A Planarian Motility Assay to Gauge the Biomodulating Properties of Natural Products
Summary
Planarian motility is used to gauge the stimulant and withdrawal properties of natural products when compared to the movement of the animals in spring water alone.
Abstract
A straightforward, controllable means of using the non-parasitic planarian, Dugesia tigrina, a free-living aquatic flatworm, to study the stimulant and withdrawal properties of natural products is described. Experimental assays benefitting from unique aspects of planarian physiology have been applied to studies on wound healing, regeneration, and tumorigenesis. In addition, because planarians exhibit sensitivity to a variety of environmental stimuli and are capable of learning and developing conditioned responses, they can be used in behavioral studies examining learning and memory. Planarians possess a basic bilateral symmetry and a central nervous system that uses neurotransmitter systems amenable to studies examining the effects of neuromuscular biomodulators. Consequently, experimental systems monitoring planarian movement and motility have been developed to examine substance addiction and withdrawal. Because planarian motility offers the potential for a sensitive, easily standardized motility assay system to monitor the effect of stimuli, the planarian locomotor velocity (pLmV) test was adapted to monitor both stimulation and withdrawal behaviors by planarians through the determination of the number of grid lines crossed by the animals with time. Here, the technique and its application are demonstrated and explained.
Introduction
The protocol described uses planarian motility to provide a means of assessing the biomodulating effects of natural substances. It was specifically adapted to determine if these substances function as stimulants, and if they then were associated with a measurable withdrawal behavior1. This assay, known as the planarian locomotor velocity (pLmV) test, was first used to test known pharmacological agents2,3. The application of this planarian motility-based assay has since grown in popularity and has been adopted by different laboratories interested in substances other than natural products4,5. For this assay, a planarian is placed in a Petri dish containing spring water or spring water containing a dissolved biomodulator. Because the dish itself is placed on graph paper, the number of grid lines crossed by the animal with time as it moves about the container can be used to determine the rate of movement in each condition. The light/dark test, otherwise referred to as the conditioned place preference test (CPP), is another variation on the theme of monitoring planarian motility, and assesses how quickly the animals respond and migrate to a darkened environment6,7. Video tracking of planarian movements can also be analyzed using computer programs and center of mass (COM) tracking8,9,10,11.
Using the planarian as an animal model for such studies offers several advantages over other animals in that the experimenter can easily control the assay environment. Specifically, starving the planarians before experimentation can prevent their exposure to other nutritional or pharmacological agents that might otherwise confound the results, and the specific biomodulator under investigation can be introduced to the planarians simply by adding it directly to the culture water, thereby standardizing exposure. Since planarians have a nervous system and neurotransmitters that are reminiscent of ‘higher-order’ animals, the physiology and experimental responses of these animals to neuromuscular stimuli are considered biologically relevant to other organisms12,13,14,15,16. Also, because planarians are relatively inexpensive and straightforward to maintain in the laboratory, they offer an accessible biological model for many investigators.
As an experimental animal, planarians are suitable to a wide range of studies. For example, our group, as well as other investigators use planarians to study tumorigenesis17,18,19. Planarians also exhibit a host of response behaviors to chemical, thermal, gravitational, electrical, photo, and magnetic stimuli that have formed the basis of other assay systems. Some of these effects have been used to study learning and memory in these animals20,21,22,23,24,25,26,27. The primary use of the planarian model in the literature at present focuses on the activity of planarian pluripotent stem cells, called neoblasts, and their role in regeneration28,29,30. Thus, adopting the model described here allows for further study using other planarian-based assays to provide a broader understanding of how natural products and other biomodulators affect the organism.
Protocol
1. Planarian husbandry
- Use planarians purchased from a biological supply company or wild-caught if needed. The planarians used in this protocol are Dugesia tigrina, as listed in the supply list. This species is also referred to as Girardia tigrina31. Other aquatic planarian species are also acceptable2,3.
NOTE: The protocol described is geared to animals purchased from a biological supply company. This assay system has not been tested with wild-caught planarians. However, if wild-caught planarians are used, it is recommended that they be habituated to the water used in the experiments, as well as the laboratory environment for at least 1 week before use. - Upon arrival, transfer planarians to plastic food storage containers containing clean spring water and keep the lids ajar.
- Maintain planarians in a darkened environment.
- Feed newly delivered planarians after 24–36 h in their new environment.
- Allow planarians to acclimate to the laboratory at least 1 week prior to experimentation.
- Feed planarians on a regular twice-a-week schedule.
- Allow planarians to feed ad libitum on chopped organic boiled eggs or blended organic beef liver for 1–2 h.
- Place fed planarians into a clean container after feeding.
- Remove the soiled water off the planarians.
- Use a small flat watercolor paintbrush (number 3–6) to transfer food debris and slime adhering to the container from around planarians to a paper towel.
- With fresh spring water and gentle swirling or agitation, dislodge planarians and pour them into a clean container.
- Any planarians remaining adhered to the container can be transferred using a round watercolor paintbrush (number 3–6) or transfer pipette with a wide bore.
- Decant the transfer water.
- Cover the planarians with clean spring water.
- After 24 h, remove the planarians from any expelled food waste by transferring them to a clean container as described above (steps 1.6.2.1–1.6.2.6).
- To clean containers and utensils used for planarian husbandry, do not use soap or detergent. Clean these items by rinsing them well with clean water (tap water is acceptable) and drying them with a clean cloth or paper towel.
2. Preparation of planarians for experiments
- Allow newly delivered planarians to acclimate to their environment at least 1 week prior to experimentation.
- Starve planarians for 5–10 days prior to experimentation.
- Change the culture water at least 1x during the starvation period.
3. Planarian locomotor velocity (pLmV) test: Stimulant behaviors
- Prior to experimentation, ensure starved planarians are fully formed, with a complete and pigmented head and tail.
- Prepare a glass or plastic 10 cm Petri dish and a habituation container for the pLmV test prior to starting the experiment.
- Place a clean 10 cm diameter Petri dish to be used for the pLmV test on prelaminated grid paper (with 0.5 cm squares).
- Add 20 mL of unadulterated spring water for controls, or spring water containing the appropriate concentration of the natural product being tested, to the 10 cm diameter Petri dish to be used for the pLmV test.
- Position a camera (e.g., cell phone or high-resolution camera) above the prepared 10 cm diameter Petri dish to record planarian motility over the grid paper during the experiment. A ring stand is a convenient way to position the camera at a distance that can record the entire view of the 10 cm Petri dish and grid lines.
- Prepare a habituation container with 5–10 mL of unadulterated spring water (controls), or spring water containing the appropriate concentration of the natural product being tested. A container similar to a scintillation vial or small 5 cm Petri dish is suitable.
- Use a small, clean, flat, or round watercolor paintbrush to gently transfer a planarian from the stock container with spring water to the habituation container having 5–10 mL of unadulterated spring water or spring water containing the natural product being tested.
NOTE: When manipulating planarians for the pLmV assay, use a small, clean, flat, or round watercolor paintbrush (number 3–6). When moving planarians, the brush should be placed under the animal to lift it gently. To ensure planarians are not damaged when using the brush, the bristles of the brush should not be splayed out under the animal. Spreading out of the bristles could harm the planarian if it is caught between the fibers of the brush.
NOTE: A wide bore transfer pipette can also be used for transferring the planarian into a clean and dry habituation container.- If using the pipette, remove excess water moved with the planarian from the habituation container using the transfer pipette.
- Carefully add the habituation solution (i.e., spring water for controls or spring water containing the concentration of natural product being tested) to the habituation container containing the planarian.
- Habituation periods will depend on the stimulation dynamics assessed for the natural product being tested. A habituation time of 2 min proved acceptable to detect stimulation in this work1.
- Following the 2 min habituation period use a watercolor paintbrush to gently transfer the planarian to the center of the prepared 10 cm Petri dish for the pLmV stimulation experiment.
- Start the camera to record the movement of the planarian. Record 10–11 min of video.
- Prepare the habituation container and the 10 cm Petri dish for the pLmV experiment with fresh solutions for each planarian.
- Use dedicated pipettes, dishes, containers, and paintbrushes for each experimental concentration of the natural product being tested to avoid inadvertently exposing planarians to the wrong solution during experimentation.
- Because planarians exhibit learned behaviors, each planarian (control or test) should only be used once21,22.
4. Planarian locomotor velocity (pLmV) test: Withdrawal behaviors
- Prior to experimentation, ensure starved planarians are fully formed, with a complete and pigmented head and tail.
- Prepare a 10 cm Petri dish (glass or plastic) for the pLmV experiment, a 5 cm Petri dish (glass or plastic) for rinsing the planarian following habituation, and a habituation container prior to starting the experiment.
- Place a clean 10 cm diameter Petri dish to be used for the pLmV experiment on prelaminated grid paper with 0.5 cm squares).
- Add 20 mL of unadulterated spring water to the 10 cm diameter Petri dish to be used for the pLmV experiment.
- Position a camera above the prepared 10 cm diameter Petri dish as in step 3.2.3 to record planarian motility over the grid paper during the experiment.
- Prepare the planarian rinse container by adding 5 mL of spring water alone to the 5 cm Petri dish.
- Prepare a habituation container with 5–10 mL of unadulterated spring water (controls) or spring water containing the natural product being tested. A container similar to a scintillation vial or small 5 cm Petri dish (glass or plastic) is suitable.
- Use a small, clean, flat, or round watercolor paintbrush to transfer a planarian from spring water to the prepared habituation container having 5–10 mL of unadulterated spring water (controls) or spring water containing the natural product being tested. Gently move the animal from the stock container to the habituation container. Ensure the planarian is not damaged by the brush.
NOTE: When manipulating planarians for the pLmV assay, use a small, clean, flat, or round watercolor paintbrush (number 3–6). When moving planarians, the brush should be placed under the animal to lift it gently. To ensure planarians are not damaged when using the brush, the bristles of the brush should not be splayed out under the animal. Spreading out of the bristles could harm the planarian if it is caught between the fibers of the brush.
NOTE: A wide bore transfer pipette can also be used to transfer the planarian into a clean and dry habituation container.- If using the pipette, excess water moved with the planarian should be removed from the habituation container using the transfer pipette.
- Carefully add the habituation solution (i.e., unadulterated spring water for controls or spring water containing the natural product being tested) to the habituation container containing the planarian.
- Habituation periods for withdrawal will depend on the stimulation dynamics assessed for the natural product being tested; 2–5 min have proven sufficient.
- Following the habituation period use a watercolor paintbrush to gently transfer the planarian to the prepared 5 cm Petri dish containing spring water to rinse off any natural product from the habituation container. Ensure the planarian is not damaged by the brush.
- Immediately transfer the planarian to the center of the prepared 10 cm Petri dish containing spring water for the pLmV withdrawal experiment. Ensure the planarian is not damaged by the brush.
- Start the camera to record the movement of the planarian. Record 10–11 min of video.
- Prepare the habituation container, the rinse container, and the 10 cm Petri dish for the pLmV experiment with fresh solutions for each planarian.
- Use dedicated pipettes, dishes, containers, and paintbrushes for each experimental concentration of the natural product being tested to avoid inadvertently exposing planarians to the wrong solution during experimentation.
- Because planarians exhibit learned behaviors, use each planarian (control or test) only once26,27.
5. Data analysis
- Prepare a data collection table to document the behavior and the motility of planarians as the number of grid lines crossed for each minute during the pLmV run. The table should allow for the accumulated number of lines per minute crossed by the planarian to be documented as well. Include lines for notes and a table of definitions to tally the observation of behaviors during the experimental period, such as ‘wander’ and ‘stop’ (see Discussion).
- Using the video, count the number of full grid lines crossed by the planarian per minute for 10 min, and record that number on the data table. Typical planarian behavior consists of continuous velocity, forward-directed, horizontal movement, with periodic turns, and without stops.
- Begin to time the experiment at the point that the planarian has moved off the paintbrush used to transfer it to the 10 cm Petri dish. Record this start time.
- To determine when the animal crosses a full grid square, focus on the head and score one line when the head fully crosses a square.
- To score a full grid when the worm moves around the edge of the dish, visualize a distance of 0.5 cm by referring to the lines as they extend out from the margins of the dish. If the planarian crosses the corner of a box, refer to the second line crossed to score one grid line. Again, focus on the head to make these determinations.
- Stop the video after each minute to record the data.
- When restarting the video to count the next minute, if the head of the worm was between grid lines when the video was stopped, record the first line crossed as a full box.
- Score the number of grid lines crossed for 10 min.
- If the animal stops moving during a pLmV test and no longer crosses grid lines during the 10 min recording time, document the behavior of the planarian in the behavioral chart (e.g., ‘wander’ or ‘stop’). Animals that cease their forward track during the pLmV assay should instead be taken note of and the data presented as a frequency of the total number of animals exposed to that reagent concentration. Coiling or convulsive behavior (known as a C behavior) preventing any forward movement during the habituation period indicates that the concentration of the natural product is not appropriate for use in a pLmV assay because the pLmV assay is motility-based. C-type behaviors can be analyzed using a different type of analysis (see Discussion).
- If possible, test multiple experimental concentrations of the natural product using at least 9–12 worms on different days and different times of the day if determining the overall effect of the reagent on planarian physiology. However, if researchers endeavor to reduce circadian rhythm-induced variability, experiments can be conducted with constant lighting at a set time of day using worms that are cultured with timed light/dark cycles and set feeding times. Have at least two experimenters involved in the project to allow for the option of having one individual record data, while the second individual counts the grid lines ‘blind’ to the conditions used for data collection. Having different individuals involved in data collection, as well as statistical calculations and analysis, also reduces possible bias.
- Calculate grid line counts for each natural product concentration on each day as relative to control counts for each minute so that data from different days, times, and experimenters can be combined. These data can be averaged and then analyzed using Student’s T-tests. P-values for each test can be assessed per minute compared to the control and between reagent concentrations. ANOVA assessments using data sets derived from different experimental concentrations provide a further method of analysis.
Representative Results
The laboratory set up and preparation of the workspace for running the pLmV assay should be completed before experimentation begins. This includes the preparation of the habituation container, rinse container if needed (for withdrawal experiments), Petri dish over laminated grid paper, and properly placed camera (Figure 1). Once all the videos are taken, it is advisable to use a common datasheet to standardize data collection and presentation between investigators (Figure 2 and Figure 2 Supplement).
The camera set up should allow for a clear view of the planarian and the grid paper to permit an accurate assessment of the progress of the animal for the duration of the experiment (Figure 3A and Figure 3A Supplement). Data collection should include the number of grid lines crossed, as well as the cumulative total number of lines crossed per minute of the experiment (Figure 3B). During pLmV analysis, planarians have a continuous velocity, forward-directed, horizontal movement, with periodic turns, and do not stop. When beginning, the first full grid box crossed should be scored as one, not the first line as determined in the example video. It is important to record the start time of directional movement after the planarian is free of the paintbrush used to transfer it to the pLmV dish, and then follow through for each minute thereafter. If a planarian is part of the way through a box at the minute time, the next grid line crossed after restarting the video should be counted as a full box. When the animal moves around the edge of the dish, refer to the lines as they extend out from the dish to determine a distance of 0.5 cm. When worms encounter the corner of a box, refer to the second line crossed to score one grid line. Always focus on the head to make these determinations. If the planarian begins to cover a tight area, the experimenter should again follow the head of the worm to monitor the distance of a full grid box. An example of this behavior is included in the supplementary video (Figure 3A supplement).
To standardize the results from each trial, the pLmV runs should be calculated and plotted as the number of boxes crossed relative to the progress of the matching control worm (Figure 3B). Each user should be trained to perform the assay and counting grid lines prior to beginning tests using reagents of interest. As a benchmark, using the experimental set up, planarians in spring water typically cover approximately 24 boxes in 3 minutes (Figure 4A; data from 4 users, average of 24.8 ± 4.8). A range of test reagent concentrations should be examined to determine the type of behavioral analysis to be used for each. For pLmV analysis, researchers should determine if the animals display motility when exposed (Figure 4B). Other types of behavioral analyses may be more effective for different types of behaviors (see discussion). Relative to the spring water control, stimulant data will show an increasing number of grid lines crossed as the animal moves through the pLmV container with the desired concentration of the test reagent in spring water, after being habituated in the same concentration of the test reagent. In contrast, withdrawal data will show a decreasing number of grid lines crossed, relative to the spring water control, when the planarian moves through the pLmV container having spring water alone, after being habituated in the desired concentration of the reagent mixed in spring water (Figure 4C and 4D). Notably, overt withdrawal data can lead to grid counts less than those of the controls, as has been observed in drug induced withdrawal data by other groups2,6.
Spring water control planarians will move over grid lines for the duration of the experiment. It is possible, however, for test planarians to cease crossing grid lines during the assay. If this occurs, these data should be highlighted separately from data on worms maintaining their progress over the grid lines. Documenting these data as a percentage of the total number of animals exposed to that particular reagent concentration is an effective way to illustrate the relative frequency of these findings (Figure 5A-C, Figure 5A Supplement and Figure 5B Supplement). Behaviors not preventing the planarians from crossing grid lines should be included in the pLmV analysis, even if these movements impede the steady progress of the animals.

Figure 1: Representative setup for the pLmV assay.
The laboratory space should be prepared prior to beginning the assay. Shown is a typical setup with a 10 cm Petri dish placed over laminated 0.5 cm grid paper. A document camera is positioned such that a clear view of the 10 cm Petri dish can be recorded on a linked computer. However, any camera can be used for recording the progress of the planarians during the experiment including a cell phone camera positioned above the Petri dish using a ring stand. In the background is a 5 cm Petri dish to rinse worms for withdrawal experiments, as well as small white containers used for habituating the planarians. Also, in the background are labeled, dedicated round watercolor paintbrushes, pipettes, and Petri dishes for each product concentration. Please click here to view a larger version of this figure.
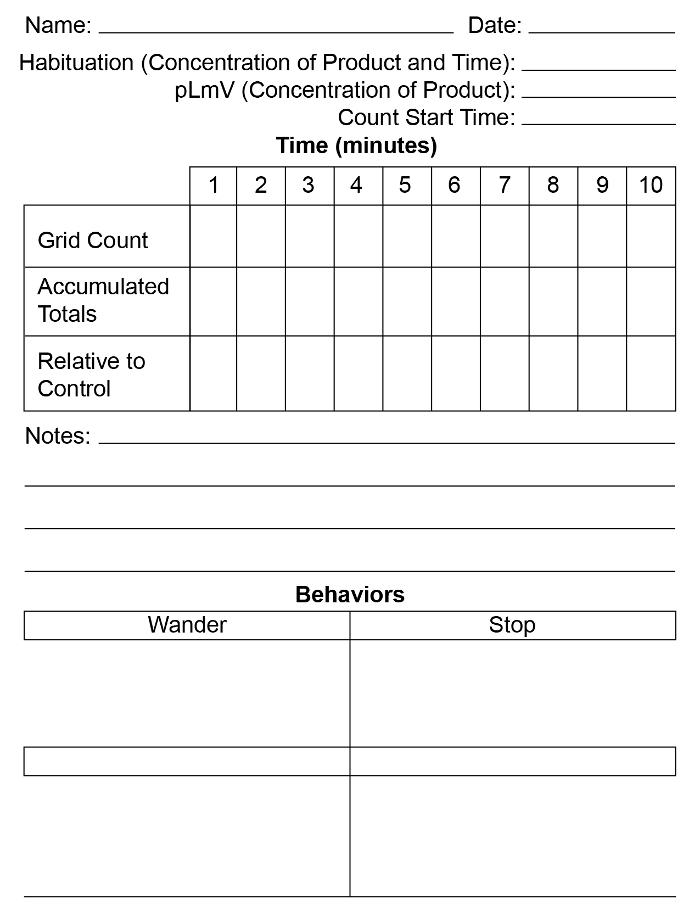
Figure 2: pLmV data sheet.
A prepared data sheet to record the number of grid lines crossed, the corresponding relative to control data, as well as a means to tally any behaviors is useful for data collection purposes. See Figure 2 Supplement for a downloadable PDF version of this figure. Please click here to view a larger version of this figure.
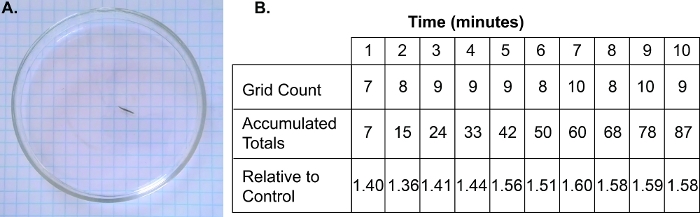
Figure 3: Representative pLmV experiment and data sheet.
(A) A camera should be placed above the 10 cm Petri dish used for the pLmV assay such that the planarian and the full 0.5 cm grid under the dish is clearly visible. The full pLmV run should be recorded (Figure 3A Supplement), and (B) the number of grid lines crossed each minute placed into a prepared data table. The accumulated total grid lines crossed should be tallied, and then these should be converted to the number of accumulated lines crossed relative to the corresponding spring water control worm. The data on the figure table provided (B) matches the counts using the supplementary video (Figure 3A Supplement). Corresponding control counts are not shown. Please click here to view a larger version of this figure.
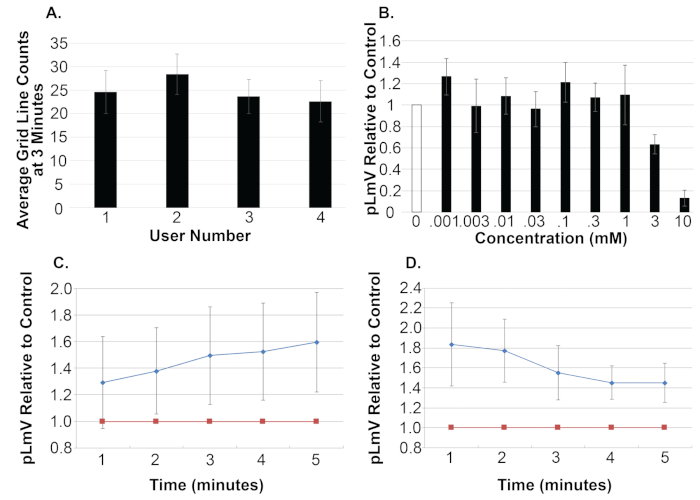
Figure 4: Representative graphs of stimulant and withdrawal data.
Researchers should be trained to use and score the pLmV assay grid lines using spring water alone prior to experimentation. Typically, planarians travel about 25 grid lines in 3 min. (A) Data from four users is shown, each with 10 spring water control worms. To appreciate the effect of a reagent on planarian motility, a series of concentrations are surveyed using the pLmV assay and the total number of grid lines crossed are examined relative to the corresponding control counts at 3 minutes. (B) Control data are represented by the white bar. Test data are represented by the black bars, with the concentrations used in mM. Relative to control data should be plotted to best represent the data collected. (C) Stimulant data represented by the blue line/diamonds, will show a rise in grid lines crossed, versus (D) withdrawal data, also represented by the blue line/diamonds, will show a decreasing slope from an initial start value, relative to the control data, as shown by the red line/squares in both (C and D). Please click here to view a larger version of this figure.

Figure 5: Observation and documentation of behavioral data.
Planarians in the pLmV assay that do not maintain directional movement during the experiment, but stop with characteristic behaviors and no longer move over the grids should be tallied. Typical behaviors may include displays as ‘wander’ (A and Figure 5A Supplement), and ‘stop’ (B and Figure 5B Supplement). These tallies of behavioral data should be presented to document the frequency of these behaviors compared to all the animals exposed to that product concentration (C). Shown are sample data documenting the percentages of spring water control animals (Ci), and animals exposed to a stimulant (Cii), having a wander (blue), stop (red) or no (green) behavior. Please click here to view a larger version of this figure.
Discussion
A straightforward and accessible planarian motility assay is described to determine the stimulant and withdrawal effects of natural products. As a behavioral model it is necessary to have stringent protocols for scoring movement and clear definitions of any behaviors to standardize observations between different experimenters. The ideas presented offer a demonstration of how this can be achieved. Each laboratory using this protocol should adapt the presented information to suit the effects of the particular product being tested. It is recommended to carefully set up the workspace to ensure tests can be done in consistent conditions by each investigator involved in the study (Figure 1). Standardized recording sheets can be compiled to assist with precise record keeping and data collection on motility and behaviors (Figure 2 and Figure 3B).
Motility tests should be done at various times of the day, using different batches of planarians if possible, to account for diverging inherent circadian activities. While these analyses have not been conducted, it is suggested that investigators monitor how the planarian circadian rhythm might affect motility by maintaining planarians using a standard light/dark cycle (e.g., 12/12), and when conducting the pLmV assay, use standard lighting conditions and run the tests at the same time of day32,33,34. Researchers should practice the mechanics of the pLmV assay and learn to count grid lines to standardize both the handling of the planarians and the assessment of planarian motility (Figure 3). This should be done using unadulterated spring water controls. Typically, after 10–20 such practice runs, data becomes quite standard for all laboratory members. Counts after a set pLmV time should be chosen to compare progress in learning the assay. Investigators typically observe approximately 24 grid lines crossed by 3 min in the pLmV assay in spring water following a 2 min habituation in spring water (Figure 4A; representative data for four investigators with 10 random spring water controls each; 24.8 ± 4.8 grid lines). Having two or three trained investigators conducting each set of tests can further improve the reliability of the results because any user-specific effects on the method and counts can be taken into account in the error analysis. In this way, tests and counts can be swapped between investigators so that counts can be done ‘blind’ to the experimental conditions. To decrease the chance of bias, different individuals could conduct statistical tests on the count data and perform the subsequent analyses. Outside of the presence of the product being tested, environmental variations in water quality such as temperature and pH should be avoided. Because planarians are light sensitive, the setup of the experiment should ensure that the lighting of the workspace is even. Finally, because planarians can exhibit learned behaviors, each worm, including controls, should only be used once26,27.
It is useful to have an experimental checklist in the lab if multiple experimenters are working with the assay, particularly in an undergraduate research laboratory, to avoid common pitfalls that might occur when running the pLmV test, which would affect the statistical value of the results. All solutions should be at room temperature because planarian motility is reduced at lower temperatures. Care should be taken to ensure that the specimens are starved 5–10 days prior to their use and that each specimen is fully formed, with a completely pigmented head and tail. It is also suggested that dedicated flat or round watercolor paintbrushes (number 3–6), habituation containers, and Petri dishes be used for each experimental concentration to make the workflow smoother for the experimenter. The technique of transferring the planarians using small flat or round watercolor paintbrushes should be practiced extensively by the investigators to ensure efficient transfer of the worms between containers without causing injury or distress to the animals (see discussion above; Figure 4A). The use of these paintbrushes minimizes the transfer of liquids between the containers and reduces potential stress on the planarians. However, planarians can be damaged by the bristles if the fibers are spread or splayed out when they contact the animal.
Because the pLmV assay relies on behavioral data, it is essential to use a sufficiently large data set to ensure robust data despite innate worm-to-worm response variability. As such, most laboratories use a minimum of nine to twelve planarians to test each concentration of the product being examined, particularly because natural products may not have as marked an effect as standard pharmaceuticals1,2,3,4,5,6,7,13,14,15,16,35,36. The number of grid lines crossed each minute is calculated relative to the spring water control data for each test day and time. These data are averaged for each test concentration for each minute and compared to the control data, as well as to other time-matched concentrations using both Student’s T-tests and ANOVA.
The pLmV assay is geared to studies of reagents affecting the motility of the planarians for the duration of the assay time. Detailed studies of planarian movements can be conducted using a number of other assessments described in the literature4,35,36,37,38. Therefore, it is prudent to conduct a series of habituation experiments to take note of how the planarians react to the test reagent using a range of concentrations and comparing any effects with the behavior of the worms in spring water prior to embarking on behavioral tests. In this way, the appropriate behavioral test can be selected to study the individual mannerisms induced by the reagent on the planarian. The planarians can be placed in various concentrations of the reagent for 5–10 min to determine if a concentration permits them to maintain their typical swimming behavior. Behaviors that do not permit motility, such as those resulting in a C-type, or convulsive or seizure-like behavior, have been the focus of studies using separate types of behavioral analyses that can be applied to this method4,39,40. pLmV analyses can be conducted using a range of concentrations after short habituation times and the number of total grid lines crossed at 3 min scored relative to spring water controls prior to time course stimulation and withdrawal analyses (Figure 4B)1,2,3,4,5. Investigators are encouraged to sample other times, such as 15, 30, and 60 min, to see if the stimulation dynamics change with time of exposure1. As has been reported, in spring water control worms maintain a steady, forward-directed horizontal movement for the 10 min assay time1,4. In contrast, product-treated worms may stop and cease their directional motility during the assay and no longer cross grid lines. The investigator can make a judgment whether to limit the length of the experimental pLmV run or derive a means to assess these behaviors as described. It is important, however, to assess the frequency of these behaviors, as they provide additional data affecting motility. Motility and movement data are discussed separately in the field because combining the information confounds the assessment of such data4,35,36,37,38. By way of an example, two behaviors were observed when the worms stopped and no longer crossed grid lines during the assay. These movements are referred to as ‘wander’ (Figure 5A and Figure 5A Supplement), and ‘stop’ (Figure 5B and Figure 5B Supplement). The frequency of these behaviors are documented as percentages of all the animals exposed to the particular product concentration together with the control data (Figure 5C). Importantly, random behaviors that do not prevent the planarian from crossing grid lines should be included in the pLmV analysis, even if these movements impede the steady progress of the animals (Figure 4B, 3 mM and 10 mM bars). As mentioned, investigators have described a number of behavioral categories that are beyond the scope of this discussion of the pLmV rate of motility protocol4,35,36,37,38.
The pLmV assay relies on the water solubility of the product under investigation. Many of these substances, however, are not fully soluble in water and, as such, only the water-soluble portions can be tested by this assay while the rest must be filtered from the solution as has been done in previous work1. If pLmV assays are run using reagents solubilized using solvents other than water, these require a volume equivalent control in addition to the spring water control. While this method has not been used with such substances, such vector controls should likely be treated as a test, and the motility scored relative to controls as would any other test substance. Another possible means to test non-soluble substances would be to feed them to the planarians by mixing them into food gels. This technique is used to introduce siRNA to planarians in gene knock-down/RNAi experiments41. Feeding planarians biological products and siRNA, however, presents complications to this assay in that the planarians would not be starved and cannot be transferred to the motility assay container within a standardized time that ensures equal exposure or intake of the product under investigation by the experimental animals prior to testing.
Once stimulant and withdrawal dynamics are established using the pLmV assay, further experimentation can involve comodulators, the introduction of siRNA, as well as biological pathway modifiers or drugs to test for the augmentation or inhibition of the observed motility effects compared to the initial results collected when not using these biomodulators1,3. For example, downregulating the expression of a gene or adding pathway inhibitors could decrease the rate of movement, while others may change the dynamics of withdrawal. Through the observation of changed pLmV results, these additional experiments can provide insight into the underlying physiology affected by a natural product or other test reagent5,42,43,44.
The procedure described is amenable to any laboratory interested in determining the stimulant and withdrawal effects of a variety of biomodulators, including many natural products. Advantages of the application of the planarian pLmV assay include how inexpensive and easy to maintain these animals are, and that they can also be subjects for other planarian-based assays to provide a broad understanding of the physiological effects of the product under investigation.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge the office of Institutional Advancement, and the Morrisville College Foundation for a publication grant to support this work, as well as the SUNY Morrisville Collegiate Science and Technology Entry Program (CSTEP) for their ongoing assistance and support of undergraduate research at SUNY Morrisville. We also wish to thank Sophia Hutchens for helpful comments on the technique described.
Materials
| Bottled Water – 1 Gal. | Poland Spring | N/A | Spring water for planarian culture and to prepare solutions |
| Brown Planaria (Dugesia tigrina) | Carolina Biological Supply Company | 132954 | Brown planaria living (other species are acceptable) |
| Flat Paintbrush | Royal Crafter's Choice | 9159 | Flat watercolor paintbrushes for cleaning planarian culture containers |
| Glass Petri Dish – 10 cm | Kimax | N/A | 10 cm diameter (glass) Petri dishes for pLmV assay |
| Glass Petri Dish – 5 cm | Kimax | N/A | 5 cm and Petri dishes for rinsing planarians during withdrawal experiments and for stimulant habituation |
| Grid Paper | Any | N/A | Standard 0.5 cm grid paper for pLmV assay |
| iPEVO Visualizer (software) | iPEVO | https://www.ipevo.com/software/visualizer | Document camera software for video capture and recording |
| Metalware Set with Support Stand and Retort Ring | Any | N/A | Standard chemistry lab ring stand to hold a cell phone camera if used |
| Organic Egg | Any | N/A | Organic egg or beef liver for feeding planarains |
| Polycarbonate Bottle w/ Screw-on Cap – 10 mL | Beckman | N/A | Plastic vials to hold 5 to 10 mL volumes for stimulant habituation |
| Round Storage Container – 10 cm | Ziploc | N/A | 10 cm Round food storage containers for approximately 90 planarians or fewer |
| Round Water Paint Brush | LOEW-Cornell | N/A | Small round watercolor paint brushes (numbers 3 to 6) – soft |
| Transfer Pipette | Any | N/A | Wide bore (5 mL) plastic transfer pipettes to move planarians |
| USB Document Camera | iPEVO | CDVU-06IP | Document camera (or other camera or cell phone camera) |
Referências
- Moustakas, D. Guarana provides additional stimulation over caffeine alone in the planarian model. PloS One. 10 (4), 0123310 (2015).
- Raffa, R. B., Valdez, J. M. Cocaine withdrawal in Planaria. European Journal of Pharmacology. 430 (1), 143-145 (2001).
- Raffa, R. B., Holland, L. J., Schulingkamp, R. J. Quantitative assessment of dopamine D2 antagonist activity using invertebrate (Planaria) locomotion as a functional endpoint. Journal of Pharmacology and Toxicological Methods. 45 (3), 223-226 (2001).
- Thumé, I. S., Frizzo, M. E. Sertraline induces toxicity and behavioral alternations in planarians. Biomedical Research International. 2017, 5792621 (2017).
- Aggarwal, S., et al. Identification of a novel allosteric modulator of the human dopamine transporter. ACS Chemical Neuroscience. 10 (8), 3718-3730 (2019).
- Zhang, C., Tallarida, C. S., Raffa, R. B., Rawls, S. M. Sucrose produces withdrawal and dompamine-sensitive reinforcing effects in planarians. Physiology & Behavior. , 8-13 (2013).
- Zewde, A. M., et al. PLDT (planarian light/dark test): an invertebrate assay to quantify defensive responding and study anxiety-like effects. Journal of Neuroscience Methods. 293, 284-288 (2018).
- Risse, B., Otto, N., Berh, D., Jiang, X., Klämbt, C. FIM Imaging and FIMtrack: two new tools allowing high-throughput and cost effective locomotion analysis. Journal of Visualized Experiments. (94), e52207 (2014).
- Inoue, T., Hoshino, H., Yamashita, T., Shimoyama, S., Agata, K. Planarian shows decision-making behavior in response to multiple stimuli by integrative brain function. Zoological Letters. 1, 7 (2015).
- Hastrom, D., Cochet-Escartin, O., Zhang, S., Khuu, C., Collins, E. M. S. Freshwater planarians as an alternative animal model for neurotoxicology. Toxicological Sciences. 147 (1), 270-285 (2015).
- Risse, B., Berh, D., Otto, N., Klämbt, C., Jiang, X. FIMtrack: an open source tracking and locomotion analysis software for small animals. PLoS One Computational Biology. 13 (5), 100553 (2017).
- Pagán, O. R. Planaria: an animal model that integrates development, regeneration and pharmacology. International Journal of Developmental Biology. 61, 519-529 (2017).
- Palladini, G. A pharmacological study of cocaine activity in planaria. Comparative Biochemistry and Physiology. 115 (1), 41-45 (1996).
- Buttarelli, F. R., Pellicano, C., Pontieri, F. E. Neuropharmacology and behavior in planarians: translation to mammals. Comparative Biochemistry and Physiology Part C. Toxicology & Pharmacology. 147 (4), 399-408 (2008).
- Nishimura, K., et al. Identification of glutamic acid decarboxylase gene and distribution of GABAergeric nervous system in the planarian Dugesia japonica. Neurociência. 153 (4), 1103-1114 (2008).
- Raffa, R. B., Rawls, S. M. . A model for drug action and abuse. , (2008).
- Hall, F., Morita, M., Best, J. B. neoplastic transformation in the planarian: I cocarcinogenesis and histopathology. The Journal of Experimental Zoology. 240 (2), 211-227 (1986).
- Voura, E. B., et al. Planarians as models of cadmium-induced neoplasia provide measurable benchmarks for mechanistic studies. Ecotoxicology and Environmental Safety. 142, 544-554 (2017).
- Van Roten, A., et al. A carcinogenic trigger to study the function of tumor suppressor genes in Schmedtea mediterranea. Disease Models and Mechanisms. 11 (9), 032573 (2018).
- Mason, P. R. Chemo-klino-kinesis in planarian food location. Animal Behaviour. 23 (2), 460-469 (1975).
- Van Huizen, A. V., et al. Weak magnetic fields alter stem cell-mediated growth. Science Advances. 5 (1), 7201 (2019).
- Brown, H. M., Ogden, T. E. The electrical response of the planarian ocellus. Journal of General Physiology. 51 (2), 255-260 (1968).
- Inoue, T., Yamashita, T., Agata, K. Thermosensory signaling by TRPM is processed by brain serotonergic neurons to produce planarian thermotaxis. The Journal of Neuroscience. 34 (47), 15701-15714 (2014).
- Byrne, T. Effects of ethanol on negative phototaxis and motility in brown planarians (Dugesia tigrina). Neuroscience Letters. 685, 102-108 (2018).
- de Sousa, N., et al. Transcriptomic analysis of planarians under simulated microgravity or 8g demonstrates that alteration of gravity induces genomic and cellular alterations that could facilitate tumoral transformation. International Journal of Molecular Sciences. 20 (3), 720 (2019).
- Best, J. B., Rubinstein, I. Maze learning and associated behavior in planaria. Journal of Comparative and Physiological Psychology. 55, 560-566 (1962).
- Shomrat, T., Levin, M. An automated training paradigm reveals long-term memory in planarians and its persistence through head regeneration. The Journal Experimental Biology. 216, 3799-3810 (2013).
- Robarts-Galbraith, R. H., Newmark, P. A. On the organ trail: insights into organ regeneration in the planarian. Current Opinion in Genetics & Development. 32, 37-46 (2015).
- Ivancovic, M., et al. Model systems for regeneration: planarians. Development. 146 (17), 167684 (2019).
- Herath, S., Lobo, D. Cross-inhibition of Turing patterns explains the self-organized regulatory mechanism of planarian fission. Journal of Theoretical Biology. 485, 110042 (2019).
- Itoh, M. T., Shinozawa, T., Sumi, Y. Circadian rhythms of melatonin-synthesizing enzyme activities and melatonin levels in planarians. Brain Research. 830 (1), 165-173 (1999).
- Itoh, M. T., Igarashi, J. Circadian rhythm of serotonin levels in planarians. Neuroreports. 11 (3), 473-476 (2000).
- Hinrichsen, R. D., et al. Photosensitivity and motility in planarian Schmedtea mediterranea vary diurnally. Chronobiology International. 36 (12), 1789-1793 (2019).
- Raffa, R. B., Desai, P. Description and quantification of cocaine withdrawal signs in planaria. Brain Research. 1032 (1-2), 200-202 (2005).
- Pagán, O. R., et al. A cembranoid from tobacco prevents the expression of induced withdrawal behavior in planarian worms. European Journal of Pharmacology. 615 (1-3), 118-124 (2009).
- Rawls, S. M., Patil, T., Yuvasheva, E., Raffa, R. B. First evidence that drugs of abuse produce behavioral sensitization and cross-sensitization in planarians. Behavioural Pharmacology. 21 (4), 301-313 (2010).
- Venturini, G., et al. A pharmacological study of dopaminergic receptors in planaria. Neuropharmacology. 28 (12), 1377-1382 (1989).
- Ouyang, K., et al. Behavioral effects of Spenda, Equal and sucrose: Clues from planarians on sweeteners. Neuroscience Letters. 636, 213-217 (2017).
- Pagán, O. R., Montgomery, E., Deats, S., Bach, D., Baker, D. Evidence of nicotine-induced, curare-sensitive, behavior in planarians. Neurochemical Research. 40 (10), 2087-2090 (2015).
- Shibata, N., Agata, K. RNA interference in planarians: feeding and injection of synthetic dsRNA. Methods in Molecular Biology. 1774, 455-466 (2018).
- Pagán, O. R., et al. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacology Biochemistry and Behavior. 89 (2), 160-170 (2008).
- Vouga, A., et al. Stereochemistry and neuropharmacology of a ‘bath salt’ cathinone: S-enantiomer of mephedrone reduces cocaine-induced reward and withdrawal in invertebrates. Neuropharmacology. 91, 109-116 (2015).
- Chan, J. D., Marchant, J. S. Pharmacological and functional genetic assays to manipulate regeneration of the planarian Dugesia japonica. Journal of Visualized Experiments. (54), e3058 (2011).

