Dynamic Measurement and Imaging of Capillaries, Arterioles, and Pericytes in Mouse Heart
Summary
Presented here is a protocol to study the coronary microcirculation in living murine heart tissue by ex vivo monitoring of the arterial perfusion pressure and flow that maintains the pressure, as well as vascular tree components including the capillary beds and pericytes, as the septal artery is cannulated and pressurized.
Abstract
Coronary arterial tone along with the opening or closing of the capillaries largely determine the blood flow to cardiomyocytes at constant perfusion pressure. However, it is difficult to monitor the dynamic changes of the coronary arterioles and the capillaries in the whole heart, primarily due to its motion and non-stop beating. Here we describe a method that enables monitoring of arterial perfusion rate, pressure and the diameter changes of the arterioles and capillaries in mouse right ventricular papillary muscles. The mouse septal artery is cannulated and perfused at a constant flow or pressure with the other dynamically measured. After perfusion with a fluorescently labeled lectin (e.g., Alexa Fluor-488 or -633 labeled Wheat-Germ Agglutinin, WGA), the arterioles and capillaries (and other vessels) in right ventricle papillary muscle and septum could be readily imaged. The vessel-diameter changes could then be measured in the presence or absence of heart contractions. When genetically encoded fluorescent proteins were expressed, specific features could be monitored. For examples, pericytes were visualized in mouse hearts that expressed NG2-DsRed. This method has provided a useful platform to study the physiological functions of capillary pericytes in heart. It is also suitable for studying the effect of reagents on the blood flow in heart by measuring the vascular/capillary diameter and the arterial luminal pressure simultaneously. This preparation, combined with a state-of-the-art optic imaging system, allows one to study the blood flow and its control at cellular and molecular level in the heart under near-physiological conditions.
Introduction
Appropriate coronary pressure-flow regulation assures sufficient blood supply to the heart to meet its metabolic demands1. However, it has only recently become clear how coronary pressure-flow is dynamically regulated in heart, despite extensive studies that have been performed in vivo and in vitro for the past decades. One of the reasons is the difficulty in establishing a physiological working model for such studies due to the constant beating of the heart. Regardless, a variety of methods have been established for the observation of the coronary micro-vessels in living tissues or animals, but none of these methods were able to achieve constant/stable focus and the measurements of the pressure, flow and microvascular diameter at the same time2,3. The direct visualization of coronary arterial micro-vessels in beating heart was introduced decades ago4,3, but the diameter measurements in small vessels was challenging and the specific functions of the many specialized cell types associated with the microcirculation was equally vexing. Even the stroboscopic method and the floating objective system could not provide the above information simultaneously5. Nevertheless, a significant amount of valuable information has been obtained using the aforementioned technologies, which have helped us understanding more about the regulation of coronary blood flow6. The method we are describing in this paper will help one investigate and understand in detail how components of coronary arteries, the arterioles and the microvasculature respond differently to stimulations and metabolic demands.
The working model we established to pursue these studies was built on the previous work of Westerhof et al.2. Following cannulation of the septal artery of the mouse heart, physiological saline solution was used to perfuse that artery to keep the myocytes and other components of the heart tissue nourished. The arterial perfusion pressure, the flow and the vascular diameter was monitored among other physiological functions using appropriate fluorescent indicators. This method enables us to visualize the coronary microvascular bed under physiological pressure in living tissue and study the cellular mechanisms underlying microcirculation regulation for the first time.
Protocol
All animal care was in accordance with the guidelines of the University of Maryland Baltimore and the Institutional Animal Care and Use Committee approved protocols.
1. Preparation of the solutions
NOTE: Prepare solutions in advance. Two types of basic solutions are used in the experiments: (1) physiological saline solutions (PSS) for bath superfusate and (2) Tyrode’s solutions for lumen perfusate. Continuous bubbling with CO2 is needed to maintain the pH of PSS. HEPES-buffered Tyrode’s solution is used in the lumen instead of PSS to avoid bubbles going into the vessels, since bubbles would damage the endothelial cells7 and occlude the flow.
- PSS solution: See the formula and composition of PSS solution in the Table 1. Make 10 L of Ca2+-free and glucose-free PSS stock solution and store at room temperature. 1 h before any procedure, take out 1 L of the stock solution and bubble with 5% CO2 (74% N2 and 21% O2) to keep the pH of solution at ~7.4. Add 1.8 g glucose and 1.8 mL CaCl2 (1 M stock)8.
NOTE: Add Ca2+ only after bubbling when pH is ~7.4, otherwise, Ca2+ will be precipitated. - Tyrode’s solution: See the formula and composition of the Tyrode’s solution in Table 1. Filter the solution (0.22 μm) and stored at 4 °C for future use9.
- Tyrode’s solution with BDM (2,3-Butanedione monoxime): Add BDM as a reversible myofilament inhibitor to prevent the contraction of the myocytes10. Weigh 600 mg BDM and add to 200 mL of Tyrode’s solution. Stir the solution with BDM until it is completely dissolved. No further filtration is required.
- Store the Tyrode’s solution containing BDM at 4 °C for future use.
2. Chamber preparation
- Coat both dissecting chamber and experimental chamber in advance with polydimethylsiloxane (PDMS) following the instructions provided by the manufacturer.
- Fill the dissecting chamber with the Tyrode’s solution containing BDM.
- Turn on the chiller 1 h before the procedure. Set the temperature at 4 °C.
3. Cannula preparation
- Turn on the micropipette puller. Select the settings as per the manufacturer’s instructions.
- Take a clean borosilicate glass tube (the outer and inner diameter of the glass tubes used was 1.2 mm and 0.69 mm respectively).
- Insert a glass tube into the grooved clamps of a Sutter pipette puller with a U-shaped platinum heating filament.
- Activate the puller to produce two cannulae, each with a long thin tip.
- Cut the tip of each cannula using a fine pair of scissors under a dissecting microscope to make the final diameter of the tip around 100 to 150 µm.
- Fire polish the tip of the cut cannula to slightly round the sharp edges.
- Bend the cannula along the shaft using a platinum wire (heated with fine control) positioned on the side of the shaft approximately 2 mm from tip. The angle of the bend in the cannula should be around 45°.
- Insert the open end of the cannula into the holder of the pressure myograph chamber and tighten the fitting. Connect a 5 mL syringe filled with Tyrode's solution to the other side of the fitting. Connect the cannula to a micromanipulator mounted on the chamber (see Figure 1 and Figure 2).
- Fill the cannula with Tyrode’s solution using the syringe (5 mL), flushing it, the tubing and connector of air bubbles.
- Prior to the cannulation procedure, measure the perfusion pressure inside the cannula over a given range of flow (50 to 300 µL/min, Figure 3). Do so only when a new cannula is used.
NOTE: The perfusion pressure inside the cannula is proportional to the flow and is linear fitted (Figure 3).
4. Extraction of mouse heart
- Put a C57BL/6 mouse (either sex, 8-16 weeks old) in the anesthesia box that is connected to the tanks of isoflurane and oxygen.
- Turn on oxygen tank and start the flow of isoflurane. Wait ~5 min until the mouse is fully anesthetized.
- After anesthesia is established, take the mouse out of the anesthesia box and inject heparin IP (into the peritoneal cavity, 360 units, 0.5 mL/mouse) to avoid blood clot formation in the vasculature. Then put the mouse back into the anesthesia box.
- 10 min after the heparin has been injected, move the mouse to the heated bed in a supine position and stabilize its paws using labeling tape. Keep the mouse under full anesthesia with isoflurane using a nose cone.
- Lift the abdominal skin of the mouse above the diaphragm with forceps and use surgical scissors to cut and expose the diaphragm.
- Cut the diaphragm and the sternum. Open the chest.
- Dissect the heart out of the thoracic cavity, cutting as close as possible to the dorsal thoracic wall.
- Place the heart into ice-cold Tyrode’s solution containing 30 mM BDM.
- Clean the heart to remove the connective tissues such as lung.
- Transfer the heart into the pre-chilled dissecting chamber filled with Tyrode’s solution containing 30 mM BDM.
5. Preparation and cannulation of septal artery
- Turn on the servo pump. Set the servo pump to “flow” mode. Let it run at high speed until the tubing is filled with the Tyrode's solution that will be used to perfuse the vascular lumen. Make sure there are no bubbles in the tubing. Then lower the speed.
- Pin the heart onto the PDMS, avoiding any damage to the middle area of the heart.
- Remove both the right and left atria. Cut open the right ventricle and remove the right ventricular free wall using a dissecting microscope.
- Expose and identify the septal artery. Place a nylon thread (30 µm diameter) under the septal artery and tie a loose knot for future use.
NOTE: The septal artery can be easily identified using a dissecting microscope. The septal artery is a major artery on the septum originating from the right coronary artery in most cases. - Remove the left ventricular free wall using fine scissors.
- Transfer the papillary muscle preparation into the experimental chamber in which the cannula was positioned. The chamber is filled with Tyrode’s solution containing BDM. The bottom of this chamber is coated with a thin (~2 mm) layer of PDMS.
- Adjust the position of the cannula (using the micromanipulator) to enable cannulation of the septal artery. Tighten the knot.
- Secure the papillary muscle using small pins to the chamber floor so that the microscope objective has a clear view of the vasculature. Pay attention to the angle and the position of the cannula and ensure that the tip of the cannula is paralleled to the arterial wall.
- Test cannulation by gradually expelling the solution from the syringe through the cannula. If all elements function properly, residual blood in the papillary muscle will exit the tissue at the beginning of this process and only Tyrode's solution will be seen later.
6. Stabilization of the preparation
- Turn on the peristaltic pump that will provide the superfusion solution. Then constantly superfuse the preparation in the bath with pre-gassed PSS at the rate of 3-4 mL/min.
- Turn on the temperature controller for the superfusion solution. Adjust the temperature of the bath superfusate to ~35-37 °C.
- Connect the cannula to the servo pump. Read the pressure displayed on the pressure servo controller.
- Monitor the flow and pressure. The flow (µL/min) and arterial perfusion pressure (mm Hg) is digitized using digitizer and recorded using an associated software (Figure 2).
- Adjust the flow to set the pressure ~10 mmHg and let it run for ~10 min.
- Increase the flow of the luminal solution to make the pressure of artery ~60 mmHg. Obtain the final perfusion pressure of the arteriole itself by subtracting the pressure drop of the cannula (Figure 3).
NOTE: If the “pressure” mode on pressure servo controller is selected, the luminal pressure is set ~60 mmHg for these experiments. Then monitor the change of the flow. - Let the sample stabilize for ~30-60 min by monitoring the flow and/or pressure levels. A gradual increase of arterial pressure is normally observed at the beginning of the perfusion after cannulation. A new steady state will get establish by itself in about 30 min (Figure 4).
- Initiate an experiment after the perfusion pressure is stabilized (at constant flow).
7. Loading the preparation with fluorescently tagged wheat-germ agglutinin (WGA)
- Prepare Tyrode's solution containing Alexa-Fluor-488 conjugated WGA by adding 100 μg of conjugated WGA into 5 mL of Tyrode's solution.
- Carefully switch the perfusate from normal Tyrode's solution to one containing fluorescently tagged WGA. It is important to carefully switch the perfusate so that no bubbles are introduced.
- After ~30 min of WGA perfusion or when WGA solution is about to run out, change the perfusate back to normal Tyrode’s solution.
8. Confocal imaging of arterioles and capillaries
- Turn on the Nipkow disk confocal microscope system.
- Use the microscope to locate the septal artery using a low power objective (4x) in transmitted light mode.
NOTE: Septal artery can be located by following the position of the cannula. - Start confocal imaging with the spinning disk confocal and choose 488 nm excitation laser, 40x objective magnification and adjust the laser intensity and the sampling rate (10 – 500 ms per image).
- Set up top and bottom imaging positions for the confocal microscope to define the imaging range and enter the parameters for the z-stack imaging.
- Set the step size as recommended for the system that is being used or set the step size as desired.
- Choose the z-stack setting and/or time series settings.
- Select or set a new folder to store the new image.
- Click “Run” to start recording imaging for future analysis (Figure 5).
9. Example vasodilator experiment: pinacidil-induced vasodilation (Video 1).
- Prepare pinacidil stock solution (100 mM in DMSO) and stored at 4 °C.
- Prepare 10 mL of Tyrode’s solution. Add 10 µL of pinacidil stock solution to make the final concentration of pinacidil 100 µM.
- Image the papillary muscle at low magnification (4x objective) to include the septal artery and the branch arterioles.
- Switch luminal solution to the pinacidil containing solution.
10. Example blood flow control experiments: vasoconstrictor-induced arterial perfusion pressure increase at constant flow (Figure 6)
- Prepare endothelin-1 (ET-1) stock solution (10 µM) and stored at -20 °C.
- Prepare 10 mL of Tyrode’s solution. Add 10 µL of ET-1 stock solution (10 µM) to make the final concentration of ET-1 10 nM.
- Record the luminal pressure with constant flow and image the papillary muscle.
- Switch luminal solution to the ET-1 containing solution.
11. Example images of capillary with pericytes (Figure 7)
- Sacrifice an NG2DsRedBAC transgenic mouse as described in section 4 of this protocol.
- Extract the heart, cannulate the septal artery and load the sample with Alexa-Fluor-488 conjugated WGA following protocols 5-7.
- Image the papillary muscle at 488 nm and 560 nm excitation, and 510-525 nm, 575-625 nm emission using confocal. The arteriole pressure will be around 40 mmHg.
Representative Results
When a fluorescence vascular marker is perfused in vascular lumen (here WGA conjugated with Alexa Fluor-488), it is possible to visualize whole vascular trees as shown in Figure 5 (Left panel) using high-speed confocal microscope. Further magnification enables the imaging of capillary in detail (Figure 5, Right Panel). Since the pressurized system supports a constant monitoring of luminal pressure, this preparation can be used for associate changes in arterial diameter with arterial pressure. Video 1 shows that when pinacidil, an ATP-sensitive K+ channel (KATP) agonist was served from lumen, the diameter of the arterioles was increased. Figure 6 shows that when vasoconstrictor ET-1 was applied from the lumen, the diameter of the arteriole was decreased, and the luminal pressure was increased when the flow was set constant.
Due to high-resolution capabilities of confocal microscopy in combination with specific cell markers, this procedure can also be used to visualize many other cells types associated with the microcirculation. Here, we used a mouse (NG2DsRedBAC transgenic mouse) that expresses DsRed fluorescence protein under a pericyte specific promoter (NG2) and labeled the vessels with WGA-Alexa Fluor 488. This allows us to image simultaneously both the capillary (green) and pericytes (red) in the mouse papillary muscle (Figure 7) under conditions that better mimic physiology in live animals.
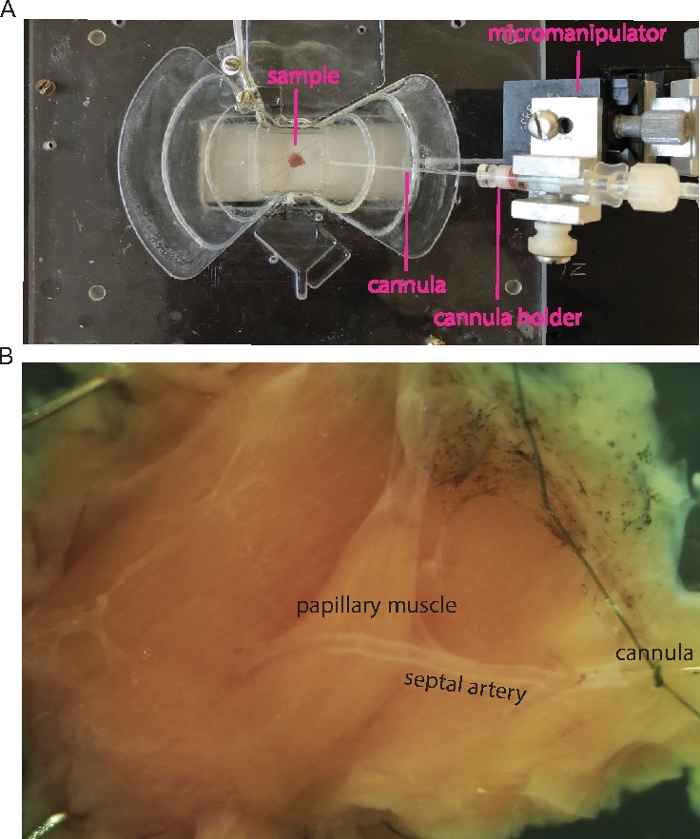
Figure 1: Cannulation of the mouse septal artery.
(A) Transmitted light image shows an example of the cannulated papillary muscle. Micromanipulator, cannula and sample (septum with right ventricle papillary muscle) are indicated as labels. (B) Zoomed-in sample in A shows the papillary muscle and the cannulated septal artery. Please click here to view a larger version of this figure.
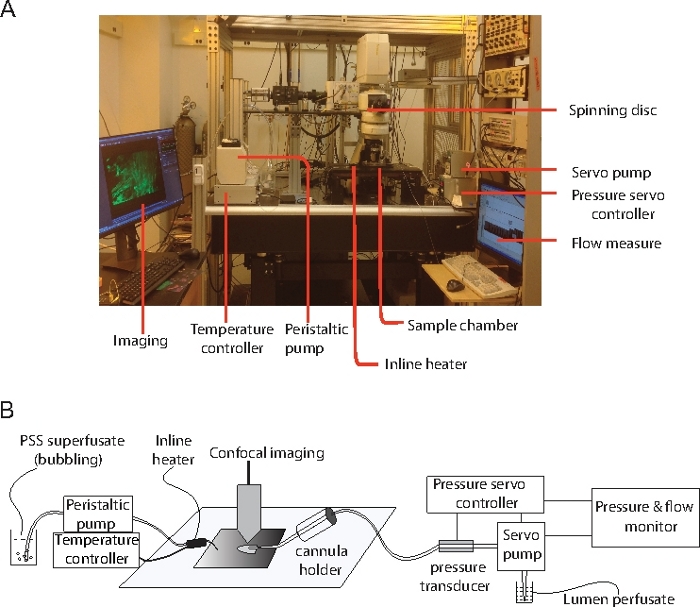
Figure 2: Equipment that was used in all the experiment.
(A) The main components of the setup that are used in the experiment. (B) Diagram illustrates the connections between the papillary muscle preparation and the physiological control experimental equipment. Please click here to view a larger version of this figure.
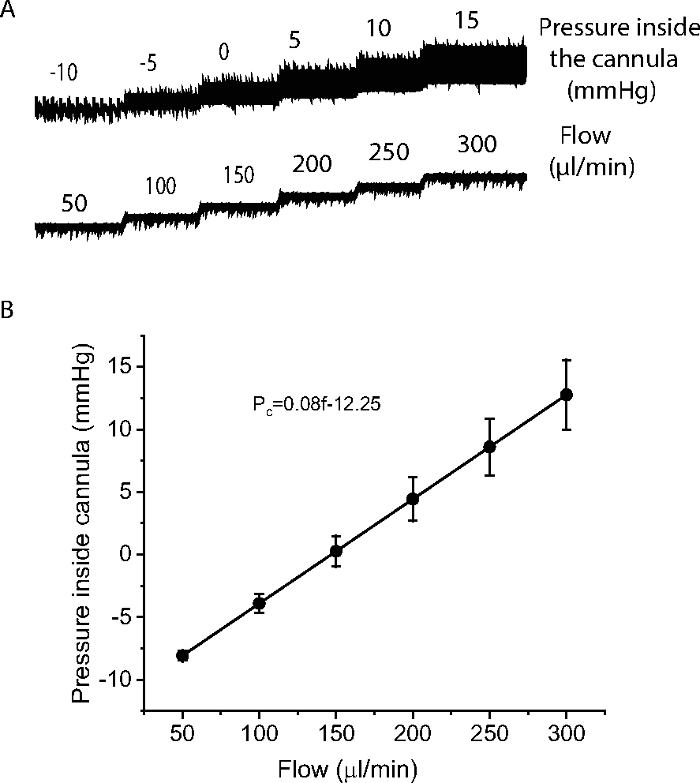
Figure 3: Measurement of pressure inside the cannula.
(A) An example to show how a cannula resistance was determined by measuring pressure inside the cannula over a range of flow (50-300 µl/min). (B) Relationship of the pressure inside the cannula with flow from 6 cannulae. The pressure inside the cannula was proportional to the flow and was fit by the expression Pc=0.08f-12.25, where Pc as pressure inside the cannula, f as flow. N=6 cannulae. Please click here to view a larger version of this figure.
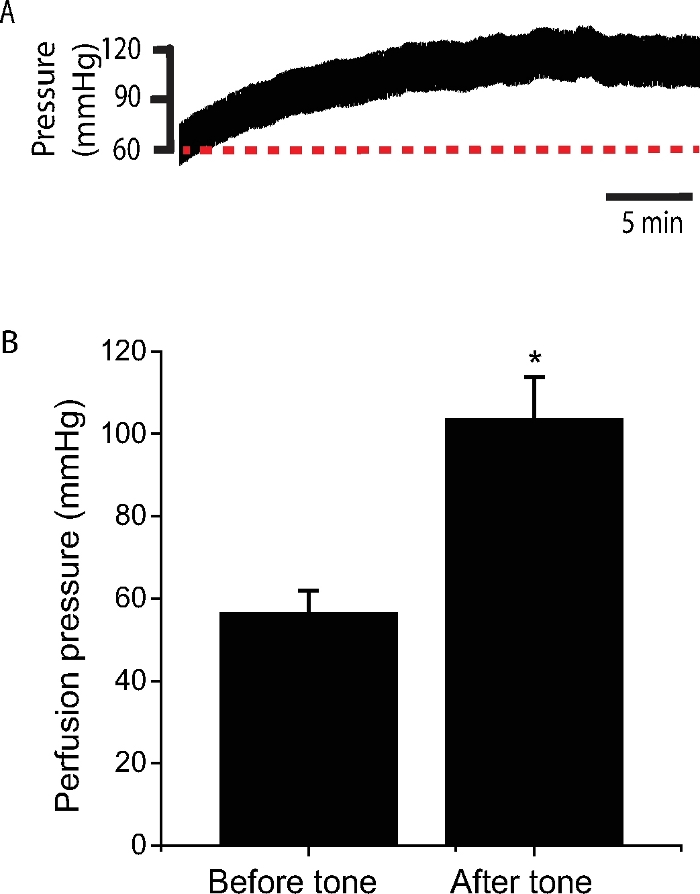
Figure 4: Perfusion pressure change during stabilization at a constant flow.
(A) A typical recording of perfusion pressure during stabilization at a constant flow (~250 µL/min). Note the increase of perfusion pressure after 30 min of stabilization. (B) Statistics show the perfusion pressure before and after the stabilization when tone was developed. The average flow of the arterioles to maintain the initial pressure (~60 mmHg) is 201.7 ± 8.6 µL/min (n= 45 mice). Please click here to view a larger version of this figure.
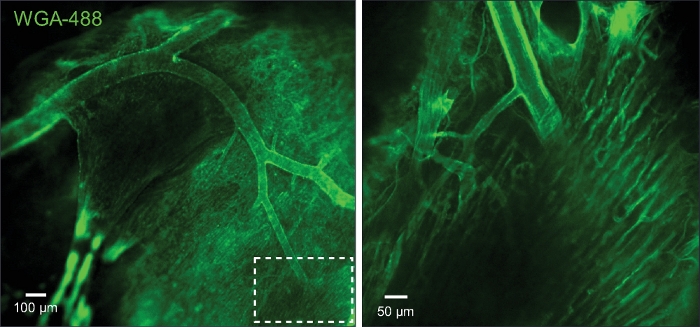
Figure 5: Imaging of capillaries and arterioles.
(A) The image shows the arterioles and capillaries that were loaded with wheat germ agglutinin (WGA). (B) Zoom in of the boxed area in A. Please click here to view a larger version of this figure.
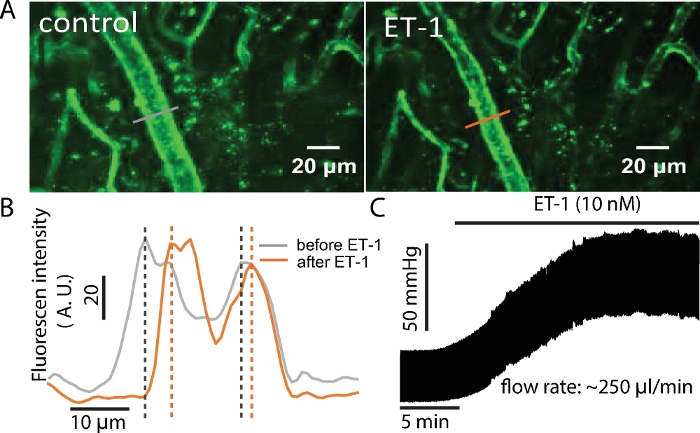
Figure 6: ET-1 increases luminal pressure and decreases arterial diameter.
(A) The arterial diameter changed with the application of ET-1 (10 nM). (B) The WGA fluorescence profiles showing the diameter change by ET-1. The diameter of the arteriole was reflected by the distance between the peak intensity of the fluorescence on the arteriole wall. (C) The luminal pressure increased in the presence of ET-1 (10 nM) at a constant flow. Please click here to view a larger version of this figure.
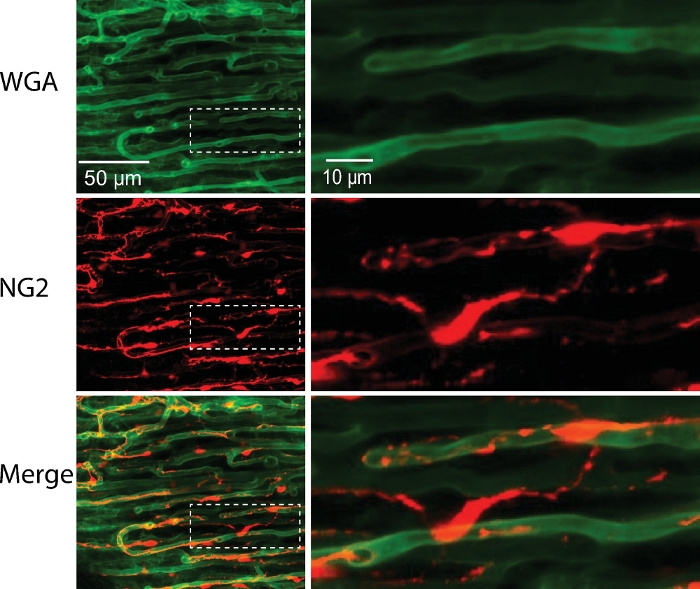
Figure 7: Capillary with pericytes from NG2-DsRed mouse.
Cardiac pericytes (red) and capillaries (green) were imaged in pressurized (40 mmHg) and perfused mouse right ventricular papillary muscle. Right panel, the zoomed-in images of the boxed areas in the left panels. Please click here to view a larger version of this figure.
Video 1: Pinacidil-induced vasodilation. Pinacidil (100 µM) was applied from the lumen. Vasodilation was seen in the arterial tree. Please click here to download this video.
| The composition of physiological saline solution (PSS) | |||
| Reagents | Final concentration (mM) | Molecular weight | g/10 Liters |
| NaCl | 112 | 58.44 | 65.45 |
| KCl | 5 | 74.55 | 3.73 |
| MgSO4 | 1.2 | 120.37 | 1.44 |
| NaH2PO4 | 1.2 | 119.98 | 1.44 |
| NaHCO3 | 24 | 84.01 | 20.16 |
| glucose | 10 | 180.16 | add 1.8 g glucose to 1 Liter PSS before use |
| CaCl2 | 1.8 | 110.99 | add 1.8 ml 1 M CaCl2 to 1 Liter PSS before use |
| The composition of Tyrode's solution | |||
| Reagents | Final concentration (mM) | Molecular weight | g/L |
| NaCl | 140 | 58.44 | 8.18 |
| KCl | 5 | 74.55 | 0.37 |
| NaH2PO4 | 0.33 | 119.98 | 0.04 |
| HEPES | 10 | 238.3 | 2.38 |
| glucose | 5.5 | 180.16 | 0.99 |
| CaCl2 | 1.8 | 110.99 | add 1.8 ml 1 M CaCl2 solution |
| MgCl2.6H2O | 0.5 | 203.3 | add 0.5 ml 1 M MgCl2 solution |
| Note: Adjust pH to 7.4 with 1 M NaOH. | |||
Table 1: The composition of the solutions.
Discussion
In the present work, we have introduced a remarkably simple yet highly practical ex vivo method to study the coronary microcirculation in heart under physiological conditions. This method was modified from mechanical investigations using rats2. The challenging addition was the imaging technology with high speed and high optical resolution. We, therefore, were able to take advantage of the advanced optical imaging systems that are now commercially available. By careful dissection and placement of the functioning papillary muscle preparation in a favorable position, we were able to visualize arterioles, pre-capillary arterioles, capillaries, and venules, as well as the pericytes and were able to measure arterial pressure and/or flow while controlling the other. The arteriole and capillary diameter changes were monitored using a highspeed spinning disk confocal microscope. The combination of the optical images and the internal perfusion of the physiological saline solution makes this preparation helpful in the evaluation of the effect of the biomedical treatments, as well as in the study of the mechanisms that are involved in the physiological and pathological regulation of blood flow in heart1.
Heart papillary muscles have been widely used in the study of cardiac physiology for many years. However, the absence of perfusion detracts from the physiology if characteristics of "work" or "metabolism" or "electrical activity" are being considered. Clearly, non-perfused papillary muscles are obviously not physiological. Nevertheless, decades ago, Westerhof et al. made the pressurized thin papillary preparation from rat to study the blood flow regulation2, but this preparation was not used in mice as we did here, due to the handling difficulties. These investigators also were not able to image the details needed to determine how blood flow regulation occurred. We were fortunate that the mouse septal artery is around 100 µm in diameter at ~60 mmHg perfusion pressure, smaller than that in rat, but large enough for our work. As a practical note, special attention needs to be given to the preparation of the cannula and the connected tubing. Avoid any bubbles in the tubing and cannula. Bubbles are easily trapped in the connected area and will block or distort blood flow. Therefore, double check these areas. In addition, it would be helpful to use a cannula with a relatively large tip as long as it is small enough to get into the artery, because a larger cannula keeps the artery open and enables smooth flow. If a sudden and unexpected increase in pressure (at constant flow) is observed during recording, it is very likely that the tip of the cannula has become stuck to the arterial wall or the cannula has been blocked by tissue debris. Therefore, the position of the cannula is very important to keep flow and pressure stable. The faster the cannulation, the better for the tissue. This leads to a more rapid recovery of its physiological function. We recommend that the time spent on cannulation not exceed ~45 min (from extraction of the heart till the completion of cannulation).
However careful one may be, this seemingly simple method takes practice to perfect. Sometimes the preparation does not respond to any stimulation, regardless of the care taken during the dissection or the rapidity and efficiency of the whole procedure. The bath temperature should be constant and between 35 to 37 °C. Another important thing is to run a routine reference check for tissue function (for example, the response to KCl) for each and every experiment, either before or after your experiment is done. We use ET-1 or KCl (70 mM) as references. Finally, it is very important to check and determine that the cannula is still in place when the experiment is done. Disregard the data if the cannula is out of place or the artery is broken or twisted by the tip of the cannula. Two important things worth noting center on the "movement of the preparation" and "data analysis". Even in “quiescence”, the preparation still moves (Video 1). Minor movement is not a problem for high speed spinning disk confocal imaging. Lowering perfusion pressure or using Na+ channel blockers can prevent or minimize the movement without interfering with microvascular vessel function. We did offline analysis of the acquired data using ImageJ-win64 (Fiji) and/or MATLAB with custom software for vessel diameter measurements. We do not detail the diameter analysis here because any software (e.g., Vasotracker11 ) should be able to analyze the diameter changes.
By combining the physiological investigations with optic imaging techniques and high speed confocal microscopy, this preparation can be widely used to 1) test the regulatory effect of new drugs on blood flow in heart; 2) Study intercellular communication (between and among cardiomyocytes, capillary endothelial cells, pericytes and arterial smooth muscle cells, and fibroblasts); and 3) Study the dynamic functional change of different cell types using fluorescence-tagged transgenic mice1. This method includes the visualization of parts of the heart tissue with networks under “near” physiology. Although we might be able to mimic in vivo conditions by pacing the preparation and/or including some red blood cells in the perfusate, it cannot replace true in vivo imaging with a full range of metabolic and work burdens. It might not be used on the large animals either due to limited depth of view of a confocal microscope. However, it should be a useful tool in the study of the molecular and cellular mechanisms underlying coronary microcirculation regulation in small animals including mouse and rat. Similar notions can be expanded and adopted in the study of blood flow control in other tissues or organs including but not limited to gastrointestinal system, pancreas, thyroid, lymph nodes, liver, kidney, skeletal muscle, brain12, retina, ganglia, bone, skin, uterus, testis, ovaries, adrenals, fat etc. This approach will improve and advance the understanding of blood flow control and regulatory mechanisms at the cellular and molecular levels under physiological conditions.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by the Center for Biomedical Engineering and Technology (BioMET); NIH (1U01HL116321) and (1R01HL142290) and the American Heart Association 10SDG4030042 (GZ), 19POST34450156 (HCJ).
Materials
| 1 M CaCl2 solution | MilliporeSigma, USA | 21115 | |
| 1 M MgCl2 solution | MilliporeSigma, USA | M1028 | |
| AxoScope software | Molecular Devices, San Jose, CA, USA | ||
| Chiller/water incubator | FisherScientific, USA | Isotemp 3016S | |
| Confocal | Nikon Instruments, USA | A1R | |
| Custom glass tubing | Drummond Scientific Company | 9-000-3301 | |
| Digidata 1322A | Molecular Devices, San Jose, CA, USA | ||
| Dissecting microscope | Olympus, Japan | SZX12 | |
| Endothelin-1 | MilliporeSigma, USA | E7764 | |
| Forceps | Fine Scientific Tools | 11295-51 | |
| Heparin Sodium Salt | Sigma-Aldrich, USA | H3393 | |
| Inline solution Heater | Warner Istruments, Hamden, CT, USA | SH-27B | |
| Isoflurane | VETone, Idaho, USA | 502017 | |
| Micropipette puller | Sutter Instruments, Novato, CA, USA | P-97 | |
| Micropipette/cannula holder | Warner Istruments, Hamden, CT, USA | 64-0981 | |
| NG2DsRedBAC transgenic mouse | The Jackson Laboratory | #008241 | |
| Nylon thread for tying blood vessels | Living Systems Instrumentation, Burlington, Vt, USA | THR-G | |
| PDMS (polydimethylsiloxane) | SYLGARD, Germantown, WI, USA | 184 SIL ELAST KIT | |
| Peristaltic pump | Gilson, Middleton, WI, USA | minipuls 3 | |
| Pressure Servo Controller | Living Systems Instrumentation, Burlington, Vt, USA | PS-200-S | |
| Scissors | Fine Scientific Tools, Foster City, CA, USA | 15000-10 | |
| Servo Pump | Living Systems Instrumentation, Burlington, Vt, USA | PS-200-P | |
| Temperature controller | Warner Instruments, Hamden, CT, USA | TC-324B | |
| Wheat Germ Agglutinin, Alexa Fluor 488 Conjugate | ThermoFisher Scientific, Waltham, MA USA | W11261 |
Referências
- Zhao, G., Joca, H. C., Nelson, M. T., Lederer, W. J. ATP- and voltage-dependent electro-metabolic signaling regulates blood flow in heart. Proceedings of the National Academy of Sciences of the United States of America. 117, 7461-7470 (2020).
- Schouten, V. J., Allaart, C. P., Westerhof, N. Effect of perfusion pressure on force of contraction in thin papillary muscles and trabeculae from rat heart. Journal of Physiology. 451, 585-604 (1992).
- Tillmanns, H., et al. Microcirculation in the ventricle of the dog and turtle. Circulation Research. 34, 561-569 (1974).
- Martini, J., Honig, C. R. Direct measurement of intercapillary distance in beating rat heart in situ under various conditions of O2 supply. Microvascular Research. 1, 244-256 (1969).
- Nellis, S. H., Liedtke, A. J., Whitesell, L. Small coronary vessel pressure and diameter in an intact beating rabbit heart using fixed-position and free-motion techniques. Circulation Research. 49, 342-353 (1981).
- Marcus, M. L., et al. Understanding the coronary circulation through studies at the microvascular level. Circulation. 82, 1-7 (1990).
- Ralevic, V., Kristek, F., Hudlicka, O., Burnstock, G. A new protocol for removal of the endothelium from the perfused rat hind-limb preparation. Circulation Research. 64, 1190-1196 (1989).
- Zhao, G., Adebiyi, A., Blaskova, E., Xi, Q., Jaggar, J. H. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Amercian Journal of Physiology-Cell Physiology. 295, 1376-1384 (2008).
- Zhao, G., Li, T., Brochet, D. X., Rosenberg, P. B., Lederer, W. J. STIM1 enhances SR Ca2+ content through binding phospholamban in rat ventricular myocytes. Proceedings of the National Academy of Sciences of the United States of America. 112, 4792-4801 (2015).
- Stowe, D. F., Boban, M., Graf, B. M., Kampine, J. P., Bosnjak, Z. J. Contraction uncoupling with butanedione monoxime versus low calcium or high potassium solutions on flow and contractile function of isolated hearts after prolonged hypothermic perfusion. Circulation. 89, 2412-2420 (1994).
- Lawton, P. F., et al. a Low-Cost and Open Source Pressure Myograph System for Vascular Physiology. Frontiers in Physiology. 10, 99 (2019).
- Kim, K. J., Filosa, J. A. Advanced in vitro approach to study neurovascular coupling mechanisms in the brain microcirculation. Journal of Physiology. 590, 1757-1770 (2012).

