Stability and Structure of Bat Major Histocompatibility Complex Class I with Heterologous β2-Microglobulin
Summary
The protocol describes experimental methods to obtain stable major histocompatibility complex (MHC) class I through potential β2-microglobulin (β2m) substitutions from different species. The structural comparison of MHC I stabilized by homologous and heterologous β2m were investigated.
Abstract
The major histocompatibility complex (MHC) plays a pivotal role in antigen peptide presentation and T cell immune responses against infectious disease and tumor development. The hybrid MHC I complexed with heterologous β2-microglobulin (β2m) substitution from different species can be stabilized in vitro. This is a feasible means to study MHC I of mammals, when the homologous β2m is not available. Meanwhile, it is indicated that mammalian β2m substitution does not significantly affect peptide presentation. However, there is limited summarization regarding the methodology and the technology for the hybrid MHC I complexed with heterologous β2-microglobulin (β2m). Herein, methods to evaluate the feasibility of heterologous β2m substitution in MHC I study are presented. These methods include preparation of expression constructs; purification of inclusion bodies and refolding of the MHC complex; determination of protein thermostability; crystal screening and structure determination. This study provides a recommendation for understanding function and structure of MHC I, and is also significant for T cell response evaluation during infectious disease and tumor immunotherapy.
Introduction
The major histocompatibility complex (MHC) exists in all vertebrates and is a set of genes that determines the cell-mediated immunity to infectious pathogens. MHC class I presents endogenous peptides, such as viral components produced upon virus infection, to T cell receptors (TCR) on the surface of CD8+ T cells to mediate cellular immunity and participate in immune regulation1. A structural study of MHC I binding to peptides provides information regarding peptide binding motifs and presentation features by MHC I molecules, which plays vital roles in evaluation of CD8+ T cell immune responses and vaccine development.
Since the first crystallization and structural determination of MHC I molecular by Bjorkman et al.2, the crystal structure analysis of MHC I molecules has greatly promoted the understanding of how peptides bind to MHC I molecules, and helps to understand the interaction of light chains with heavy chains and peptides. A series of follow-up studies indicated that although the genes encoding the light chain is not associated with the MHC, the light chain is a key protein for the assembly of MHC I molecules3,4. It interacts with the three domains of MHC class I molecules on multiple surfaces. When the light chain is absent, MHC class I molecules cannot be correctly expressed on the surface of antigen-presenting cells and cannot interact with TCR to exert their immunological functions.
MHC I is comprised of a heavy chain (H chain) and light chain (i.e., β2-microglobulin (β2m)), and is assembled through binding to a suitable peptide5. The extracellular segment of the H chain consists of α1, α2 and α3 domains6. The α1 and α2 domains form the peptide binding groove (PBG). The β2m chain acts as a structural subunit of the assembly complex in MHC I, stabilizing the conformation of the complex, and is a molecular chaperone for MHC I H chain folding7,8,9. A series of studies have shown that MHC I H chains from various mammals such as bat (Chiroptera) (Ptal-N*01:01)10, rhesus macaque (Primates) (Mamu-B*17)11 (Mamu-A*01)12 (Mamu-A*02)13, mouse (Rodentia) (H-2Kd)14,15, dog (Carnivora) (DLA-88*50801)16, cattle (Artiodactyla) (BoLA-A11)17 and equine (Perissodactyla) (Eqca-N*00602 and Eqca-N*00601)18 can combine with heterologous β2m (Table 1). These hybrid molecules are often used in structural and functional studies. However, the methodology for the functional and structural study of the hybrid MHC I with heterologous β2m is not yet summarized. Meanwhile, the structural basis for the interchanged β2m between different taxa remain unclear.
Herein, the procedure for MHC I expression, refolding, crystallization, crystal data collection and structure determination are summarized. In addition, potential substitutions of β2m from different species are analyzed through comparing the structural conformation of MHC I stabilized by homologous and heterologous β2m. These methods will be helpful for further MHC I structural study and CD8+ T cell immune response evaluation in cancer and infectious disease.
Protocol
1. Preparation of expression constructs
- Retrieve the sequences of MHC class I genes (including predicted genes) from bats from the NCBI database.
- Retrieve higher mammal MHC I heavy chain sequences from the Immuno Polymorphism Database (IPD) (www.ebi.ac.uk/ipd/mhc) and the UniProt database (www.uniprot.org).
- To obtain soluble MHC complexes, mutagenize the sequences to remove the cytosolic and transmembrane regions.
- Clone the genes encoding the ectodomains of the bat Ptal-N*01:01 (GenBank no. KT98792919) (residues 1–277) and bat β2m (GenBank no. XP_006920478.1) (residues 1–98) into pET-28a vectors (Novagen, Beijing, China), respectively.
- Insert the optimized (for Escherichia coli) sequences were inserted between the NcoI and EcoR1 sites of a modified pET-28a vector.
- Construct human β2m expression plasmids as previously described20.
2. Peptide synthesis
- Peptides prediction
- As described previously10, to screen for peptides that may bind to Ptal-N*01:01, predict candidate peptides using the protein bodies of bat-related viruses hendra virus (HeV). To obtain high affinity peptides based on the structural model from the online server, NetMHCpan 4.0 server (http://www.cbs.dtu.dk/services/NetMHCpan/)21 and Rosetta FlexPepDock22 predicted potential binding in the selected peptide fraction. In addition, other software and databases including BIMAS, the Immune Epitope Database (IEDB), NetCTL 1.2, and SYFPEITHI can also be used, all of them integrating prediction of peptide–MHC class I binding affinity, transporter associated with antigen processing (TAP) transport efficiency, proteasomal C-terminal cleavage and half-time of dissociation of peptide–HLA class I molecules in their readouts.
- To predict high-binding peptides, use both the NetMHCpan and Rosetta FlexPepDock server.
NOTE: Unfortunately, neither produced predictions matched the experimental data. This may indicate that current MHC binding peptide predictions were not suitable for non-human and non-mouse mammals such as bats, which may have a different manner of peptide binding. Therefore, we need to combine various prediction software and experimental results for comprehensive analysis to obtain high affinity peptides.
- Peptides preparation and preservation
- Rather than generating custom peptides, use specialized service providers. Purchase all peptides commercially following peptide prediction tools, which have a higher binding score. The peptide purity was determined to be >95% by HPLC and mass spectrometry.
- Store all purchased peptides as freeze-dried powder at −80 °C and dissolve in dimethyl sulfoxide (DMSO) before use.
3. Purification of inclusion bodies
- Transformation of E. coli
- Transform 10 ng of plasmid containing bat MHC I Ptal-N*01:01 H chain, or human β2m or bat β2m into 100 µL of BL21(DE3) E. coli. Bathe in ice for 30 min.
- Heat shock at 42 °C for 90 s, followed by bathing in ice for 2 min.
- Add 800 µL of lysogeny broth (LB: yeast extract, tryptone and NaCl; see the Table of Materials) to step 3.1.2 and shake at 200 rotations per minute (rpm) on a rocking platform at 37 °C for 20 min.
- Apply 100 µL of bacterial suspension to the plate containing the corresponding antibiotic resistance (ampicillin: 100 ng/mL), which are same as previous constructs.
- Inoculate cultures
- Pick a single recently transformed bacterial clone into 3 mL of LB with antibiotic medium (ampicillin: 100 ng/mL) and culture at 37 °C, while shaking at 200 rpm on a rocking platform to activate the bacteria. Cultures can be inoculated late at night, to be ready for induction the next morning.
- One night in advance, transfer 500 µL of the activated bacterial stock into 50 mL of LB with antibiotic medium (ampicillin: 100 ng/mL) and incubate overnight at 37 °C, shaking at 200 rpm. For convenience when preparing the next inoculate, freeze the remaining activated bacterial stock in 1 mL aliquots (500 μL of culture with 500 μL of 40% glycerol) at −80 °C until the next preparation is needed.
- To generate large amounts of recombinant protein, transfer the bacterial preparation into 2 L of LB with antibiotic medium (ampicillin: 100 ng/mL) at a ratio of 1:100, incubate at 37 °C while shaking at 200 rpm. Culture until an absorbance of 0.6 at 600 nm is achieved. Add 1 mM isopropyl-1-thio-β-D-galactopyranoside (IPTG) (vary the concentration of IPTG for different MHCs, mostly between 0.1 mM-1 mM) to the flask for induction of protein expression.
- Harvest bacteria
- Transfer the bacteria to centrifuge bottles and centrifuge at 5000 x g for 20 min at 4 °C. All steps from now on should be performed at 4 °C.
- Resuspend the bacteria in 60 mL of phosphate buffer saline (PBS) and liberate the expressed recombinant protein by ultrasonic cell disruptor.
NOTE: In general, the program we set on this machine is ultrasonic 6S, interval 12S, 300 W, 99 times).
- Purification of inclusion bodies
- Centrifuge the sonicated bacterial suspension at 12,000 x g for 30 min. Discard the supernatant and resuspend the pellet in an appropriate volume of washing buffer (0.5% Triton-100, 50 mM Tris pH 8.0, 300 mM NaCl, 10 mM EDTA, 10 mM DTT). Repeat this process once more.
- Centrifuge at 12,000 x g for 10–20 min. Discard the supernatant and resuspend the inclusion bodies in resuspension buffer (50 mM Tris pH 8.0, 100 mM NaCl, 10 mM EDTA, 10 mM DTT). Remove a 20 µL sample (inclusion bodies in resuspension buffer) for SDS-PAGE to test the purity of inclusion bodies.
- Centrifuge the remaining preparation at 12,000 x g for 10–20 min. Discard the supernatant. Weigh the pellet containing the inclusion bodies and add dissolution buffer (6 M Gua-HCl, 10% glycerin, 50 mM Tris pH 8.0, 100 mM NaCl, 10 mM EDTA) to a final concentration of 30 mg/mL. Stir slowly using a magnetic stirrer at 4 °C until the inclusion bodies are dissolved in the dissolution buffer.
- Centrifuge 12,000 x g for 10–20 min. Discard the precipitates. Store the inclusion bodies at −20 °C or −80 °C.
NOTE: Before proceeding with the purification of inclusion bodies, confirm that the induction and expression of recombinant protein were successful. Run samples from each culture (taken before and after IPTG induction) on a protein gel; 15% SDS-PAGE (SDS-PAGE concentrated gum: H2O, 30% acrylamide, 1 M Tris-HCl pH 6.8, 10% SDS, 10% APS and TEMED; SDS-PAGE separation gel: H2O, 30% acrylamide, 1.5 M Tris-HCl pH 8.8, 10% SDS, 10% APS and TEMED; see the Table of Materials ) for the β2m, 10% SDS-PAGE for the H chain.
4. Refolding of MHC complex
NOTE: The efficiency of inclusion bodies refolded will affect the yield of protein obtained. Folding competes with polymerization, so it is generally accepted that refolding at low protein concentrations is the most successful method. In this paper, the inclusion bodies concentration is 30 mg/mL.
- Prepare refolding buffer.
- Vary the composition of the refolding buffer depending on the protein. For example, the pH, ionic strength, redox conditions and the presence of ligands will affect the refolding results. The most common is to use Tris or HEPES-based buffers at a neutral pH with the NaCl concentration of 50-500 mM, but this also depends on the target protein. The following is the refolding buffer formula used in this article.
- Prepare the folding buffer with 100 mM Tris-HCl pH 8.0, 400 mM L-arginine, 2 mM EDTA-2Na.
- Cool the buffer to 4 °C in a 250–300 mL flask, and then add 5 mM reduced glutathione (GSH) and 0.5 mM oxidized glutathione (GSSG). Stir slowly using a magnetic stirrer at 4 °C for a further 10–20 min before adding the inclusion bodies and peptides.
- Injection and dilution of MHC H chain and β2m
NOTE: The temperature at which refolding is performed may vary, although generally, in order minimize aggregation, 4 °C is best. We use dilution to refold the proteins.- Using a needle from a 1 mL syringe, inject 1 mL of hβ2m inclusion bodies or bβ2m inclusion bodies into two refolding buffers (1 liter each), respectively. Inject near the vigorously rotating stirring rod to obtain fast and efficient dilution. β2m refolds relatively easy and remains stable even in the absence of the H chain.
- After the β2m has been dissolved in refolding solution, dissolve peptides (5 mg/mL) in DMSO and quickly inject 200 µL into the refolding solution. Stir slowly for 10–20 min before adding the H chain.
- Inject the H chain with the same procedure described above for β2m. The H chain is very unstable; therefore, the order of injection is quite important. Inject 3 mL of H chain inclusion bodies into two different refolding buffers (1 liter each) with hβ2m or bβ2m, respectively. The consecutive injection of small aliquots instead of the single application of the whole amount of protein increases the yield of refolded MHC. Allow refolding to proceed at 4 °C for 8–10 h.
- Concentration of refolded protein
- Use ultrafiltration in a pressurized chamber with 10 kDa MMCO membrane for concentration of the refolding proteins. This method is convenient and can be combined with buffer exchange. Add exchange buffer (20 mM Tris-HCl, 50 mM NaCl pH 8.0) to the chamber and concentrate to a final volume of 30–50 mL.
- Transfer the refolding solution to a centrifuge tube, spin at 12,000 x g for 15 min at 4 °C to remove precipitates.
- Carefully transfer the supernatant and concentrate further to a final volume of ~1 mL.
- Centrifuge at 12,000 xg for 10–20 min. Transfer the supernatant to a sterile tube and purify the proteins using a 10/300 GL size-exclusion column.
- Collect the samples at the peak and analyze them using SDS–PAGE (15% polyacrylamide gel).
- Collect the MHC complex peak and concentrate to a final concentration of 15 mg/mL.
- Dilute the complex to 7.5 mg/mL and 15 mg/mL for crystallization.
5. Crystallization, data collection, and processing
- Perform crystallization of complexed MHC and peptide using the sitting drop vapor diffusion technique.
- Screen the Ptal-N*01:01/peptide complexes with commercial crystal kits (e.g., Crystal Screen kit I/II, Index Screen kit, PEGIon kit I/II, and the PEGRx kit).
- Seal the resulting solution and equilibrate against 100 µL of reservoir solution at 4 or 18 °C.
- Observe the crystal growth in culture for 3 days, 1 week, 2 weeks, 1 month, 3 months and 6 months. Use a microscope to see if each drop in the crystal plate has crystals.
NOTE: Ptal-N*01:01/HeV1(bβ2m) crystals were observed in 0.2 M NaCl, 0.1 M Bis-Tris (pH 5.5), and 25% (w/v) polyethylene glycol 3,350 at a concentration of 7.5 mg/mL. Ptal-N*01:01/HeV1(hβ2m) crystals were observed in 0.1 M HEPES, pH 7.0, 2% w/v polyethylene glycol 3,350 at a concentration of 7.5 mg/mL. - For cryogenic protection, transfer the crystals to a storage solution containing 20% glycerol and then cool rapidly in a 100 K gaseous nitrogen stream.
- Collect X-ray diffraction data from the beamline BL19U of the Shanghai synchrotron radiation facility (Shanghai, China).
6. Structure determination and analyses
- With high-resolution structural data, process and scale the strength of the collection using the Denzo program and the HKL2000 software package (https://hkl-xray.com/)23.
- After choosing a better initial model in the Protein Data Bank (PDB), determine the structures using molecular replacement with the program Phaser MR in CCP424 (http://www.ccp4.ac.uk/). The model used was the structure coordinates with Protein Data Bank (PDB) code 5F1I.
- Use the refined X-ray model for the initial joint refinement. Use Phenix refine alone and joint modes for the X-ray alone and the joint neutron and X-ray refinement, respectively.
- After every round of refinement, manually check the model against Fo − Fc and 2Fo − Fc positive nuclear density maps in Coot25 (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/). This facilitates the proper placement of waters and certain D atoms. All figures of structures were created by PyMOL (http://www.pymol.org/).
Representative Results
Previous work reported that the HeV-derived HeV1 (DFANTFLP) peptide was presented by Ptal-N*01:0110,19. Herein, the binding capacity of this peptide to Ptal-N*01:01 with homologous bat β2m (bβ2m) and heterologous human β2m (hβ2m) (Figure 1C,1D) was evaluated. Crystals with higher resolution were formed, respectively (Figure 1E,1F). A crystal is formed from the Ptal-N*01:01/HeV1 complex, which was formed through renaturation with bβ2m, and the resolution is 2.31 Å. A crystal is formed from the Ptal-N*01:01/HeV1 complex, which was formed through renaturation with hβ2m, and the resolution is 1.6 Å. The Ptal-N*01:01/HeV1 complex was successfully formed through renaturation with both bβ2m and hβ2m (Figure 1C,1D). In this context, we showed that the Ptal-N*01:01/HeV1 complex was not formed without the presence of β2m (Figure 1A) and the H-2Kd that fold correctly through the hβ2m (Figure 1B).
The structures of Ptal-N*01:01/HeV1/bβ2m and Ptal-N*01:01/HeV1/hβ2m were then analyzed. In the Ptal-N*01:01/HeV1/bβ2m structure, residues R3, H31, Q34, D53, W60, Y63 of bβ2m bound to the H chain residues through bottom of the PBG and residues Q8, Y10, R12, N24, Y28, N98, N99 bound to the α3 domain of the H chain. Similar to the Ptal-N*01:01/HeV1/bβ2m complex, in the Ptal-N*01:01/HeV1/hβ2m structure, conserved residues H31, D53, W60, Y63 of hβ2m which correspond to bβ2m, made contact with the bottom of the PBG and conserved residues Q8, Y10, R12, N24 which correspond to bβ2m bound to α3 domain (Figure 2A,2B).
In the overall structures Ptal-N*01:01/HeV1/bβ2m and Ptal-N*01:01/HeV1/hβ2m, the average root-mean-square deviation (RMSD) of residues 1–184 of the H chains (forming the α1α2 PBG) was 0.248 under all Cα atoms superposition (Figure 3A). This finding indicated that there was no difference between these two complexes. The conformations of the similar peptides in the complexes with different β2m were then compared. The structure of the peptide alignment showed that the conformations of HeV1 peptides in these two complexes were quite similar (Figure 3B). In addition, the structures of gp33(KAVYNFATM) presented by H-2Db and complexed with mouse β2m (mβ2m) or hβ2m were aligned. The RMSD of the α1α2 PBG of H-2Db was 0.283 and the overall conformations of peptides in these two structures were also similar (Figure 3C,3D). These data indicate that the β2m substitution between bβ2m and hβ2m, and mβ2m and hβ2m do not affect the conformations of presented peptides.
Sequence alignment showed that the amino acids of β2m from different species are highly conserved (Figure 4). Following analysis of the β2m from different species showed that most of the amino acids of β2m that were forming the hydrogen bonds with the H chain of MHC I were conserved (Figure 4, Table 2). Meanwhile, the diverse residues were also amino acids with similar chemical properties in mammals. However, the key residues involved in β2m binding to the H chain of MHC I showed polymorphisms in chickens, fish and amphibians (Figure 4).
Table 1: The various mammals combine with heterologous β2m. Please click here to download this table.
Table 2: Hydrogen bond interactions between heterologous β2m and heavy chain in MHC I of various species. Please click here to download this table.
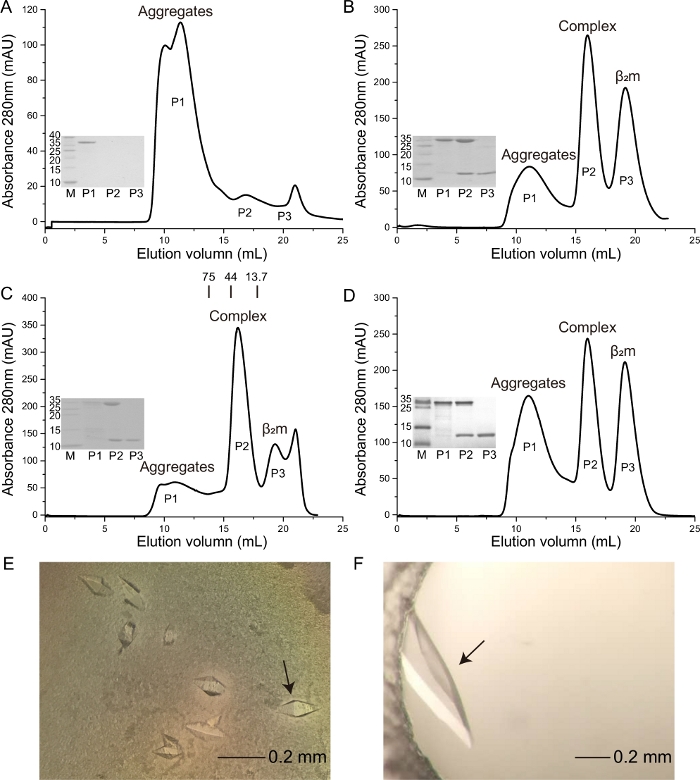
Figure 1: Purification of the soluble and refolded Ptal-N*01:01/HeV1 complex proteins and photographs of the crystal used for diffraction analysis. The M is molecular weight markers in kDa. The P1 is the aggregates. The P2 is the MHC complex. The P3 is the β2m. (A) Ptal-N*01:01/HeV1 complex was not formed without the presence of β2m. (B) H-2Kd complex was formed through renaturation with hβ2m. (C) Ptal-N*01:01/HeV1 complex was formed through renaturation with bβ2m. The profile is marked with the approximate positions of the molecular mass standards of 75.0, 44.0, and 13.7 kDa. (D) Ptal-N*01:01/ HeV1 complex was formed through renaturation with hβ2m. (E) The crystal is formed from Ptal-N*01:01/HeV1 complex, which was formed through renaturation with bβ2m. The black arrow represents the crystal used to collect data during X-ray diffraction. (F) The crystal is formed from Ptal-N*01:01/HeV1 complex which was formed through renaturation with hβ2m. The black arrow represents the crystal used to collect data during X-ray diffraction. Please click here to view a larger version of this figure.
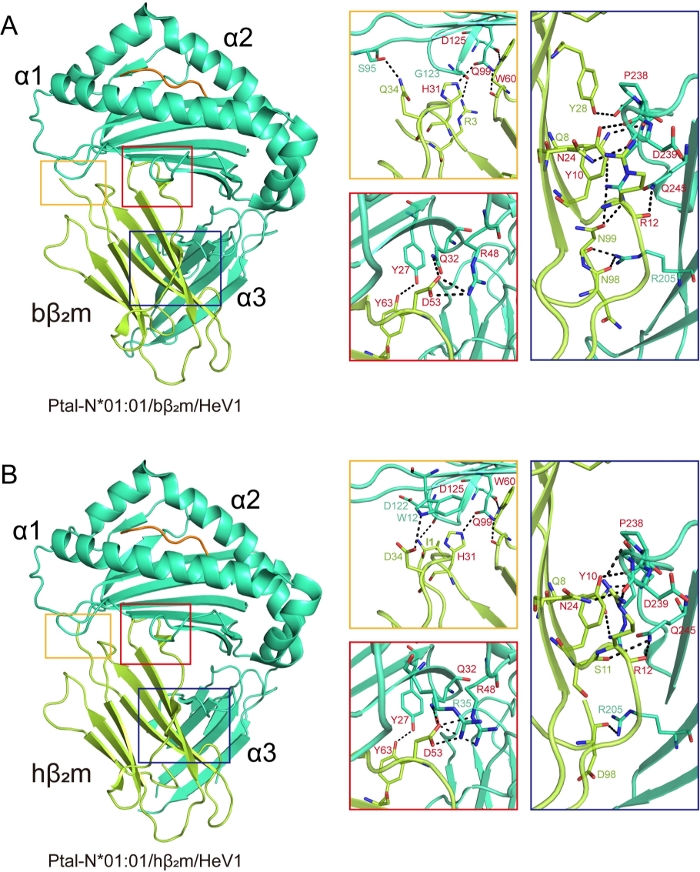
Figure 2: Hydrogen bonding between β2m and heavy chain in hybrid MHC I complexes. Hydrogen bonding between β2m and H chain in (A) Ptal-N*01:01/bβ2m/HeV1 and (B) Ptal-N*01:01/hβ2m/HeV1 MHC complexes. Hydrogen bond interactions are represented by a black dotted line. The square represents the area zoomed in and shown to the right in the corresponding colored boxes. The red represents that the homologous β2m and the heterologous β2m use the same amino acids to form hydrogen bonds with the H chain. Please click here to view a larger version of this figure.
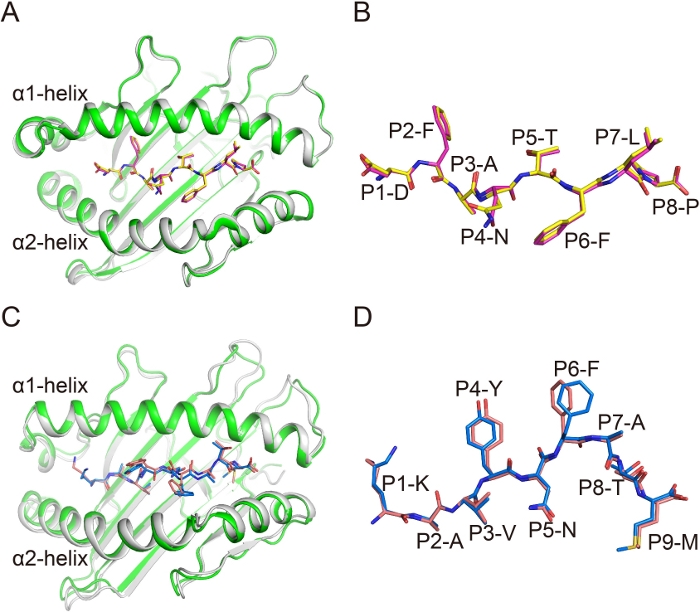
Figure 3: Similar conformation of the MHC complex and the antigenic peptides in hybrid MHC I complexes. (A) The superimposition of α1α2 domains of Ptal-N*01:01/bβ2m (green) and Ptal-N*01:01/hβ2m (gray). (B) The superposition of HeV1 peptide with the superimposition of α1α2 domain of each Ptal-N*01:01 molecule. HeV1 Peptide is represented as pink in Ptal-N*01:01/bβ2m and as yellow in Ptal-N*01:01/hβ2m. (C) The superimposition of α1α2 domains of H-2Db /mouse β2m (mβ2m) (green) and H-2Db /hβ2m (gray). (D) The superposition of gp33 peptide with the superimposition of α1α2 domain of each H-2Db molecule. Peptide gp33 is represented as blue in H-2Db /mβ2m and as pink in H-2Db /hβ2m. Please click here to view a larger version of this figure.
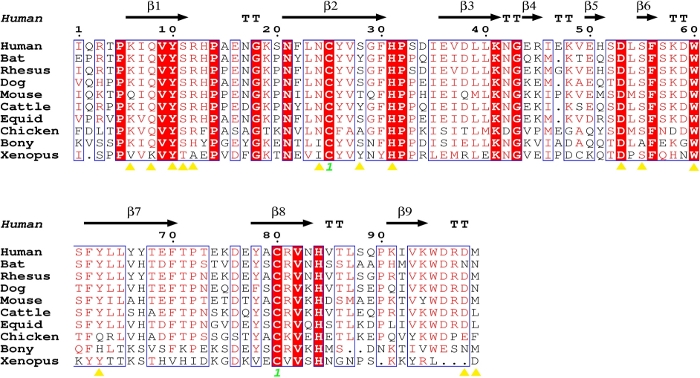
Figure 4: Structure-based sequence alignment of hβ2m with β2m of other species. The black arrows denote β-strands. The residues highlighted in red are completely conserved, and the residues in blue boxes are highly (>80%) conserved. The yellow triangles represent the key amino acids for the interaction between the β2m and H chains. The sequence alignment was generated using Clustal X32 and ESPript33. Please click here to view a larger version of this figure.
Discussion
The construction of a hybrid protein complex through heterologous substitution from different taxa is a common strategy for functional and structural investigations when the homologous complex is not available, such as in the MHC I and its ligands. However, there is limited summarization regarding the methodology and the technology. Herein, the structure of bat MHC I, Ptal-N*01:01, stabilized by bβ2m or hβ2m was analyzed. The key amino acids of β2m binding to Ptal-N*01:01 were found to be conserved between bat and human. Upon further analysis, the key residue involved in β2m binding to the H chain of MHC I was found to be conserved in mammals but polymorphous in chickens, fish and amphibians. These data indicate that heterologous β2m substitution is a feasible means by which to study the MHC I of mammals. However, substitution between mammals and birds, fish or amphibians may not be feasible.
Structural studies play pivotal roles in understanding the molecular mechanisms of peptide presentation by MHC I molecules. Heterologous β2m substitution is commonly used in MHC I structural studies14,16,17,18,26,27,28. Previous work has shown that in bovine MHC I, N*01801, murine β2m and bovine β2m behaved similarly during binding to the α1α2 domains of the N*01801 H chain17. Herein, the structure of a single peptide presented by the same H chain of MHC I but different β2m was analyzed. These data show that the conformations of peptide are similar when stabilized by cross-taxa β2m, thus indicating that heterologous β2m substitution does not affect peptide presentation.
Antigen peptides presented by MHC I are recognized by TCR to mediate T cell activation29. During viral infection, evaluation of antigen-specific T cell immune responses will greatly improve the understanding of viral infections and host immune responses. The peptide-MHC tetramer is an important technique to evaluate T cell responses; it is a technique to tetramerize the MHC monomer molecule, improve its affinity, and combine it with multiple TCRS on T cells. Tetramers are widely used in research and clinical diagnosis14,29,30. Recently, TCR-engineered T cells (TCR-T) have become a hot topic in tumor immunotherapy for their potential efficacy in the treatment of malignant tumors31. MHC I tetramer staining is a crucial method for screening specific TCR binding to cancer-related antigen peptides presented by MHC I molecules29. Therefore, MHC I tetramer preparation plays a vital role in TCR screening. In addition to binding of the MHC I H chain, β2m also binds to CD8 on the T cell surface, which may lead to non-specific staining during TCR screening by MHC I tetramer staining. Heterologous β2m substitution may decrease this non-specific binding of the MHC I tetramer.
However, there are some limitations to the protocol. Firstly, although E. coli remains the dominant expression host for recombinant proteins, many post-translational modifications cannot be performed and the expressed protein products form insoluble inclusion bodies. Then, due to the similar substitution of some non-mammalian β2m with mammalian β2m, it is not known whether non-mammal β2m can be replaced by heterologous β2m to assist and stabilize their MHC structure. In the protocol, we have included a description on our tried analyses using both the NetMHCpan and Rosetta FlexPepDock server. Unfortunately, neither produced predictions matched the experimental data. This may indicate that current MHC binding peptide predictions were not suitable for non-human and non-mouse mammals such as bats, which may have a different manner of peptide binding. Therefore, we need to combine various prediction software and experimental results for comprehensive analysis to obtain high affinity peptides.
In the protocol described here, the key step is that the MHC can be renatured correctly. It is very important to obtain a MHC complex with high refolding efficiency. Therefore, it is crucial to pay attention to select suitable peptides and increase the purity of inclusion bodies.
In conclusion, herein is summarized a protocol for MHC I expression, refolding, crystallization, crystal data collection and structure determination. Furthermore, the feasibility of heterologous β2m substitution in MHC I study was analyzed. This study provides a sound reference for understanding MHC I in structural and functional studies. In addition, these data are also significant for evaluation of T cell responses during infectious disease and tumor immunotherapy.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was supported by the open fund of state key laboratory of Pharmaceutical Biotechnology, Nan-jing University, China (Grant no. KF-GN-201905), the National Natural Science Foundation of China (grants 81971501). William J. Liu is supported by the Excellent Young Scientist Program of the NSFC (81822040) and Beijing New-star Plan of Science and Technology (Z181100006218080).
Materials
| 10 kDa MMCO membrane | Merck millipore | PLGC07610 | |
| 30% Acrylamide | LABLEAD | A3291-500ml*5 | |
| 5×Protein SDS Loading | Novoprotein | PM099-01A | |
| AMICON ULTRA-15 15ML-10 KDa cutoff | Merck millipore | UFC901096 | |
| Ampicillin | Inalco | 1758-9314 | |
| APS | Sigma | A3678-100G | |
| BL21(DE3) strain | TIANGEN | CB105-02 | |
| DMSO | MP | 219605580 | Wear suitable gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| DTT | Solarbio | D1070 | Gloves and goggles should be worn and operated in a ventilated kitchen. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| EDTA-2Na | KeyGEN BioTECH | KGT515500 | |
| Glycerin | HUSHI | 10010618 | |
| GSH | Amresco | 0399-250G | |
| GSSG | Amresco | 0524-100G | |
| Guanidine hydrochloride | Amresco | E424-5KG | |
| hβ2m | our lab | Zhang, S. et al. Structural basis of cross-allele presentation by HLA-A*0301 and HLA-A*1101 revealed by two HIV-derived peptide complexes. Mol Immunol. 49 (1-2), 395-401, (2011). | |
| IPTG | Inalco | 1758-1400 | |
| L-Arginine Hydrochloride | Amresco | 0877-5KG | |
| NaCl | Solarbio | S8210 | |
| Protein Marker | Fermentas | 26614 | |
| SDS | Boao Rui Jing | A112130 | |
| Superdex Increase 200 10/300 GL | GE Healthcare | 28990944 | |
| TEMED | Thermo | 17919 | Gloves and goggles should be worn and operated in a ventilated kitchen. |
| Tris-HCl | Amresco | 0497-5KG | |
| Triton X-100 | Bioruler | RH30056-100mL | |
| Tryptone | Oxoid | LP0042 | |
| Yeast extract | Oxoid | LP0021 |
Referências
- Vyas, J. M., Van der Veen, A. G., Ploegh, H. L. The known unknowns of antigen processing and presentation. Nature Reviews Immunology. 8 (8), 607-618 (2008).
- Bjorkman, P. J., et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 329 (6139), 506-512 (1987).
- Seong, R. H., Clayberger, C. A., Krensky, A. M., Parnes, J. R. Rescue of Daudi cell HLA expression by transfection of the mouse beta 2-microglobulin gene. Journal of Experimental Medicine. 167 (2), 288-299 (1988).
- Zijlstra, M., et al. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 344 (6268), 742-746 (1990).
- Gao, G. F., et al. Crystal structure of the complex between human CD8alpha(alpha) and HLA-A2. Nature. 387 (6633), 630-634 (1997).
- Bjorkman, P. J., Parham, P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annual Review of Biochemistry. 59, 253-288 (1990).
- Achour, A., et al. Structural basis of the differential stability and receptor specificity of H-2Db in complex with murine versus human beta 2-microglobulin. Journal of Molecular Biology. 356 (2), 382-396 (2006).
- Kubota, K. Association of serum beta 2-microglobulin with H-2 class I heavy chains on the surface of mouse cells in culture. Journal of Immunology. 133 (6), 3203-3210 (1984).
- Bernabeu, C., van de Rijn, M., Lerch, P. G., Terhorst, C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 308 (5960), 642-645 (1984).
- Lu, D., et al. Peptide presentation by bat MHC class I provides new insight into the antiviral immunity of bats. PLoS Biology. 17 (9), 3000436 (2019).
- Wu, Y., et al. Structural basis of diverse peptide accommodation by the rhesus macaque MHC class I molecule Mamu-B*17: insights into immune protection from simian immunodeficiency virus. Journal of Immunology. 187 (12), 6382-6392 (2011).
- Chu, F., et al. First glimpse of the peptide presentation by rhesus macaque MHC class I: crystal structures of Mamu-A*01 complexed with two immunogenic SIV epitopes and insights into CTL escape. Journal of Immunology. 178 (2), 944-952 (2007).
- Liu, J., et al. Diverse peptide presentation of rhesus macaque major histocompatibility complex class I Mamu-A*02 revealed by two peptide complex structures and insights into immune escape of simian immunodeficiency virus. Journal of Virology. 85 (14), 7372-7383 (2011).
- Liu, W. J., et al. Protective T cell responses featured by concordant recognition of Middle East respiratory syndrome coronavirus-derived CD8+ T cell epitopes and host MHC. Journal of Immunology. 198 (2), 873-882 (2017).
- Mitaksov, V., Fremont, D. H. Structural definition of the H-2Kd peptide-binding motif. Journal of Biological Chemistry. 281 (15), 10618-10625 (2006).
- Xiao, J., et al. Diversified anchoring features the peptide presentation of DLA-88*50801: first structural insight into domestic dog MHC class I. Journal of Immunology. 197 (6), 2306-2315 (2016).
- Li, X., et al. Two distinct conformations of a rinderpest virus epitope presented by bovine major histocompatibility complex class I N*01801: a host strategy to present featured peptides. Journal of Virology. 85 (12), 6038-6048 (2011).
- Yao, S., et al. Structural illumination of equine MHC class I molecules highlights unconventional epitope presentation manner that is evolved in equine leukocyte antigen alleles. Journal of Immunology. 196 (4), 1943-1954 (2016).
- Wynne, J. W., et al. Characterization of the antigen processing machinery and endogenous peptide presentation of a bat MHC class I molecule. Journal of Immunology. 196 (11), 4468-4476 (2016).
- Zhang, S., et al. Structural basis of cross-allele presentation by HLA-A*0301 and HLA-A*1101 revealed by two HIV-derived peptide complexes. Molecular Immunology. 49 (1-2), 395-401 (2011).
- Hoof, I., et al. NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics. 61 (1), 1-13 (2009).
- Raveh, B., London, N., Zimmerman, L., Schueler-Furman, O. Rosetta FlexPepDock ab-initio: simultaneous folding, docking and refinement of peptides onto their receptors. PLoS One. 6 (4), 18934 (2011).
- Otwinowski, Z., Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods in Enzymology. 276, 307-326 (1997).
- Brunger, A. T., et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallographica Section D: Biological Crystallography. 54, 905-921 (1998).
- Emsley, P., Lohkamp, B., Scott, W. G., Cowtan, K. Features and development of Coot. Acta Crystallographica Section D: Biological Crystallography. 66, 486-501 (2010).
- Glithero, A., et al. Crystal structures of two H-2Db/glycopeptide complexes suggest a molecular basis for CTL cross-reactivity. Immunity. 10 (1), 63-74 (1999).
- Tungatt, K., et al. Induction of influenza-specific local CD8 T-cells in the respiratory tract after aerosol delivery of vaccine antigen or virus in the Babraham inbred pig. PLoS Pathogens. 14 (5), 1007017 (2018).
- McCoy, W. H. t., Wang, X., Yokoyama, W. M., Hansen, T. H., Fremont, D. H. Structural mechanism of ER retrieval of MHC class I by cowpox. PLoS Biology. 10 (11), 1001432 (2012).
- Altman, J. D., et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 274 (5284), 94-96 (1996).
- Zhao, M., et al. Heterosubtypic protections against human-infecting avian influenza viruses correlate to biased cross-T-cell responses. mBio. 9 (4), (2018).
- Zhao, L., Cao, Y. J. Engineered T cell therapy for cancer in the clinic. Search Results. 10, 2250 (2019).
- Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., Higgins, D. G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 25 (24), 4876-4882 (1997).
- Gouet, P., Robert, X., Courcelle, E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Research. 31 (13), 3320-3323 (2003).

