such as K48 these properties are good, while for others, they are less desirable. For example, K33 chromatography in a typical reverse-phase setup is poor due to the formation of a stretched elution profile, and the poor ionization properties of the K27 peptide reduce its visibility by MS12.
In this protocol, we describe how to perform ubiquitin topology assessment of a biological sample by PRM. Example data for the procedure are presented using a perturbation of the proteasome using MG-132 inhibitor treatment in several different cell types.
Protocol
1. Preparation of a heavy peptide standard
- Depending on the supplier and quality of the heavy peptides purchased, the heavy peptides will need to be diluted. This protocol used the peptide sequences reported in Table 1, with the C–terminal amino acid modified to Lysine (13C615N2-lysine) or Arginine (13C615N4-arginine).
- Mix the heavy peptides, diluting the mix with 50% acetonitrile (ACN) to create a peptide mix with each peptide at 10 µM.
- Create aliquots of the peptide mix and store at -80 °C.
2. Sample preparation
- Sample lysis
NOTE: The stability of ubiquitin chains must be considered during sample preparation. Keep biological samples and buffers chilled at 4 °C and add samples to ubiquitin stabilization buffer as rapidly as possible.- Prepare a solution of 1 M NH4HCO3 (ammonium bicarbonate) in water and adjust the pH to 8 using 1 M NaOH.
- Prepare a solution of 200 mM N-ethylmaleimide (NEM) in water.
- Prepare ubiquitin stabilization buffer using 8 M urea in 50 mM NH4HCO3/10 mM NEM. Ubiquitin stabilization buffer must be prepared freshly each time.
NOTE: A 5 mL solution of 8 M urea will require considerably less than 5 mL of water given the expansion of water when in a saturated solution with urea.
CAUTION: Do not prepare the buffer above 50 °C due to the tendency of urea to form polyurea at high temperatures. - Resuspend the biological sample to be investigated in ubiquitin stabilization buffer aiming for 0.5 µg/µL of protein and lyse the cells with an appropriate method for the sample. A sonication method consisting of three 10 s pulses with an SFX 150 Branson sonifier set at 70% intensity with 10 s breaks on ice between each pulse, was used for the cell lines in this protocol.
- Centrifuge sample at 18,000 x g for 10 min at 4 °C in a tabletop centrifuge.
- Transfer supernatant to a fresh low protein binding microcentrifuge tube. Samples can be stored at -20 °C at this point if needed.
- Preparation of sample and controls
- If the samples are frozen, mix (e.g., with a thermomixer) while they are thawed to rapidly dissolve the urea.
CAUTION: Do not use temperatures above 50 °C due to the tendency of urea to form polyurea at high temperatures. - Centrifuge sample at 18,000 x g for 10 min at 4 °C in a tabletop centrifuge.
- Transfer 20 µg of sample to a fresh low-bind microcentrifuge tube and adjust the volume to 50 µL with ubiquitin stabilization buffer.
- Create a negative control using a normalized volume of ubiquitin stabilization buffer (50 µL). In this sample, no light version of each peptide should be present, allowing observation of any light contamination arising from the spike-in heavy peptides. This negative control was also used for retention time scheduling of the PRM described in section 3.1.
- A positive control was also created with ubiquitin stabilization buffer and the addition of commercially available chain types to the normalized volume of ubiquitin stabilization buffer (50 µL). Commonly, K63, K48, and M1 are available. In the representative results 20 ng of K48, K63, and M1 were utilized.
- If the samples are frozen, mix (e.g., with a thermomixer) while they are thawed to rapidly dissolve the urea.
- Reduction, alkylation, and digestion
- Prepare a solution of 50 mM ammonium bicarbonate (NH4HCO3) in water and adjust the sample to pH = 8 with 1 M NaOH.
- A culture of E. coli grown in LB broth was centrifuged 5,000 x g for 5 min to form a pellet, which was then washed 2x with PBS before resuspension in ubiquitin stabilization buffer at 5 μg/μL.
- Add 1 µg of E. coli lysate to each sample and control. This protocol uses E. coli (DH10B), but any complex lysate without ubiquitin can be used. Note that ubiquitin is common to all eukaryotes.
NOTE: Using an E. coli lysate matrix reduces the loss of peptides due to nonspecific adhesion to plasticware. This is particularly noticeable in the negative control or samples with a limited protein content and has a strong impact on the observation of K6312. - Prepare a solution of 500 mM tris(2-carboxyethyl)phosphine (TCEP) in MS grade water (DTT can be used as an alternative).
- Reduce the samples and controls by the addition of TCEP to a final concentration of 50 mM. Vortex briefly before incubating for 30 min at room temperature (RT). If DTT is used, the temperature has to be elevated to 50 °C.
CAUTION: Do not use temperatures above 50 °C due to the tendency of urea to form polyurea at high temperatures. - Prepare a solution of 550 mM chloroacetamide (CAA) in NH4HCO3. Store in the dark.
- Alkylate the samples and controls by adding CAA to a final concentration of 55 mM. Vortex briefly before incubating for 20 min at RT in the dark.
NOTE: CAA is used instead of iodoacetamide as the reduction agent to decrease the chance of a double carbamidomethyl modification (114.0429) on a lysine residue being mistaken for a -GG modification (114.0429)13. - Add endopeptidase LysC to the samples at 1:25 (w/w) ratio to the protein content. Incubate for 3 h at 37 °C.
- Dilute the samples and controls with 200 µL of 50 mM NH4HCO3 pH = 8 (1:5 v/v).
- Add trypsin to the samples at 1:25 w/w ratio to the protein content. Incubate for 12 h at 37 °C.
- Add 10% formic acid (FA) solution in MS grade water at a 1:10 ratio v/v to each sample and control sample. Check that pH is <3) prior to the C18 cleanup and add more formic acid if needed.
- To each sample and control add 0.5 µL of the heavy peptide standard created in section 1.
- Peptide cleanup
- Several manufacturers offer C18 cleanup tips or plates. Follow the manufacturer's instructions for the product used, and dry eluted peptides in a vacuum centrifuge, ready for MS analysis.
3. Analysis by LC-MS/MS
- Execute initial PRM analysis on the heavy peptide standard in an unscheduled fashion.
- Resuspend the dried samples in 10 µL of MS loading buffer 2% ACN/0.05% trifluoroacetic acid (TFA).
- Equip an HPLC with a reverse-phase C18 analytical column (75 µm x 15 cm, 2 µm 100 Å C18 beads) designed for nanoflow MS. Form linear gradients exchanging MS grade water supplemented with 0.1% FA with ACN containing 0.1% FA. A sacrificial trap column (75 µm x 2 cm, 3 µm, 100 Å C18 beads) to preserve the life of the analytical column was used here but is not required.
- Couple the output of the analytical column to a nano-ESI source on a high-resolution mass spectrometer.
- Utilize an appropriate targeted proteomics method for the mass spectrometer. For example, on a Q-Exactive plus a two-experiment method, cycle between a Full MS and a PRM experiment. For the Full MS experiment, a resolution of 120,000 with an AGC target of 1e6 and 240 ms as Maximum IT was used. For the PRM experiment, the same AGC target and maximum IT was used with a lower resolution of 60,000 and an isolation window of 1.2 m/z.
- Inject 200 ng of the negative control onto the analytical column using the HPLC autosampler. Apply a linear gradient to the sample on the analytical column increasing the ACN concentration from 2–25% over a period of ~45 min.
NOTE: The 25% ACN concentration is lower than usual for proteomics due to the low hydrophilic nature of typical ubiquitin topology-characteristic peptides. If other peptides are to be quantified, a higher ACN concentration may be desired to achieve good target separation. - Analyze the results of the scheduling run as described in section 4.2 to create a scheduled inclusion list.
- Analysis of the samples
- Run the remaining samples as described for the scheduling in 3.1 apart from the use of the scheduled inclusion list created in section 4.2.
NOTE: A blank must be run after the positive control to ensure no carryover from the previous run is being detected in subsequent samples.
- Run the remaining samples as described for the scheduling in 3.1 apart from the use of the scheduled inclusion list created in section 4.2.
4. Software analysis
- Create an inclusion list of appropriate formatting for the utilized MS. This protocol used the open source software Skyline (version 19.1.0.193) (https://skyline.ms), which offers well-documented support and help. An inclusion list can be created for several mass spectrometers using the export isolation list facility.
- Open a new Skyline document.
- Select heavy isotope modifications as appropriate to heavy topology-characteristic peptides. In Settings|Peptide Settings under the Modification tab, click on the name field to see the potential modification. Selection is based on the isotopic state of the heavy peptides purchased. In this example, synthetic peptides carrying either a heavy Lysine (13C615N2-lysine) or a heavy Arginine (13C615N4-arginine) at the C-terminus were used. Appropriate labels would be: “Label:13C(6)15N(2) (C-term K)” and “Label:13C(6)15N(4) (C-term R)”.
- Select Settings|Transition. Under the Filter tab include Precursor Charges 2, 3, 4.
- Insert peptides by selecting Edit|Insert|Peptides.
- Fill in the relevant fields including the topology-characteristic peptide sequence and a protein name (e.g., "Chain–K63").
- Expand each peptide now shown in the Targets panel. Delete undesired charge states. The preferred charge state and collision energy, which may differ based on mass spectrometer used, for each topology-characteristic peptide is shown in Table 1.
- For each topology-characteristic peptide (except for M1 where the GG is included in the sequence) right-click on the sequence and select Modify. Add a GG as a structural modification by clicking the name field and selecting <Show all…> before selecting "GlyGly (K)".
- Export the isolation list File|Export|Isolation List, select the instrument type, and set the method type to Standard. Selecting OK will open a prompt to save a *.csv file that can be used to create a PRM method.
- Create a scheduled inclusion list based on a heavy peptide scheduling run explained here using the open source Skyline software.
- Create a target list as described in section 4.1.
- Import the scheduling run in 3.1 by selecting File|Import|Results.
- Review the identifications. The heavy signal for each peptide is observable by clicking on the mass for each heavy peptide entry. Correct recognition of the peak is often determined automatically, but the software may require manual curation by selecting the peak using click and drag under the x-axis.
- Retention times selected for the heavy variants are also applied to the light versions, thus creating a schedule for the PRM. The scheduling window can be modified under Settings|Peptide Settings using the Prediction tab, by changing the Time Window.
- A scheduled inclusion list can now be exported using File|Export|Isolation list and selecting the appropriate instrument type and setting method type to Scheduled.
- Analyze the data.
- Import the samples in the same fashion as for the scheduling run analysis in section 4.2.
- After the complete import of all samples, curation of transitions is recommended. This is the process by which transitions with interference or poor signal-to-noise ratios are removed. An example of a chromatogram before and after curation is shown in Figure 4.
- Data can now be exported either by right clicking on relevant graphs and selecting Copy data or by selecting File|Export|Results.
Representative Results
To demonstrate the use of a ubiquitin chain analysis by PRM, three cell lines were selected: a mouse melanoma cell line B16, and the two common human cell lines A549 (adenocarcinomic alveolar basal epithelial cells) and HeLa (cervical cancer cells). These cultures grew to midexponential phase in appropriate media before being treated with 0, 10, or 100 mM MG-132 for 4 h prior to harvest. MG-132 is a proteasome inhibitor preventing the degradation of ubiquitin-conjugated proteins by the proteasome14. Given such, it is an appropriate test condition to demonstrate ubiquitin chain analysis. The resultant increase in the K48 chain can be seen in Figure 3. Proteasome inhibitor treatment induced an increase in K48 chains15.
As described in the introduction, unlike SRM or MRM, PRM performs a full product ion scan after selection of the precursor ion. While this means that product ions do not need to be specified before the run, they should still be curated post run. Curation is the process by which transitions truly representative of the intended peptide are selected. Figure 4 shows the product ion chromatogram for K48 before and after curation. Product ions that have a signal with an inconsistent elution profile likely due to interference were removed. Low intensity product ions were then removed given that the signal-to-noise ratio is less favorable for such transitions. The optimal transitions selected during curation will commonly be consistent between experiments, and may differ depending on mass spectrometer used, chromatographic conditions, analysis settings, and the biological background of the sample. Thus, for each investigation proper curation of transitions is required. A balance is also required between inclusion of more transitions and thus technical replicates and cleaner data for quantification.
Typical product ion chromatograms for each of the identified ubiquitin chain topologies in this experiment are shown in Figure 5. K27 and K29 are omitted here because under these biological conditions the signal was below detection. The elution profile of K33 as shown here was considerably broader than would be commonly accepted for PRM analysis. However, alternative selection of peptides with better elution profiles is not possible in ubiquitin chain analysis and an interpretation must be made based on this profile. If K33 is of particular interest, altered chromatographic conditions may improve quantification of this chain type, but likely to the detriment of other chain types.
In designing a PRM experiment, it is preferable to maximize the signal of the product ions used for quantification by increasing signal-to-noise. The optimization of collision energy (i.e., the energy used by the mass spectrometer to fragment precursor peptide ions to product ions) is one means by which the signal can be improved16. A collision energy range from 14–28 was applied to repeated injections of a single sample with the resulting observed peak areas of K63 and M1 shown in Figure 6. As can be seen, for K63 a higher collision energy of 26 was optimal, while for M1 a lower energy of 18 was ideal. The collision energy for each topology-characteristic peptide and a selection of unmodified ubiquitin fragments is shown in Table 1. These collision energies may need to be optimized based on the mass spectrometer and fragmentation method used.
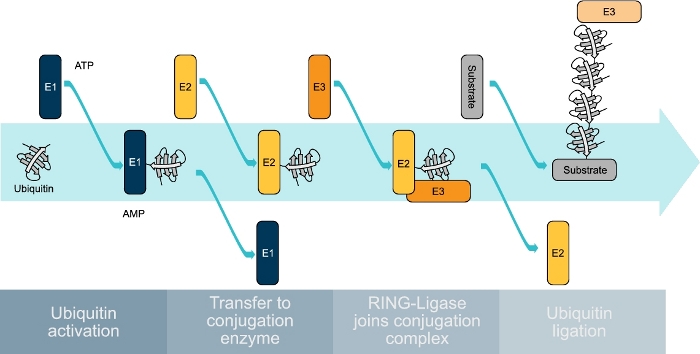
Figure 1: Schematic representation of the ubiquitin-conjugating cascade. Ubiquitin is first bound by an E1 enzyme in an ATP-dependent manner. The activated ubiquitin is then transferred to an E2 enzyme that is then joined by an E3-ligase to transfer the ubiquitin to the substrate protein. This process is repeated several times until a ubiquitin chain is formed on the substrate protein. Please click here to view a larger version of this figure.
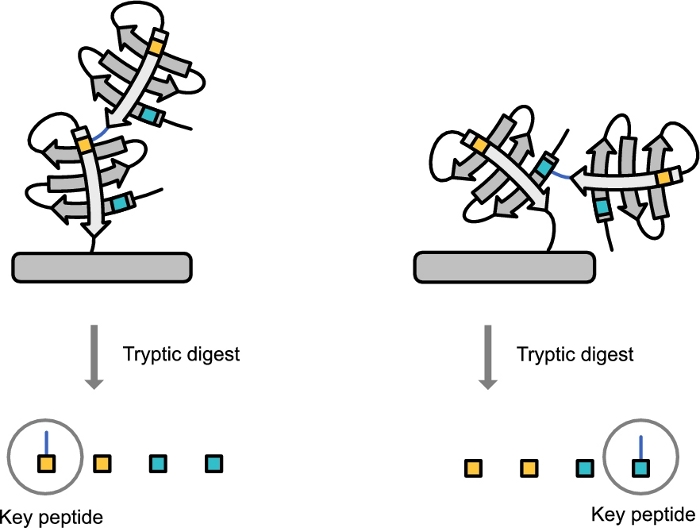
Figure 2: Diagram showing the derivation of the ubiquitin topology-characteristic peptide. Post tryptic digestion the upstream binding site is derived with the -GG C-terminal of the downstream ubiquitin attached. Please click here to view a larger version of this figure.
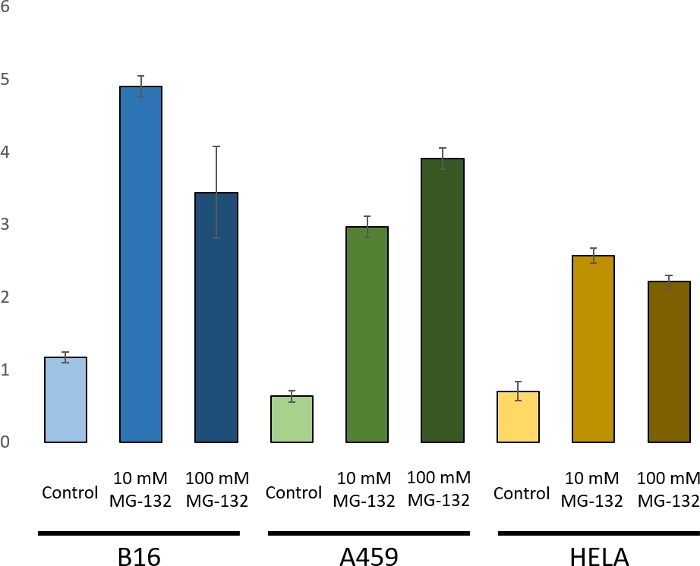
Figure 3: Comparison of K48 chain under MG-132 treatment for a mouse cell line B16 and the two human cell lines A459 and HeLa. In A459 increasing concentrations of MG-132 led to stabilization of K48 chains. With B16 and HeLa cells an increase was seen under treatment with either 10 mM or 100 mM MG-132. The decrease observed from 10 mM to 100 mM can be explained by the increased sensitivity of B16 and HeLa cells to MG-132, leading to cell death. Error bars show the standard deviation of the transitions. Please click here to view a larger version of this figure.
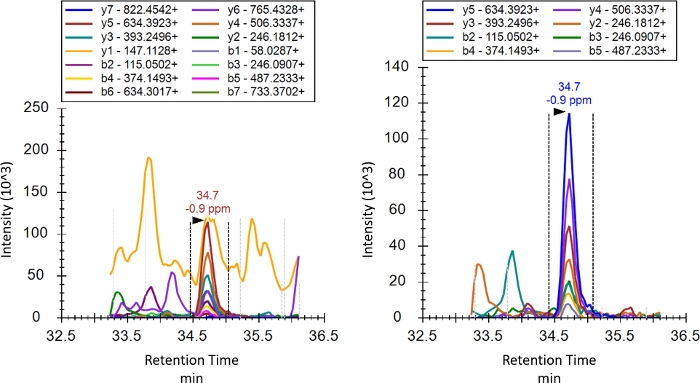
Figure 4: Product ion chromatograms of the M1 topology-characteristic peptide before and after curation of transitions for interference or low intensity. Retention time on the x-axis is shown in minutes. Please click here to view a larger version of this figure.
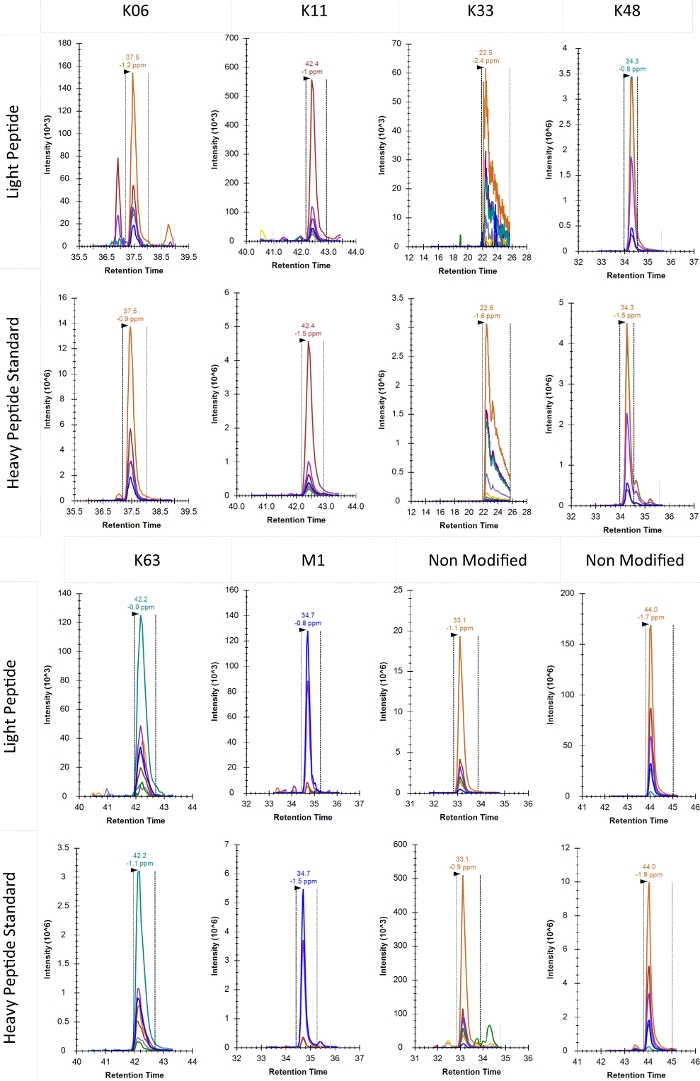
Figure 5: Typical product ion chromatogram of various topology-characteristic peptides as well as two non-modified ubiquitin peptides. The elution time in minutes and observed mass error for each peptide is reported above the apex of the relevant peak. Dotted lines also show the window for determination of the peak area. Retention time on the x-axis is shown in minutes. Please click here to view a larger version of this figure.
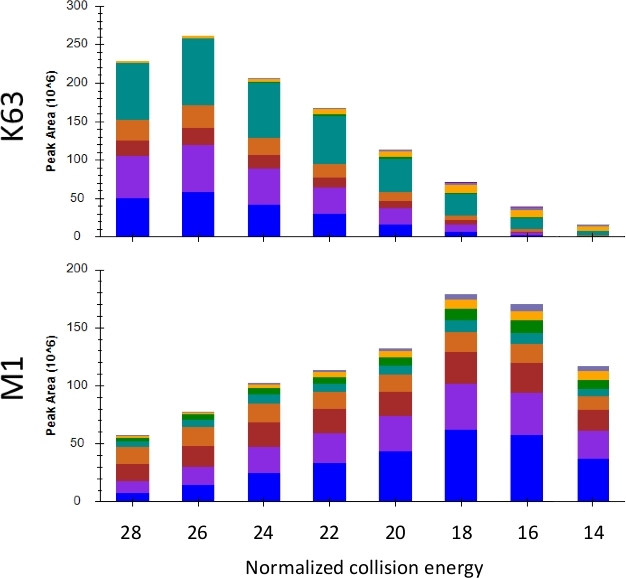
Figure 6: Observed peak area for K63 and M1 over a series of normalized collision energies. The different colors represent the ion intensity contributed by each transition. Please click here to view a larger version of this figure.
| Chain type | Topology Characteristic Peptide | Charge state | Normalised Collision Energy |
| K06 | MQIFVK[GG]TLTGK | 3 | 18 |
| K11 | TLTGK[GG]TITLEVEPSDTIENVK | 3 | 18 |
| K27 | TITLEVEPSDTIENVK[GG]AK | 3 | 18 |
| K29 | AK[GG]IQDK | 2 | 22 |
| K33 | IQDK[GG]EGIPPDQQR | 3 | 18 |
| K48 | LIFAGK[GG]QLEDGR | 3 | 24 |
| K63 | TLSDYNIQK[GG]ESTLHLVLR | 4 | 26 |
| M1 (linear) | GGMQIFVK | 2 | 18 |
| Non Modified Ubqiuitin | ESTLHLVLR | 2 | 26 |
| Non Modified Ubqiuitin | TITLEVEPSDTIENVK | 2 | 18 |
| Non Modified Ubqiuitin | TLSDYNIQK | 2 | 18 |
Table 1: Sequences for the human ubiquitin topology-characteristic peptides, the most observable charge state, and favorable normalized collision energy.
Discussion
Analysis of the ubiquitin state within a proteome is of increasing importance to a wide variety of biological questions. The description of the ubiquitination state of a sample must focus not only on the profile of proteins being ubiquitinated but also on the topology of such ubiquitination. The assessment of this topology by targeted MS, as described here, has a role in a wide range of biological investigations.
It should be understood that the protocol outlined here provides a global topology profile. Use of a bottom-up proteomics approach allows for the determination of peptides that were ubiquitinated, including ubiquitin peptides. The observation of the ubiquitination of ubiquitin peptides allows the determination of the chain topology, but the link to the target of ubiquitin is lost. Thus, the particular chain topology on any given protein cannot be determined, but a global topology profile is derived. The method can also be combined with an enrichment strategy to provide more targeted results. Such an enrichment must consider the chain stability and the abundance of chains post enrichment. The detection of the chain topology is limited by the purity the enrichment strategy provides, as it is not possible to distinguish a ubiquitin topology linked to a contaminating protein from the ones linked to the protein of interest. The critical step in this protocol, facilitating a successful analysis, is the balancing of preserving the ubiquitin chain while digesting the ubiquitin protein. This is challenging due to the propensity of chain breakdown coupled to the resistance of ubiquitin itself to enzymatic digestion. Further, the addition of a support matrix and careful consideration of MS conditions are required given the less favorable nature of some of the topology-characteristic peptides to liquid chromatographic coupled mass spectrometric analysis.
While we have outlined in this protocol a means by which all ubiquitin chain types can be assessed concurrently, it is also possible to adjust several aspects of the protocol based on the particular chain topology of interest. Manipulating the chromatographic conditions or varying the heavy peptide concentrations can increase the accuracy of an investigation, improving results for a particular chain topology and tailoring the investigation to the biological question proposed.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Céline Jeanty for her assistance in creation of cellular pellets with treatment of MG-132 as described in the representative results and Elise Mommaerts for her provision of E. coli pellets used in the protocol.
Materials
| Acetonitrile (ACN) | Merck | 100029 | |
| Ammonium bicarbonate (ABC) | Fluka | 9830 | |
| Centrifuge | Beckman Coulter | Microfuge 16 | |
| Chloroacetamide (CAA) | Sigma | 22790 | |
| Eppendorf LoBind | Eppendorf | 22431081 | |
| Formic acid (FA) | Thermo Fisher Scientific | 85178 | |
| Heavy Peptides | JPT Peptide Technologies | ||
| HPLC | Dionex | Ulitimate 3000 | |
| LC Column | Thermo Fisher Scientific | 160321 | |
| Lys C | Wako | 125-05061 | |
| Mass Spectrometer | Thermo Fisher Scientific | Q-Exactive Plus | |
| N-ethylmaleimide (NEM) | ACROS Organics | 156100050 | |
| Positive Control Chain K48 | Boston Biochem | UC-240 | |
| Positive Control Chain K63 | Boston Biochem | UC-340-100 | |
| Positive Control Chain M1 | Boston Biochem | UC-710B-025 | |
| Sodium Hydroxide (NaOH) | Sigma | S5881 | |
| Sonifier | Branson sonifier | SFX 150 | |
| Thermomixer | Eppendorf | Thermomixer Comfort | |
| Trifluoroacetic acid (TFA) | Sigam | T6508 | |
| Tris(2-carboxyethyl)phosphine (TCEP) | Thermo Fisher Scientific | 77720 | |
| Trypsin | Promega | V1511A | |
| Urea | Sigma | 51456 | |
| Waters μElution C18 plates | Waters | 186002318 |
Referências
- Khoury, G. A., Baliban, R. C., Floudas, C. A. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Scientific Reports. 1, 90 (2011).
- Goldstein, G., et al. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proceedings of the National Academy of Sciences of the United States of America. 72 (1), 11-15 (1975).
- Glickman, M. H., Ciechanover, A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 82, 373-428 (2002).
- Pickart, C. M., Eddins, M. J. Ubiquitin: structures, functions, mechanisms. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 1695 (1-3), 55-72 (2004).
- Akutsu, M., Dikic, I., Bremm, A. Ubiquitin chain diversity at a glance. Journal of Cell Science. 129 (5), 875-880 (2016).
- Komander, D., Rape, M. The Ubiquitin Code. Annual Review of Biochemistry. 81 (1), 203-229 (2012).
- Mattern, M., Sutherland, J., Kadimisetty, K., Barrio, R., Rodriguez, M. S. Using Ubiquitin Binders to Decipher the Ubiquitin Code. Trends in Biochemical Sciences. 44 (7), 599-615 (2019).
- Newton, K., et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 134 (4), 668-678 (2008).
- Spence, J., et al. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 102 (1), 67-76 (2000).
- Borràs, E., Sabidó, E. What is targeted proteomics? A concise revision of targeted acquisition and targeted data analysis in mass spectrometry. PROTEOMICS. 17 (17-18), (2017).
- Lesur, A., Domon, B. Advances in high-resolution accurate mass spectrometry application to targeted proteomics. PROTEOMICS. 15 (5-6), 880-890 (2015).
- Longworth, J., Dittmar, G. Assessment of Ubiquitin Chain Topology by Targeted Mass Spectrometry. Methods in Molecular Biology. 1977, 25-34 (2019).
- Nielsen, M. L., et al. Iodoacetamide-induced artifact mimics ubiquitination in mass spectrometry. Nature Methods. 5 (6), 459-460 (2008).
- Tawa, N. E., Odessey, R., Goldberg, A. L. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. The Journal of Clinical Investigation. 100 (1), 197-203 (1997).
- Kim, W., et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Molecular Cell. 44 (2), 325-340 (2011).
- MacLean, B., et al. Effect of Collision Energy Optimization on the Measurement of Peptides by Selected Reaction Monitoring (SRM) Mass Spectrometry. Analytical Chemistry. 82 (24), 10116-10124 (2010).

