Time-lapse Imaging of Mouse Macrophage Chemotaxis
Summary
Here we describe methods using time-lapse, phase-contrast microscopy to image mouse resident peritoneal macrophages in a chemotactic complement C5a gradient. The protocols can be extended to other immune cells.
Abstract
Chemotaxis is receptor-mediated guidance of cells along a chemical gradient, whereas chemokinesis is the stimulation of random cell motility by a chemical. Chemokinesis and chemotaxis are fundamental for the mobilization and deployment of immune cells. For example, chemokines (chemotactic cytokines) can rapidly recruit circulating neutrophils and monocytes to extravascular sites of inflammation. Chemoattractant receptors belong to the large family of G protein-coupled receptors. How chemoattractant (i.e., ligand) gradients direct cell migration via G protein-coupled receptor signaling is not yet fully understood. In the field of immunology, neutrophils are popular model cells for studying chemotaxis in vitro. Here we describe a real-time two-dimensional (2D) chemotaxis assay tailored for mouse resident macrophages, which have traditionally been more difficult to study. Macrophages move at a slow pace of ~1 µm/min on a 2D surface and are less well suited for point-source migration assays (e.g., migration towards the tip of a micropipette filled with chemoattractant) than neutrophils or Dictyostelium discoideum, which move an order of magnitude faster. Widely used Transwell assays are useful for studying the chemotactic activity of different substances, but do not provide information on cell morphology, velocity, or chemotactic navigation. Here we describe a time-lapse microscopy-based macrophage chemotaxis assay that allows quantification of cell velocity and chemotactic efficiency and provides a platform to delineate the transducers, signal pathways, and effectors of chemotaxis.
Introduction
Immune cells typically migrate singly on a 2D surface in an amoeboid fashion1,2, which involves repeated cycles of protrusion of the front, integrin-mediated cell adhesion, and retraction of the rear. A prerequisite step is cell polarization, in which cells form front and rear ends3. Chemotaxis starts with the detection of chemoattractants by G protein-coupled receptors and a complex signaling network mediated by membrane-anchored heterotrimeric G proteins and small monomeric G proteins, as well as phospholipid-bound guanine nucleotide exchange factors (GEFs)4,5. Activation of Rho GTPases of the Cdc42 and Rac subfamilies induce protrusions at the front6 and members of the Rho subfamily, especially RhoA, activate contraction of the rear5,7. In a three-dimensional (3D) environment, integrins are largely redundant for leukocyte migration and RhoA becomes more important for squeezing cells through narrow passages8, whereas Cdc42- or Rac-induced Arp2/3 activation remains important for chemotactic steering9,10.
Immune cells may face different chemoattractants, especially in the settings of tissue injury, pathogen invasion, and inflammation. The endogenous chemoattractants expressed on phagocytes complement C3a and C5a, are rapidly generated by activation of the complement cascade, and are recognized by complement C3a and C5a receptors. Similarly, necrotic cells recruit phagocytes via formyl peptide receptors, which recognize mitochondria-derived as well as bacteria-derived formyl peptides11. Immune cells also express G protein-coupled receptors for chemokines, a large family of chemoattractant peptides involved in the regulation of immune cell trafficking during both homeostasis and inflammation. Chemokines are classified into four groups depending on the spacing of the first two cysteine (C) residues: C, CC, CXC, and CX3C cytokines, where X is an amino acid. Thus, in vivo immune cells need to appropriately respond to highly complex spatial and temporal signals, making the study of chemotaxis a daunting task. Below we provide a brief history of chemotaxis, which began with intravital imaging approaches.
The study of leukocyte chemotaxis dates back to 188812, when the ophthalmologist Theodor Karl Gustav Leber clearly described the directed migration of leukocytes to, and the accumulation at, sites of inflammation in a model of mycotic (fungal) keratitis. Leber stressed that the attraction of excess leukocytes by pathogen-derived substances is important for elimination of harmful microorganisms via phagocytosis, which had been described by Metchnikoff (also known as Metschnikoff) earlier in the same decade13. In vivo experiments were also performed in the 1920s by Clark and Clark14,15, who took advantage of the transparency of tadpoles and showed that sterile inflammation induced by croton oil14 or other irritants15 caused leukocytes to adhere to blood vessels, followed by diapedesis (transendothelial migration) and rapid migration through the tissue spaces towards the irritant. In vitro experiments using the microcinematography method developed by Jean Comandon16 showed that leukocytes migrated towards a particulate chemoattractant source such as bacteria17. At that time, the molecular identities of chemotactic factors were unknown. In the 1960s, Stephen Boyden18 recognized that techniques to study the chemotactic activity of soluble substances were lacking. He devised a chamber, subsequently known as the Boyden chamber, with two compartments separated by a filter paper membrane. A cell suspension is added to the upper compartment and the test substance is added to either both compartments or only to the lower compartment. After an incubation period, the filter membrane is removed, and the cells are fixed and stained. By comparing how many cells migrate across the filter membrane towards the lower well with the test substance in both compartments, in neither compartment, or only in the lower compartment, chemotactic activity can be determined. Transwell assays are still popular today and have been modified in various ways, including the use of different polycarbonate membranes with defined pore sizes and densities19,20. A major drawback of Transwell assays is that it is impractical to directly visualize cells migrating and the migration path across the membrane typically does not exceed the diameter of an immune cell.
Sally H. Zigmond developed a chemotaxis chamber21 that enabled visualization of both gradient formation and cell morphology using fluorescent dyes. The chamber consists of a plexiglass (acrylic) slide with two parallel linear wells, each with a volume of ~100 µL, separated by a 1 mm wide bridge 3–10 µm below the upper plane of the slide. A coverslip seeded with cells is inverted and placed onto the slide such that it spans the two wells. After addition of a chemoattractant to one of the wells, a steep chemoattractant gradient forms across the bridge, typically within 30–90 min. Human polymorphonuclear leukocytes (granulocytes) in the Zigmond chamber are observed orienting themselves towards the chemoattractant. Variations of the Zigmond chamber have been reported, including the Dunn22 and Insall23 chambers, both of which use a coverslip seeded with cells placed across two wells separated by a 1 mm wide bridge. The Dunn chamber consists of concentric wells separated by a circular bridge, whereas the Insall chamber is more closely related to the Zigmond chamber, but provides bridges of two different widths, 0.5 mm and 1 mm. A novel chemotaxis chamber, termed µ-Slide Chemotaxis and manufactured by plastic injection molding, was described by Zemgel et al.24. The chemotaxis chamber consists of two 40 µL reservoirs separated by a 1 mm wide channel with a length of 2 mm and a height of 70 µm. The bottom of the chamber is formed by a gas permeable, thin plastic sheet with the same thickness and optical properties of a No. 1.5 glass coverslip24. Here we describe a chemotaxis assay using the µ-Slide Chemotaxis chamber to visualize the migration of mouse resident peritoneal macrophages for up to 14 h in a chemotactic (complement C5a) gradient.
Protocol
The protocols follow the guidelines of our local research ethics committee, as well as the animal care guidelines.
NOTE: Figure 1 shows a workflow of the chemotaxis assay.
1. Prefilling Chemotaxis Slides
- Prefill the 1 mm wide and 2 mm long connecting channels of one or two chemotaxis slides using modified RPMI 1640 HEPES medium, consisting of bicarbonate-free RPMI 1640 medium containing 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 10% heat-inactivated fetal bovine serum (FBS), and antibiotics such as penicillin (100 U/mL) and streptomycin (100 µg/mL), prepared by diluting 100x penicillin/streptomycin, and 1 µg/mL lipopolysaccharide (from E. coli), and a Toll-like receptor 4 ligand used to activate the cells.
- Place a chemotaxis slide (Figure 2A) into a round (10 cm diameter) cell culture dish, both preheated to 37 °C, and set the dish onto a heated (37 °C) aluminum block. Insert plugs into ports 1 and 4 (Figure 2B).
NOTE: An aluminum block maintained at 37 °C and placed inside the laminar flow hood is useful for preparing chemotaxis chambers. Ideally, the heated block should provide a flat working area and wells for various tubes, such as 50 mL tubes and 2 mL microcentrifuge tubes. - Using a 10–200 µL pipette tip with a beveled tip, deposit 15 µL of modified RPMI 1640 HEPES medium into filling port 3 (Figure 2B). Next, with the volume still set at 15 µL and the control button of the (2–20 µL volume) pipette depressed, insert the pipette tip into port 2 and aspirate 15 µL at a moderately fast rate (Figure 2B). This will prefill the 1 mm x 2 mm connecting channel (observation area), as well as the two flanking supply channels (between the central observation area and ports 2 and 3, respectively). Cover filling ports 2 and 3 with caps.
- After prefilling, place the chemotaxis slides onto a rack kept in a closed humidity chamber within an otherwise dry and CO2-free incubator at 37 °C.
NOTE: It is important to use the correct pipette tip for filling the chemotaxis slide. A beveled pipette tip wedges into the top of the filling port, whereas commonly used pointed pipette tips can be inserted more deeply into the filling port and may greatly increase resistance to fluid flow.
- Place a chemotaxis slide (Figure 2A) into a round (10 cm diameter) cell culture dish, both preheated to 37 °C, and set the dish onto a heated (37 °C) aluminum block. Insert plugs into ports 1 and 4 (Figure 2B).
2. Isolation of Mouse Resident Peritoneal Macrophages
- Sacrifice a 3–4 month-old mouse using a high concentration of the volatile anesthetic isoflurane (>5% in air) or carbon dioxide25, followed by cervical dislocation. Loss of the righting reflex in rodents correlates with loss of consciousness in humans26. Clean the abdomen of the mouse with 80% ethanol in water and then make a 1–2 cm midline skin incision using surgical scissors with blunt tips. Peel back the skin to expose the underlying abdominal wall.
- Insert a 24 G plastic catheter into the peritoneal cavity. Using a 5 mL plastic syringe, lavage the cavity using 2 x 4.5 mL ice-cold Hank’s buffered salt solution (HBSS), without Ca2+ and Mg2+. Leave around 0.5 mL of residual HBSS in the syringe so that tissue inadvertently sucked onto the tip of the catheter can be expelled.
- Transfer the lavaged medium, typically 8–8.5 mL in total, into a 14 mL polypropylene round bottom tube. Centrifuge the tube at 300 x g for 6.5 min at room temperature.
NOTE: The round bottom tube allows the supernatant to be fully decanted and reduces cell clumping. - Discard the supernatant and resuspend the peritoneal cells (typically ~4 x 106 cells per mouse) in 200 µL of modified RPMI 1640 HEPES medium. Dilute a sample of the cell suspension 1:20 and use a counting device, such as a Neubauer improved counting chamber, to count the cells. Next, dilute the cell suspension to a final concentration of 10 x 106 cells/mL and maintain the cells in a round bottom 2 mL polypropylene microcentrifuge tube at 37 °C using a heated aluminum block (see NOTE in step 1.1.1).
3. Seeding Peritoneal Cells into Chemotaxis Slides
- After pipetting the cell suspension up and down 5x with the pipette volume set at 100 µL (or at half the suspension volume) to reduce clumping, gently deposit 10 µL of the cell suspension into port 3 of a chemotaxis chamber (Figure 2C). Place the pipette tip into port 2 and slowly draw the cell suspension into the connecting channel (Figure 2C). As soon as the cell suspension has been introduced, remove the plugs at ports 1 and 4, which will help to arrest the flow of the cell suspension. Place caps on all four filling ports.
- Repeat step 3.1 for all chemotaxis chambers. Using a small inverted microscope and a 10x phase-contrast objective lens, inspect the chemotaxis slides for unwanted air bubbles.
- Place the chemotaxis slides seeded with peritoneal cells in a humidity chamber at 37 °C for 2–3 h.
4. Filling the Reservoirs and Adding Chemoattractant
- Inspect the observation area (channel connecting the two 40 µL reservoirs) using an inverted microscope.
NOTE: At this stage, the cell density will be higher than after filling the reservoirs, because weakly adherent cells, predominantly CD19+ cells (B1 cells), will be washed out of the observation area during the filling procedure (Figure 2C–E). - Place plugs into filling ports 1 and 2 (Figure 2D). Ensure that filling port 3 is filled to the top with medium and free of air bubbles. Use a sterile 27 G syringe needle to dislodge unwanted air bubbles, if required.
- Using a 10–100 µL volume mechanical pipette, aspirate ~60 µL of modified RPMI 1640 HEPES medium and place the pipette tip into filling port 3. Use the volume setting ring of the pipette to slowly and steadily inject medium into the reservoir such that medium reaches the top of filling port 4 after 1–2 min (Figure 2D).
- Fill the second reservoir. Move the plug from port 1 and slowly insert it into port 3 (Figure 2D). Next, aspirate ~50 µL of modified RPMI 1640 HEPES medium and place the pipette tip into filling port 4. Use the volume setting ring of the 10–100 µL volume pipette to slowly and steadily inject medium into the second reservoir such that medium reaches the top of filling port 1 after 1–2 min (Figure 2D).
- Place 495 µL of modified RPMI 1640 HEPES medium into a (round bottom) 2 mL microcentrifuge tube and add 5 µL of Patent Blue V (stock solution: 10 mg/mL in phosphate-buffered saline [PBS]), a blue dye used as a visual indicator of concentration gradient formation. Mix by brief vortexing. Add 5.4 µL of recombinant mouse complement C5a (stock solution: 50 µg/mL in PBS with 0.1% bovine serum albumin) and mix by brief vortexing.
- Deposit 15 µL of blue, complement C5a-containing medium into filling port 1 (Figure 3A), after making sure that the shallow depression at the top of the port is medium free (otherwise the drop may spillover).
- Insert a 10–200 µL pipette tip into filling port 4 and slowly and steadily rotate the volume setting ring of the 10–100 µL volume pipette to draw the drop of blue, complement C5a-containing medium into the opposite reservoir (Figure 3B). Air will start to enter the short vertical column of filling port 1. Draw air in until the fluid-air interface is midway in the vertical column, and then slowly insert a plug into the port.
- Gently lift the pipette from port 4, using the other hand to ensure that the slide remains fixed in place. Finally, slowly plug port 4 (Figure 3B).
- Inspect the chemotaxis slide on an inverted microscope.
NOTE: The remaining adherent cells in the observation area should be predominantly macrophages. This can be confirmed using fluorescently tagged anti-F4/80 antibodies (F4/80 is a specific marker for mouse macrophages). B cells can be identified using fluorescently tagged anti-CD19 antibodies and F4/80–/CD19– cells can be detected using a blue fluorescent nucleic acid stain (Figure 4).
5. Imaging Macrophage Migration by Time-lapse, Phase-contrast Microscopy
- Place a chemotaxis slide on the stage of an inverted microscope fitted with a stage incubator. Maintain the temperature at 37 °C.
- Image the 1 mm x 2 mm observation area using a 10x phase-contrast objective lens and focus on the macrophage lamellipodia: thin, sheet-like membrane protrusions. Capture images for 14 h at a rate of 1 frame every 2 min.
6. Analysis of Time-lapse Images
- Analyze the time-lapse, phase-contrast images using automated image analysis software or the Manual Tracking plugin, produced by Fabrice P. Cordelières, for ImageJ.
NOTE: Automated tracking programs can be used to analyze cells imaged by either time-lapse, phase-contrast, or fluorescence microscopy. For example, the Java-based software iTrack4U produced by Cordelières et al.27 can be used for automated cell tracking and analysis using time-lapse, phase-contrast, or fluorescence images as input. Manual tracking is more time consuming, but the tracks generated by the ImageJ plugin Manual Tracking can be directly imported and automatically analyzed by the ImageJ plugin Chemotaxis and Migration Tool28,29.
Representative Results
A schematic diagram of the chemotaxis slide used for time-lapse video microscopy of mouse peritoneal macrophages migrating in a chemotactic gradient is shown in Figure 2A. The slide contains three chemotaxis chambers, each of which has four filling ports. Ports can be individually closed using the plugs shown above the slide. Alternatively, a non-sealing cap can be placed over an unplugged port to maintain sterility. After plugging ports 1 and 4, the observation area (1 mm wide x 2 mm long x 70 µm high channel connecting the two reservoirs) between ports 2 and 3 can be prefilled with medium by placing a 15 µL drop into port 3 and aspirating with a 2–20 µL volume pipette at port 2 (Figure 2B). A suspension of mouse resident peritoneal cells (10 x 106 cells/mL) was seeded into the observation area by placing a 10 µL drop of suspension into port 3 and slowly aspirating at port 2 (Figure 2C). A typical image of cells seeded in the observation area taken by phase-contrast microscopy using a 10x objective lens is shown in Figure 2C. After incubating for 2–3 h, the chemotaxis slide was slowly filled with medium (Figure 2D). After plugging ports 1 and 2, medium was slowly injected via port 3 until it emerged from port 4. Next, the plug was switched from port 1 to port 3, and then the second reservoir was filled by slowly injecting medium via port 4 until it emerged at port 1. At this stage, cells in the observation area were reinspected using an inverted microscope (Figure 2E). By comparing images shortly before (Figure 2C) and after (Figure 2E) filling of the reservoirs, up to two-thirds of the cells had been washed out of the observation area. Generally, weakly adherent CD19+ cells (B1 cells) were washed out and the remaining cells were predominantly F4/80+ cells (macrophages). This was demonstrated by fluorescence microscopy after labeling each cell type with fluorescently labeled specific antibodies (Figure 4). In Figure 4A, freshly isolated mouse resident peritoneal cells were labeled with green fluorescent fluorophore-conjugated anti-F4/80 antibodies and red fluorescent fluorophore-conjugated anti-CD19 antibodies, and the nuclei of cells were labeled with a blue fluorescent nucleic acid stain. F4/80 is a specific marker for mouse macrophages30, whereas CD19 is a B cell marker. Figure 4B shows F4/80+ cells imaged by spinning disk confocal microscopy in the observation area of a chemotaxis chamber. The cells were labeled after an overnight chemotaxis assay recorded by time-lapse, phase-contrast microscopy.
Complement C5a (chemoattractant) was introduced to one of the two reservoirs by placing a 15 µL drop of medium containing 0.54 µg/mL (recombinant mouse) complement C5a and 10 µg/mL Patent Blue V into filling port 1 (Figure 3A) after plugging ports 2 and 3. The chemoattractant medium was slowly drawn into the reservoir by slow aspiration with a pipette via port 4. Figure 3B shows the diffusion of the blue dye after drawing the 15 µL drop into a reservoir. Patent Blue V was used as an indirect visual indicator of chemoattractant diffusion. Complement C5a molecules are considerably larger than those of Patent Blue V (9.0 kDa versus 0.57 kDa) and diffuse more slowly. After diffusion of complement C5a in the reservoir, its concentration was ~0.2 µg/mL (15 µL/40 µL [reservoir volume] x 0.54 µg/mL = 0.2 µg/mL), equivalent to ~22.5 nM. A modestly steep gradient formed across the observation area after 3 h and continued to increase, reaching a maximum at around 12 h31. Figure 3C shows the migration tracks of macrophages migrating in a complement C5a gradient, between 6–12 h after adding the chemoattractant. Cell velocity and chemotactic efficiency, indexed as y-FMI (y-forward migration index; range: -1 to +1) and x-FMI, of individual macrophages was calculated from the migration plots (Figure 3D). Figure 3D also shows a migration plot produced after normalizing the start point of each migration track to X = 0 and Y = 0 below the box plots. The inset in the migration plot shows how the y-FMI was calculated for each migration track.
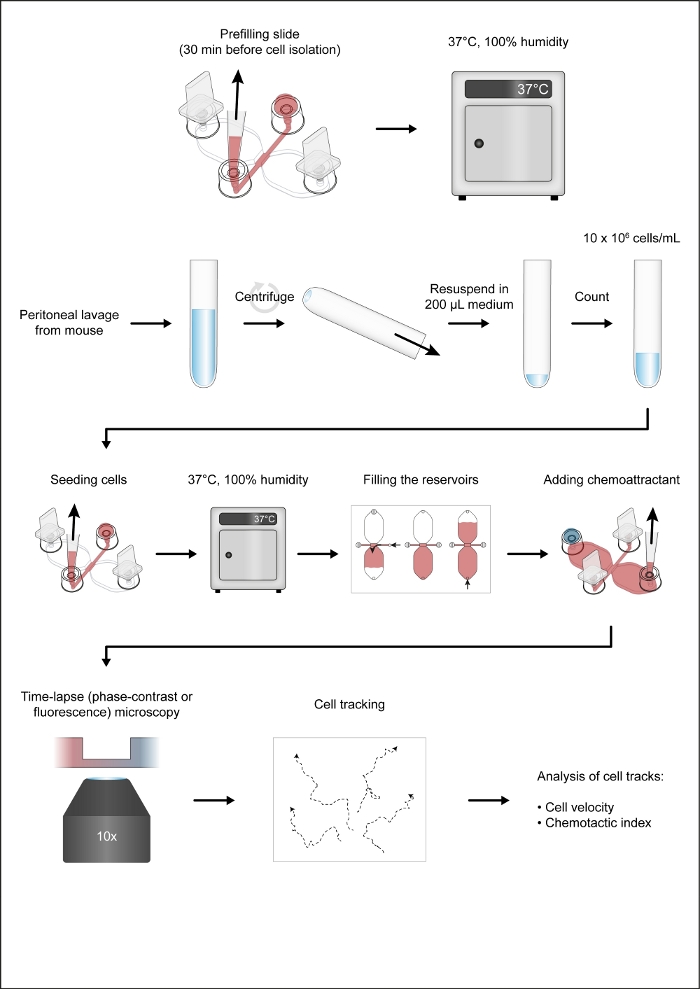
Figure 1: The workflow of the chemotaxis assay. Please click here to view a larger version of this figure.
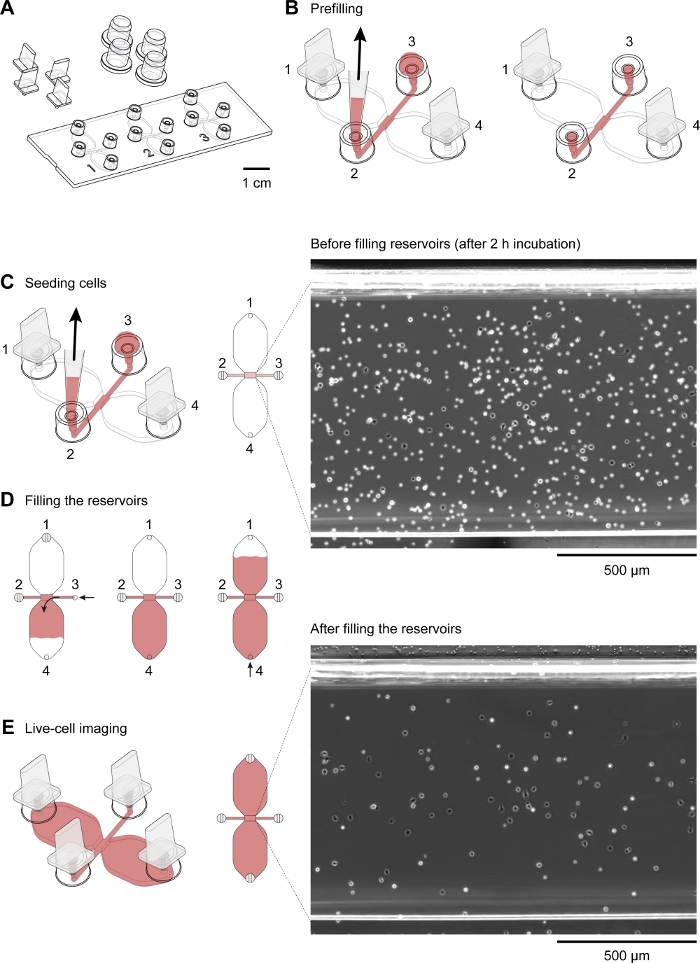
Figure 2: Handling of chemotaxis slides. (A) A 3D view of a chemotaxis slide with four plugs and four caps. The slide contains three chemotaxis chambers, each of which consists of two 40 µL reservoirs connected by a 1 mm x 2 mm channel, which is 70 µm high, termed the observation area. (B) The connecting channel extends at both ends to filling ports 2 and 3. After inserting plugs into filling ports 1 and 4, the observation area was prefilled with medium (red) by applying a drop of medium to port 3 and aspirating at port 2 with a 10–200 µL pipette tip. Subsequently, caps were applied to ports 2 and 3 before incubating the slide at 37 °C and preparing the cell suspension. (C) The observation area, where the chemoattractant gradient formed, was seeded with macrophages by applying a 10 µL drop of mouse resident peritoneal cells at port 3 and slowly aspirating at port 2. The slide was then incubated in a humidity chamber at 37 °C for 2–3 h. The phase-contrast image shown on the right, obtained via a 10x objective lens, shows peritoneal cells after seeding and incubation at 37 °C for 2 h. Scale bar = 500 µm. (D) Chemotaxis chambers were filled with medium by plugging ports 1 and 2 and then slowly injecting medium via port 3 until it emerged at port 4. Slow and steady filling can be achieved by turning the volume setting ring of a 20–100 µL volume pipette. After filling the first reservoir, the second reservoir can be filled by plugging ports 2 and 3 and then slowly injecting medium at port 4 until it emerges at port 1. (E) Phase-contrast image of the same observation area shown above (C) after filling the two reservoirs. Scale bar = 500 µm. Graphic elements provided by Elias Horn. Please click here to view a larger version of this figure.
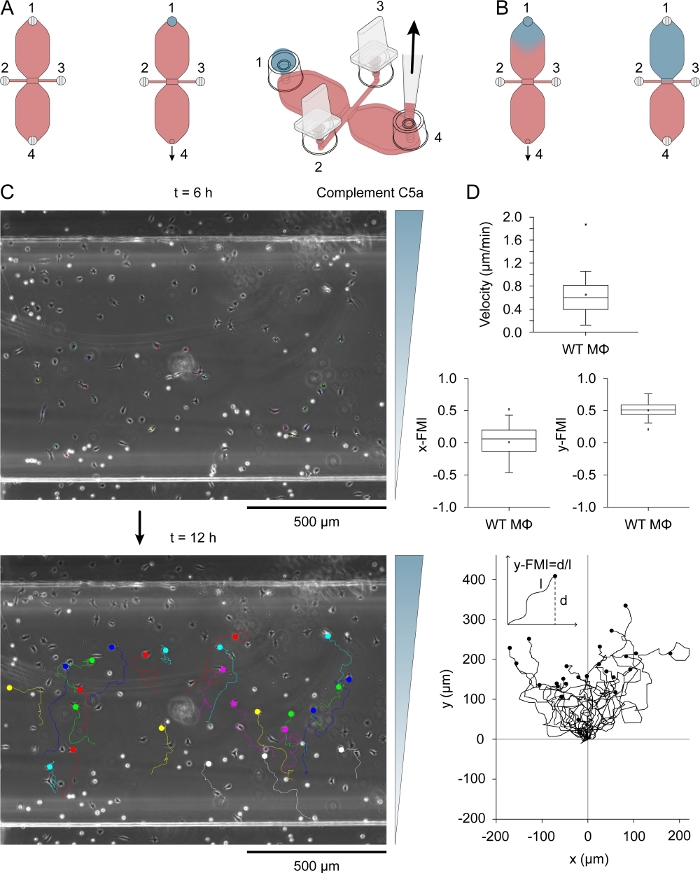
Figure 3: Chemotaxis assay. (A) Chemoattractant was introduced to one of the two reservoirs of a chemotaxis chamber by applying a 15 µL drop of medium containing 0.54 µg/mL complement C5a and 10 µg/mL Patent Blue V to filling port 1, followed by slow aspiration at port 4. (B) Initially after being drawn into the reservoir the blue, chemoattractant-containing medium had a roughly inverted drop shape, and then slowly diffused throughout the reservoir. (C) Migration tracks of macrophages migrating in a chemoattractant (complement C5a) gradient between 6–12 h after introducing chemoattractant to one of the reservoirs. The direction of the gradient is indicated on the right. The end of each migration track is indicated by a filled circle. (D) Blox plots of velocity, x-FMI (x-forward migration index) and y-FMI (y-forward migration index), an index of chemotactic efficiency that ranges from -1 to +1. Data were obtained by analysis of 25 macrophage migration tracks. Macrophages in the lower half of the observation area and showing displacement of at least one cell width over 6 h were randomly selected for analysis. Below is a plot of migration tracks after normalizing the start point to X = 0 and Y = 0. The chemotaxis index (y-FMI) was calculated by dividing the net displacement along the Y-axis (d) by the accumulated length (l) of the migration path, as schematically shown. Graphic elements in panels A and B provided by Elias Horn. Please click here to view a larger version of this figure.
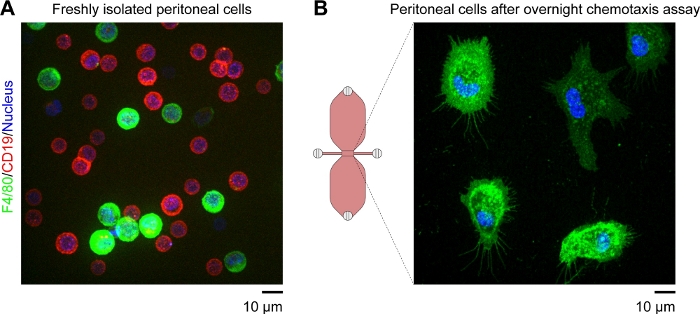
Figure 4: Fluorescent images of living mouse resident peritoneal cells obtained by spinning disk confocal microscopy. (A) Extended focus image (brightest point merge of all Z-planes) of freshly isolated mouse peritoneal cells labeled with green fluorescent anti-F4/80 (macrophage marker) antibodies, red fluorescent anti-CD19 (B cell marker) antibodies, and a blue fluorescent nucleic acid stain. Scale bar = 10 µm. (B) Snapshot (single Z-plane) of F4/80+ cells (macrophages) in the observation area of a chemotaxis chamber taken after an overnight chemotaxis assay. Cells were labeled with green fluorescent anti-F4/80 antibodies and a blue fluorescent nucleic acid stain. The complement C5a and Patent Blue V gradients were washed out by the cell labeling procedure, which explains why the upper reservoir in the schematic diagram of the chemotaxis chamber is not blue. Scale bar = 10 µm. Graphic element provided by Elias Horn. Please click here to view a larger version of this figure.
Discussion
Intravital imaging dates back to the 19th century and provides a means to study the behavior of living immune cells in their natural environment. However, even with today’s advanced microscopy and genetic techniques it is difficult to study the response of cells to specific chemoattractants in vivo. To circumvent this problem, Boyden18 developed Transwell assays in the 1960s, but these end-point assays did not provide visualization of how cells actually migrated towards chemoattractants, making it difficult to distinguish chemokinesis, stimulated random migration by a chemical cue32, and chemotaxis, migration towards higher concentrations of chemical stimuli from each other33. This problem was solved by designing various open chambers with a bridge, typically 1 mm wide, situated between two reservoirs and accessible by an objective lens21,22,23. Applying an inverted cover slip, seeded with adherent cells, closes the chambers and chemoattractant added to one of the reservoirs diffuses across the bridge to the opposing reservoir, creating a concentration gradient. Here we describe a chemotaxis assay using the same principle but using a closed chamber featuring four filling ports. Using this system and time-lapse, phase-contrast microscopy, we developed an assay to image mouse resident peritoneal macrophages migrating in a chemotactic complement C5a gradient31,34,35,36. This assay, combined with knockout mouse models, proved instrumental in the investigation of the roles of various Rho GTPases and motor proteins in macrophage morphology, motility, and chemotaxis31,34,35,36,37. We have also used this approach to image human peripheral blood monocytes migrating on a 2D surface or in a 3D collagen type I matrix38. Moreover, the assay is suitable for mouse bone marrow-derived macrophages or macrophages derived from conditionally immortalized myeloid precursor cells39,40. We have previously used polytetrafluoroethylene (PTFE) bags with luer adapters to culture bone marrow cells and obtain macrophages34. The advantage of PTFE bags is that the cells can be readily resuspended and ready for use after placing the bag on ice for 20–30 min. Note that we prefill the chemotaxis slide observation area before introducing the cells. This approach has the advantage that unwanted air bubbles can be subsequently flushed out (with variable success) and the presoaked observation area enables the slow introduction of a cell suspension by pipetting. Prefilling, though, increases the likelihood that the medium will partially flow into one or both of the flanking reservoirs, which will promote the seeding of cells beyond the observation area. Alternatively, the cell suspension can be directly pipetted into a dry observation area, but unwanted air bubbles cannot be subsequently expelled.
The peritoneal cavity of the mouse contains two main populations of cells: F4/80+ macrophages and (smaller) CD19+ B cells, at a ratio of about 1:2 (Figure 4A). These two cell populations account for over 95% of peritoneal cavity cells, whereas the remaining F4/80–/CD19– cells can usually be identified as CD11c+ cells (dendritic cells) or CD3+ cells (T cells). Weakly adherent B cells are washed out of the observation area during the filling of the reservoirs with the medium (Figure 2). After adding chemoattractant to one of the two reservoirs, time-lapse, phase-contrast microscopy can be used to image the remaining cells (macrophages) migrating in an evolving chemoattractant gradient. The formation of the complement C5a gradient in the observation area, via diffusion from one reservoir to the other, can be simulated using a fluorescent dye with similar molecular weight. A good substitute for recombinant mouse complement C5a (predicted molecular weight, 9.0 kDa) is fluorescently labeled dextran (10 kDa)31. Using confocal microscopy, the fluorescence gradient in the narrow channel (observation area) connecting the two reservoirs of the chemotaxis slide can be measured at fixed intervals and concentration profiles at the different time points can be plotted24,31. We routinely add a nonfluorescent, blue dye (Patent Blue V) to the chemoattractant medium to provide a convenient visual indicator of diffusion and gradient formation. Within 1 h of introducing 15 µL of blue, chemoattractant-containing medium into a reservoir, the reservoir appears uniformly blue and, according to Fick’s laws of diffusion, a gradient will form across the narrow observation area connecting the reservoirs (Figure 3B). Several days are required for the solute (blue dye or chemoattractant) to become uniformly distributed.
Fluorescence microscopy can be substituted for phase-contrast microscopy, which offers advantages for automated cell tracking, because fluorescently labeled cells can be readily distinguished from the background. Another advantage is that specific populations of immune cells can be selectively tracked after labeling surface markers with fluorescent antibodies. We used this approach to image human peripheral blood CD14+ cells (monocytes) migrating in a chemotactic fMLP (N-formylmethionine-leucyl-phenylalanine) gradient38. Similarly, fluorescent anti-F4/80 antibodies could be used to image mouse macrophages migrating in a chemotactic complement C5a gradient. Phototoxicity is a potential disadvantage of using fluorescence imaging41. This can be reduced by various means42, including using fluorophores excited with longer wavelengths and adding antioxidants to the medium. Alternatively, labeled cells could initially be identified by fluorescence microscopy and subsequently imaged by time-lapse, phase-contrast microscopy. However, in practice, cells moving at moderately low velocities, such as ~1 µm/min (macrophages) or ~4 µm/min (monocytes), can be intermittently imaged by fluorescence microscopy at intervals of minutes, which is well tolerated38. We previously used fluorescence microscopy and the chemotaxis slide described here for 3D chemotaxis assays38,43. In this case, both reservoirs were prefilled with medium and 15 µL chemoattractant-containing medium was drawn into one of the reservoirs immediately before slowly pipetting fluorescently labeled cells suspended in medium containing collagen type I into the observation area. The difficult part of this procedure is the handling of collagen type I, which is concentrated in acidic solution. The pH of the collagen solution needs to be neutralized by addition of alkaline solution before mixing the ice-cold collagen solution with the cell suspension. Transfer of the collagen-cell mixture to an incubator at 37 °C will initiate collagen polymerization. During incubation, the slide should be slowly rotated around its long axis so that the cells remain evenly distributed in the X-, Y- and Z-axis directions while the collagen polymerizes into a gel. A related closed chemotaxis slide suitable for 3D chemotaxis assays, with six plugs instead of four plugs, has recently been described29. This system allows the collagen-cell mixture to be introduced into the observation area before independently filling each of the flanking reservoirs, because each reservoir has two filling ports, rather than a single port.
In summary, we describe a real-time chemotaxis assay that allows the visualization of cells navigating in a chemotactic gradient over a period of 6 or more hours. Herein we focus on macrophages, which play major roles in inflammatory diseases but have been underrepresented in real-time chemotaxis assays compared to faster moving cells like neutrophils and Dictyostelium amoebae.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant (HA 3271/3-2) from the DFG (Deutsche Forschungsgemeinschaft).
Materials
| µ-Slide (anodized aluminium) rack | Ibidi, Martinsried, Germany | 80003 | Autoclavable stackable rack for channel slides |
| µ-Slide Chemotaxis 2D (chemotaxis slide) | Ibidi, Martinsried, Germany | 80306 | Slide containing chemotaxis chambers (tissue culture treated) |
| 100x penicillin/streptomycin | Thermo Fisher Scientific | 15140122 | Used as supplement for RPMI 1640 media |
| 10-100 µL pipette with volume control ring | Eppendorf | 3123000047 | Eppendorf Research plus pipette |
| 10-200 µL pipette tips | Greiner Bio-One International | 739261 | Pipette tips with beveled tips (96 pieces per rack: sterile) |
| 14 ml polypropylene round bottom tubes | BD Falcon | 352059 | Used to collect peritoneal cells |
| 14-bit Hamamatsu C9100-50 Electron Multiplying-Charged Couple Device (EM-CCD) peltier-cooled camera | Hamamatsu Photonics Inc., Japan | EM-CCD camera of the spinning disk confocal microscope system | |
| 2-20 µL pipette with volume control ring | Eppendorf | 3123000039 | Eppendorf Research plus pipette |
| 24-G plastic catheter | B Braun Mesungen AG, Germany | 4254503-01 | Used for peritoneal lavage |
| 405 nm solid state laser, 50 mW | Perkin Elmer, Rodgau, Germany | Laser (405 nm) source of spinning disk confocal microscope system | |
| 488 nm solid state laser, 50 mW | Perkin Elmer, Rodgau, Germany | Laser (488 nm) source of spinning disk confocal microscope system | |
| 561 nm solid state laser, 50 mW | Perkin Elmer, Rodgau, Germany | Laser (561 nm) source of spinning disk confocal microscope system | |
| Alexa Fluor 488-conjugated rat (IgG2a) monoclonal (clone BM8) anti-mouse F4/80 antibody | Thermo Fisher Scientific | MF48020 | Mouse macrophage marker and plasma membrane label |
| Alexa Fluor 594-conjugated rat (IgG2a) monoclonal (clone 6D5) anti-mouse CD19 antibody | BioLegend | 115552 | Mouse B cell marker |
| C-Chip disposable (improved Neubauer) hemocytometer | NanoEnTek (distributed by VWR International) | 631-1098 | Used to count cells |
| CSU-X1 spinning disk scanner | Yokogawa Electric Corporation, Japan | Nipkow spinning disk unit | |
| Hank’s buffered salt solution without Ca2+ and Mg2+ | Thermo Fisher Scientific | 14170120 | Used for peritoneal lavage |
| Heat-inactivated fetal bovine serum | Thermo Fisher Scientific | 10082139 | Used as supplement for RPMI 1640 media |
| Hoechst 34580 | Thermo Fisher Scientific | H21486 | Cell permeable, blue fluorescent nucleic acid stain |
| ImageJ (image processing and analysis in Java) | National Institutes of Health (NIH) | Image analysis software | |
| Lipopolysaccharides from Escherichia coliO111:B4 | Sigma-Aldrich | L4391-1MG | Toll-like receptor 4 ligand |
| Nikon Eclipse Ti inverse microscope | Nikon, Japan | Inverted microscope | |
| Patent Blue V, sodium salt | Sigma-Aldrich | 21605-10G | Blue-colored dye used as visual indicator of gradient formation |
| Recombinant mouse complement C5a protein | R&D Systems | 2150-C5-025 | Chemoattractant for mouse macrophages |
| RPMI 1640 medium containing 20 mM Hepes | Sigma-Aldrich | R7388 | Basis medium for assays |
| UltraVIEW Vox 3D live cell imaging system + Volocity software | Perkin Elmer, Rodgau, Germany | Spinning disk confocal microscope system | |
| Zeiss LSM 510 + Axiovision software | Carl Zeiss Microscopy, Oberkochen, Germany | Confocal laser scanning microscope (LSM) adapted for phase-contrast microscopy |
Referências
- Lammermann, T., Germain, R. N. The multiple faces of leukocyte interstitial migration. Seminars in Immunopathology. 36, 227-251 (2014).
- Lammermann, T., Sixt, M. Mechanical modes of ‘amoeboid’ cell migration. Current Opinion in Cell Biology. 21, 636-644 (2009).
- Woodham, E. F., Machesky, L. M. Polarised cell migration: intrinsic and extrinsic drivers. Current Opinion in Cell Biology. 30, 25-32 (2014).
- Devreotes, P. N., et al. Excitable Signal Transduction Networks in Directed Cell Migration. Annual Review of Cell and Developmental Biology. 33, 103-125 (2017).
- Kamp, M. E., Liu, Y., Kortholt, A. Function and Regulation of Heterotrimeric G Proteins during Chemotaxis. International Journal of Molecular Sciences. 17 (1), 90 (2016).
- Miao, Y., et al. Wave patterns organize cellular protrusions and control cortical dynamics. Molecular Systems Biology. 15, 8585 (2019).
- Ridley, A. J., et al. Cell migration: integrating signals from front to back. Science. 302, 1704-1709 (2003).
- Lammermann, T., et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 453, 51-55 (2008).
- Mullins, R. D., Heuser, J. A., Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proceedings of the National Academy of Sciences of the United States of America. 95, 6181-6186 (1998).
- Leithner, A., et al. Diversified actin protrusions promote environmental exploration but are dispensable for locomotion of leukocytes. Nature Cell Biology. 18, 1253-1259 (2016).
- McDonald, B., et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 330, 362-366 (2010).
- Leber, T. Ueber die Entstehung der Entzündung und die Wirkung der entzündungserregenden Schädlichkeiten. Fortschritte der Medizin. 6, 460-464 (1888).
- Tauber, A. I. Metchnikoff and the phagocytosis theory. Nature Reviews Molecular Cell Biology. 4, 897-901 (2003).
- Clark, E. R., Linton Clark, E. Reactions of cells in the tail of amphibian larvae to injected croton oil (aseptic inflammation). American Journal of Anatomy. 27, 221-254 (1920).
- Clark, E. R., Linton Clark, E. The reaction of living cells in the tadpole’s tail toward starch, agar-agar, gelatin, and gum arabic. The Anatomical Record. 24, (1922).
- Comandon, J. Phagocytose in vitro des Hématozoaires du Calfat (enregistrement cinématographique). Comptes Rendus Hebdomadaires des Séances et Mémoires de la Société de Biologie. 69, 314-316 (1917).
- McCutcheon, M. Chemotaxis in leukocytes. Physiological Reviews. 26, 319-336 (1946).
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. The Journal of Experimental Medicine. 115, 453-466 (1962).
- Horwitz, D. A., Garrett, M. A. Use of leukocyte chemotaxis in vitro to assay mediators generated by immune reactions. I. Quantitation of mononuclear and polymorphonuclear leukocyte chemotaxis with polycarbonate (nuclepore) filters. Journal of Immunology. 106, 649-655 (1971).
- Bignold, L. P. A novel polycarbonate (Nuclepore) membrane demonstrates chemotaxis, unaffected by chemokinesis, of polymorphonuclear leukocytes in the Boyden chamber. Journal of Immunological Methods. 105, 275-280 (1987).
- Zigmond, S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. The Journal of Cell Biology. 75, 606-616 (1977).
- Zicha, D., Dunn, G. A., Brown, A. F. A new direct-viewing chemotaxis chamber. Journal of Cell Science. 99, 769-775 (1991).
- Muinonen-Martin, A. J., Veltman, D. M., Kalna, G., Insall, R. H. An improved chamber for direct visualisation of chemotaxis. PLoS One. 5, 15309 (2010).
- Zengel, P., et al. mu-Slide Chemotaxis: a new chamber for long-term chemotaxis studies. BMC Cell Biology. 12, 21 (2011).
- Valentim, A. M., Guedes, S. R., Pereira, A. M., Antunes, L. M. Euthanasia using gaseous agents in laboratory rodents. Lab Animal. 50, 241-253 (2016).
- Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nature Reviews. Neuroscience. 9, 370-386 (2008).
- Cordelieres, F. P., et al. Automated cell tracking and analysis in phase-contrast videos (iTrack4U): development of Java software based on combined mean-shift processes. PLoS One. 8, 81266 (2013).
- Zantl, R., Horn, E. Chemotaxis of slow migrating mammalian cells analysed by video microscopy. Methods in Molecular Biology. 769, 191-203 (2011).
- Biswenger, V., et al. Characterization of EGF-guided MDA-MB-231 cell chemotaxis in vitro using a physiological and highly sensitive assay system. PLoS One. 13, 0203040 (2018).
- Austyn, J. M., Gordon, S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. European Journal of Immunology. 11, 805-815 (1981).
- Hanley, P. J., et al. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proceedings of the National Academy of Sciences of the United States of America. 107, 12145-12150 (2010).
- Wilkinson, P. C. Cell Locomotion and Chemotaxis: Basic Concepts and Methodological Approaches. Methods. 10, 74-81 (1996).
- Pfeffer, W. Locomotorische Richtungsbewegungen durch chemische Reize. Untersuchungen aus dem Botanischen Institut zu Tübingen. 1, 363 (1884).
- Konigs, V., et al. Mouse macrophages completely lacking Rho subfamily GTPases (RhoA, RhoB, and RhoC) have severe lamellipodial retraction defects, but robust chemotactic navigation and altered motility. The Journal of Biological Chemistry. 289, 30772-30784 (2014).
- Horsthemke, M., et al. Multiple roles of filopodial dynamics in particle capture and phagocytosis and phenotypes of Cdc42 and Myo10 deletion. The Journal of Biological Chemistry. 292, 7258-7273 (2017).
- Bachg, A. C., et al. Phenotypic analysis of Myo10 knockout (Myo10(tm2/tm2)) mice lacking full-length (motorized) but not brain-specific headless myosin X. Scientific Reports. 9, 597 (2019).
- Horsthemke, M., et al. A novel isoform of myosin 18A (Myo18Agamma) is an essential sarcomeric protein in mouse heart. The Journal of Biological Chemistry. 294, 7202-7218 (2019).
- Bzymek, R., et al. Real-time two- and three-dimensional imaging of monocyte motility and navigation on planar surfaces and in collagen matrices: roles of Rho. Scientific Reports. 6, 25016 (2016).
- Wang, G. G., et al. Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nature Methods. 3, 287-293 (2006).
- Gran, S., et al. Imaging, myeloid precursor immortalization, and genome editing for defining mechanisms of leukocyte recruitment in vivo. Theranostics. 8, 2407-2423 (2018).
- Magidson, V., Khodjakov, A. Circumventing photodamage in live-cell microscopy. Methods in Cell Biology. 114, 545-560 (2013).
- Icha, J., Weber, M., Waters, J. C., Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays : News and Reviews in Molecular, Cellular and Developmental Biology. 39 (8), 1700003 (2017).
- Isfort, K., et al. Real-time imaging reveals that P2Y2 and P2Y12 receptor agonists are not chemoattractants and macrophage chemotaxis to complement C5a is phosphatidylinositol 3-kinase (PI3K)- and p38 mitogen-activated protein kinase (MAPK)-independent. The Journal of Biological Chemistry. 286, 44776-44787 (2011).

