Distinguishing Intrapulmonary Immune Cells from Intravascular Immune Cell Populations: the Intrajugular Approach
Summary
The aim of the current study is to describe a protocol for differentiating between intravascular and intraparenchymal immune cells in studies of lung inflammation. We use an intrajugular injection of a fluorescent tagged antibody prior to lung harvest. Further, we use an inflation-based lung digestion process to improve the yield of leukocytes from the lung.
Abstract
Circadian rhythms refer to oscillations in various biological process that occur with a 24 h period. At the molecular level, such rhythms are comprised of a web of transcriptional-translational feedback loops (TTFL) of core clock genes. Individual tissues and organ systems, including the immune system, have their own clock. In the systemic circulation, various members of the CD45+ population oscillate across the day; however, many of these rhythms are not identical or even similar in the tissue resident CD45+ leukocyte population. When studying the role of circadian regulation of lung inflammation, CD45+ within the lung may need to be investigated. However, despite optimized perfusion methods, leukocytes trapped from the circulation persist in the lungs. The goal in designing this protocol was to distinguish between intravascular and intraparenchymal leukocytes. Towards this end, mice are injected with a fluorescent tagged CD45 antibody intrajugularly shortly before lung harvest. Thereafter, the lung is digested using a customized lung digestion technique to obtain a single cell suspension. The sample is stained for the regular panel of antibodies for intraparenchymal immune cells (including another CD45 antibody). Flowcytometric analyses shows a clear elucidation of the populations. Thus, the method of labeling and defining intrapulmonary CD45+ cells will be particularly important where the behavior of intrapulmonary and circulating immune cells are numerically and functionally distinct.
Introduction
We describe here efficient and reliable methods of differentiating intravascular leukocytes from pulmonary leukocytes. Even with the best perfusion techniques, studies have revealed residual CD45+ from circulation persists in the lung. This impairs the ability to distinguish between the rhythms in the circulation and the lung. This effect is further amplified in cases of lung inflammation. This is particularly relevant for the study of circadian regulation of inflammation.
Circadian rhythms refer to the diurnal oscillations in various biological processes that occur with a period of 24 h. The circadian system is an evolutionarily conserved anticipatory mechanism that confers protection on the host as it faces changes in its environment such as threat of infections. At the cellular level, the clock is organized into self-sustained transcriptional-translational feedback loops comprising the core clock genes1. The immune system has its own clock that impacts its response to pathogens and inflammatory insults2,3. As an organ exposed to the environment constantly, circadian rhythms are particularly important in the lung4. Various immune processes in the lung are under clock control5,6,7. However, the phase of various biological processes in the lung and the systemic circulation are not the same8, which by extension, also suggests that the oscillations of leukocytes in the lung and the circulation may not be identical. Thus, having a method to efficiently distinguish between pulmonary and intravascular leukocytes will be critical in the circadian context.
The aim of this study was to devise a method that can differentiate between intravascular and intraparenchymal leukocytes reliably. For this, we used a labeling of intravascular leukocytes and lung digestion method. For the labeling of intravascular leukocytes, we use intrajugular injection, which targets a large blood vessel and can be reproducibly used in mice of all strains and sizes. Many other methods have used tail vein injection9,10, which are notoriously harder to perform in Bl6 mice11. The intrajugular injection does necessitate use of anesthesia and is best done under direct visualization with dissecting microscope or magnifying loupes. Thus, the ease and reliability of the intrajugular injection should be weighed against the need for anesthesia and special equipment. However, given the ready availability of these equipment in most research labs, we do not view this to be a limiting factor. However, a case-by-case consideration seems prudent.
Protocol
All animal studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and met the stipulations of the Guide for the Care and Use of Laboratory Animals.
NOTE: The overall process may be divided into 1) intravenous CD45 labeling, 2) harvest, 3) digestion, and 4) staining and flow cytometry. These steps have been summarized in Figure 1.
1. Solutions/Reagent preparation
- Prepare Dissociation Media by adding 5 mL of 2 mM L-glutamine, 20 mL of Fetal Bovine Serum (FBS), 1 mL of 2-mercaptoethanol, and 10 mL of Pen/Strep to 500 mL of DMEM.
NOTE: Dissociation Media is stable for up to 2 months when stored at 2-4 °C. - Prepare Fluorescence Activated Cell Sorting (FACS) Buffer by adding 10 mL of FBS and 500 mg of sodium azide to 500 mL of PBS without magnesium or calcium.
NOTE: With the addition of sodium azide, FACS Buffer can be stored at 2-4 °C for months. - On the day of sample collection, add the DNase and Liberase solutions to Dissociation Media at a 1:100 dilution (i.e., add 10 µL of DNase and Liberase for every 1 mL of Dissociation Media).
NOTE: For each mouse, digesting the whole lung requires 10 mL/mouse and half of the lung requires 5 mL/mouse.
2. Intravenous CD45 labeling
- For this experiment, use adult C57Bl6 mice aged 8-12 weeks old.
- Anesthetize the mice with agents of choice. A combination of xylazine and ketamine were used for this purpose, but other agents are acceptable as well. The aim is to get moderate to deep level of anesthesia that lasts about 5 -10 minutes.
NOTE: The xylazine and ketamine anesthesia mixture is administered intraperitoneally. Use 10-15 mg/kg of xylazine and 120-150 mg/kg of ketamine. - Once the pedal reflex is negative, position the animal on its back and tape down its limbs gently to keep the head as central as possible.
- Expose the jugular vein and pectoral muscle by lifting the skin with forceps and snipping with sharp surgical scissors.
- Inject 200 µL of anti-CD45 antibody (flow cytometry grade antibody; diluted 1:300 in PBS) into the jugular vein using a 28 G needle. Wait 2-4 minutes so the antibody can circulate throughout the vasculature.
NOTE: Entering through the pectoral muscle into the jugular vein from a shallow angle prevents considerable bleeding from occurring. - Thereafter euthanize the animal by exposure to CO2 for 10 minutes. Proceed to lung perfusion and harvest lungs and other tissues.
NOTE: Any other method of humane euthanasia that adheres to the AVMA guidelines for the euthanasia of animals is also acceptable.
3. Dissection/Harvest (Figure 1)
- Position the animal on its back on a flat board and pin the paws down, keeping the head central.
- Spray the body with 70% ethanol. Open the thoracic cavity with forceps and scissors to expose the lung, heart, and trachea.
- Perfuse the lung by making a small incision in the left ventricle of the heart and injecting 10 mL of cold PBS through the right ventricle.
- Snip an opening in the trachea and insert an intravenous cannula. Once the cannula is in, pass a suture string about 6-8 cm long underneath the trachea and tie it twice to the cannula.
- Tuck the surgical string into the cannula and attach a syringe containing Dissociation Media (5 mL for half a lung or a 10 mL for a whole lung) as depicted in Figure 1B.
- Gently cut away the lung from the rest of the body and place the syringe with the lung attached into a 50 mL conical tube.
4. Digestion to single cell suspension
- Incubate the lung at 37 °C for 30-40 minutes, instilling 1 mL (for half the lung) or 2 mL (for the whole lung) of Dissociation Media every 5 minutes.
- Once all the media is instilled, remove the syringe and cannula, and place the 50 mL conical tube into a shaking water bath at 180 rpm for the remainder of the incubation for better yield.
NOTE: Alternatively, the tubes may be manually shaken every 5 minutes. - Add 10 mL of PBS and shake vigorously for 1 minute to stop the reaction.
- Pass the solution through a cell strainer (70 µm) into a new 50 mL conical tube. Use a 5 mL syringe rubber stopper to pass any clumps of tissue through the strainer. Add PBS so that the final volume is 30 mL.
- Centrifuge the samples for 10 minutes at 1,200 x g and 4 °C. Discard the supernatant without disturbing the pellet. Pipet out any remaining solution.
- Add 1 mL of Red Blood Cell (RBC) Lysis Buffer and mix with the cell pellet by pipetting.
- Incubate at room temperature for 60-90 s. Add PBS so that the final volume is 30 mL to stop the reaction.
NOTE: Incubation time depends on the amount of blood in the cell pellet; the redder the cell pellet, the longer the incubation time. - Centrifuge the samples for 10 minutes at 1,200 x g and 4 °C. Discard the supernatant without disturbing the pellet. Pipet out any remaining solution.
- Add 1 mL of FACs buffer to the cell pellet and mix by pipetting.
5. Staining cells for flow cytometry
- Use a cell counter to determine the total number of cells in the cell suspension.
- Transfer the cell suspension into each labeled FACS tube so that there are 3 x 106 cells total per sample.
- Add Fc Block (diluted 1:100) and incubate for 15 minutes on ice.
- Centrifuge the tubes for 5 minutes at 1,200 x g and 4 °C. Discard the supernatant without disturbing the pellet.
- Stain the samples with a specified antibody mixture and incubate for 20 minutes on ice protected from light (i.e., by covering with aluminum foil). Mix by racking tubes against the tube holder halfway through incubation.
- Add 1 mL of FACS buffer to wash and mix by pipetting.
- Transfer the cell suspension into another FACS tube by pipetting the suspension slowly through a 35 µm strainer.
- Centrifuge the tubes for 5 minutes at 1,200 x g and 4 °C. Discard the supernatant without disturbing the pellet.
- Add 150 µL of FACS buffer and mix by pipetting.
- Add 10 µL of DAPI (1:100) to each tube immediately prior to running the samples.
NOTE: The samples are now ready to be run on the flow cytometer.
Representative Results
Using this technique, the total cell count of the naïve dissociated lungs (only the left lobes were used for the representative data) was between 27.3 x 106 to 71.1 x 106 cells/mL. After gating on size and gating out doublets and dead cells (gating scheme in Figure 2), the leukocyte counts ranged from 6.9 x 106 to 13.5 x 106 cells/mL. Circulating leukocytes that remain trapped even after perfusion to clear the lungs constituted approximately 4% to 13% of the live cells in the experiment. While all leukocytes in the dissociated lung stain for CD45 on the PE-Cy7, only the circulating leukocytes are double stained with the CD45 antibody on both fluorophores, PE-Cy7 and Pac blue. Despite standardizing the process of perfusion, we found that about 24% to 70% of the total leukocytes in the dissociated lung belonged to the circulating leukocyte pool. The mean leukocyte counts without accounting for the intravascular labeling was 10.2 x 106 cells/mL, while the corrected value was 4.93 x 106 cells/mL (Figure 3A). Therefore, failing to differentiate residual intravascular leukocytes that persist after perfusion results in a significant overestimate the number of pulmonary leukocytes. For this experiment, animals were 8-12 weeks of age and weighed 25-30 g at the time of dissection. Dose adjustments may need to be made for extremes of ages and weight. Other useful controls include using an intravascular sample to demonstrate good labeling of intravascular leukocytes and a sample from an unlabeled tissue (such as a lymph node) to exclude the possibility to diffuse labeling of all tissues by transmigration of labeled leukocytes from the blood.
Since research questions often involve circadian sampling, we further broke down the data by time of day (for the animal) at which the lung harvest was performed. By circadian convention ZT0 refers to the time when lights turn on. We used dawn (ZT23: onset of rest phase) and dusk (ZT11; onset of active phase) as the two time points. We note that the proportion of the intraparenchymal and the residual intravascular leukocytes varied by the time of day at dissection (Figure 3B). In this experiment, the mice were maintained in reverse light-dark (LD) cycles using light-controlled circadian boxes, so that ZT11 and ZT23 mice were in reverse phases at the same conventional time and could be harvested simultaneously. Thus, lungs from both groups were labeled, harvested and digested at the same time of the day and therefore these differences in Figure 3 cannot be attributed to differences in processing.
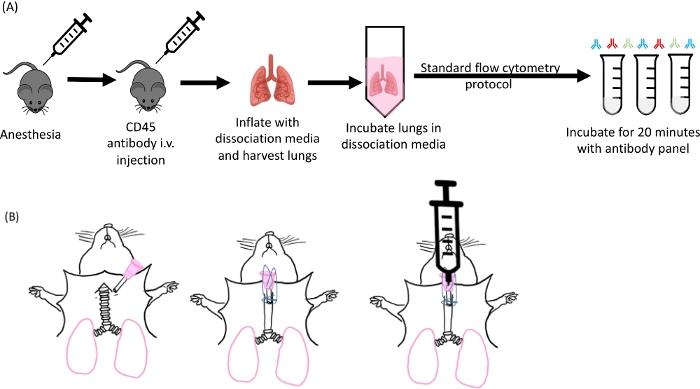
Figure 1: Schematic of lung inflation and digestion. (A) Overall experimental design starting from anesthetizing of the mice to the antibody staining step. (B) Image of the cannula insertion step of the lung harvest. Please click here to view a larger version of this figure.
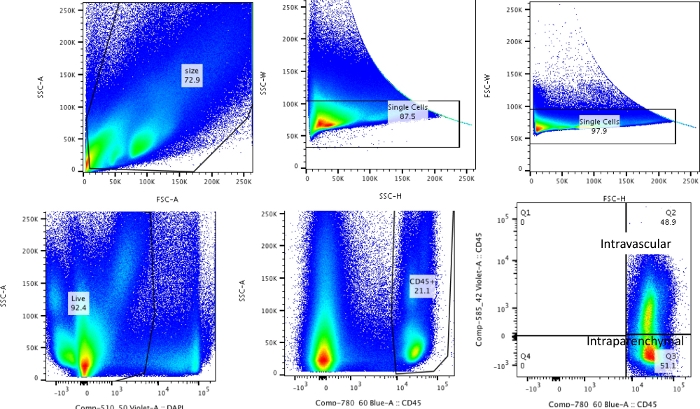
Figure 2: Gating strategy. Gating strategy used for differentiating intravascular leukocytes from leukocytes that are truly resident in the lungs. Please click here to view a larger version of this figure.
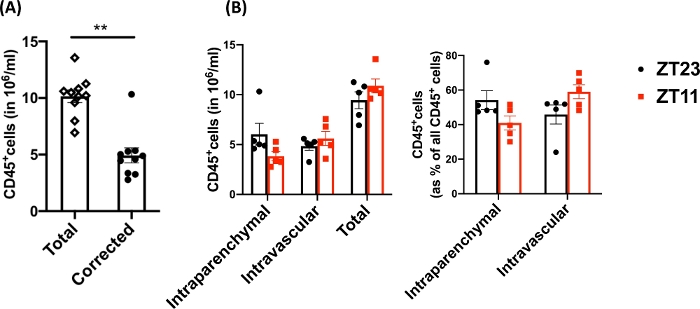
Figure 3: Comparison of intraparenchymal and intravascular leukocytes. (A) Leukocyte counts (in 106 cell/mL) using traditional digestion and labeling compared with the counts corrected for residual intravascular leukocytes that were not cleared out of the lungs. P<0.0001 by Student’s t-test. Data represented as mean±SEM. (B) Leukocyte numbers and percentage from lungs harvested at dawn (ZT23) or dusk (ZT11). (right panel) 2-way ANOVA, p >0.05 for both time of harvest and compartment labeled, but p<0.05 for interaction. Data represented as mean±SEM. Please click here to view a larger version of this figure.
Discussion
Careful studies of lung inflammation and pulmonary immune responses are crucial to the understanding of many disease conditions. Flow cytometry is routinely used to enumerate and ascribe functional relevance to pulmonary leukocytes. The function of leukocytes depends at least partly on where they are found. Although there is accumulating evidence to support that even after perfect perfusion protocols, many intravascular leukocytes persist in the lungs, most studies do not differentiate between intrapulmonary and intravascular leukocytes. Further, the residual intravascular leukocytes that remain post-perfusion in the lung are likely to be randomly distributed across the samples depending on the preparation, such that predicting how it affects the results is impossible. This hampers the generalizability of the results and is a threat to the rigor and reproducibility of those studies involving flowcytometry12.
We have described here a method to determine the residual leukocytes from the vasculature and to differentiate them from the truly intrapulmonary leukocytes. Although such methods have been used in the past9,13,14, we have refined the labeling, as well as digestion, to make it the most efficient preparation. Other reports describe using the retro-orbital or tail vein injections for the intravascular labeling10. Retro-orbital injections inflict more pain on the animal and thus require a higher level of regulatory scrutiny. Although the tail vein injection does not need anesthesia, it is technically more challenging11 especially in C57bl6 animals. This intrajugular approach overcomes both these disadvantages by making the procedure relatively painless and easy to perform under microscopy. Although we have described the procedure here for young adult mice, given the larger size of the intrajugular, this protocol may be used for smaller animals15. The ease of the procedure makes the labeling consistent across animals and thus would add to the rigor and reproducibility of the results.
The critical steps in this protocol include the reproducibility of the intravascular injection and the lung harvest. Some additional considerations that are needed for intrajugular injection are need for anesthesia and magnifying equipment (magnifying loupes or dissecting microscope). Both these steps require practice to master and may add to variability if not well mastered. With intrajugular injection, a faulty technique may lead to excessive bleeding and precipitate death. Thus, there is an emphasis on equipment to aid visualization. In the case of lung harvest, poor technique may result in rupture of the trachea. While one may still proceed with other methods of lung digestion (such as using a scalpel to chop the lung, etc.), in our experience it yields much lower cell counts and tends to increase variability between samples.
Another important concern emerging from any additional steps is that it increases the total processing time and may reduce the viability the samples, and therefore the quality of the data. By labeling intravascular leukocytes before the digestion, we ensure that the viability of the prepared sample is not adversely affected in the protocol. Further, using the inflation technique to distribute the digestive enzymes throughout the lung, we ensure that the digestion is uniform and yields reliably more cells than other methods to dissociate the lung tissue7. Few additional concerns that may be considered are common to this labeling method independent of the route of administration of the intravascular antibody. This includes possible competitive binding between the two CD45 antibodies. This may be partially mitigated by using different clones of the CD45 antibody, although care must be taken to ensure that the two antibodies used have very similar binding affinities. The other aspect to consider is if the time from injection to harvesting (here, 2-4 minutes) is enough for labeling the majority of the intravascular leukocytes. Given that the intravascular labeling precedes the overall CD45 labeling, and at a heart rate of 300-700 beats per minute in mice16, it is very likely that there is complete labeling of the intravascular CD45 compartment. This method was adapted from others who have used very similar concentrations and times from administration to harvest9,10,13. Finally, another issue to consider is the possible transmigration of the resulting leukocytes, technically in either direction in the time from the injection to the harvest. This may also vary by the underlying experimental condition. Although, not used here, one way to overcome this at least partially would be to use other standard methods of euthanasia that reduce the time from injection to harvest, such as cervical dislocation.
This work revolves around the elucidation of circadian regulation of lung injury, repair and regeneration. Although the entire body is under circadian regulation, the phases of such regulation vary by organ and cell type. Thus, the phase of the rhythms of leukocyte or their functional subtypes may be different based on the organ or site of circulation. Not differentiating these two populations may in fact result in false negative results in the analyses of various leukocyte sub-populations (if the circulating and pulmonary populations are in opposite phase, the averages may not be vastly different). Thus, differentiating between circulating leukocytes that remain in the lung even after perfusion from those that have already migrated into the lung and are actively participating in the inflammatory cascade is essential to discovering the biology of circadian regulation. The protocol described here helps this differentiation. While we have done this in the lung, this method lends itself to being adapted for other organs as well. However, adding these steps does lengthen the time to harvest and may not be suitable for all circadian experiments. This would thus entail a customized consideration on where differentiating between the intravascular and intraparenchymal leukocytes is most relevant.
In conclusion, we have described in detail a method of distinguishing residual intravascular from intrapulmonary leukocytes and inflation-based lung digestion. These techniques will enhance the yield of single cell suspension from the lung and improve the rigor and reproducibility of flowcytometry studies. Finally, this is of particular relevance for those studying circadian rhythms.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the NHLBI-K08HL132053 (SS). The authors thank Dr. G. A. FitzGerald for access to a dissecting microscope and a shaking water bath.
Materials
| Boekel Scientific Medium Water Bath | Boekel Grant Scientific | 290200 | |
| 10 mL BD Syringes with BD Luer-Lok Tip | BD Biosciences | 309604 | |
| 5 mL BD Syringes with BD Luer-Lok Tip | BD Biosciences | 309646 | |
| Anti-CD45- Pac Blue | Biolegend | 103114 | |
| Anti-CD45- Pe/Cy7 | Biolegend | 103114 | |
| Cell strainer 70 µm Nylon | Fisher | 352350 | |
| Corning Conical-Bottom Centrifuge Tube 50 mL | Avantor | 21008-714 | |
| Corning Falcon Test Tube with Cell Strainer Snap Cap | EMSCO | 10004637 | |
| Dissection Microscope | Olympus | SZX-SDO2 | |
| DMEM, high glucose | Life Technologies | 11965084 | |
| Dnase | Roche | 10104159001 | |
| DPBS without Ca++ & Mg++ | 14190136 | ||
| Fc Block | Biolegend | 101320 | |
| HyClone Fetal Bovine Serum | GE Healthcare | SH30071.03 | |
| L-Glutamine (200 mM) | Life Technologies | 25030-081 | |
| Liberase Research Grade | Sigma | 5401127001 | |
| Penicillin-Streptomycin (10,000 U/mL) | Life Technologies | 15140-122 | |
| Precision Shaking Water Bath | Thermo Fisher | TSSWB15 | |
| Red Blood Cell Lysing Buffer | Sigma | R7757 | |
| Suture Silk 4-0 | Roboz | SUT-15-2 |
Referências
- Partch, C. L., Green, C. B., Takahashi, J. S. Molecular architecture of the mammalian circadian clock. Trends in Cell Biology. 24, 90-99 (2014).
- Man, K., Loudon, A., Chawla, A. Immunity around the clock. Science. 354, 999-1003 (2016).
- Haspel, J. A., et al. Perfect timing: circadian rhythms, sleep, and immunity – an NIH workshop summary. JCI Insight. 5, (2020).
- Nosal, C., Ehlers, A., Haspel, J. A. Why Lungs Keep Time: Circadian Rhythms and Lung Immunity. Annual Review of Physiology. 82, 391-412 (2020).
- Gibbs, J., et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature Medicine. 20, 919-926 (2014).
- Ehlers, A., et al. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunology. 11, 97-111 (2018).
- Sengupta, S., et al. Circadian control of lung inflammation in influenza infection. Nature Communications. 10, 4107 (2019).
- Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E., Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America. 111, 16219-16224 (2014).
- Anderson, K. G., et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature Protocols. 9, 209-222 (2014).
- Gibbings, S. L., Jakubzick, C. V. Isolation and Characterization of Mononuclear Phagocytes in the Mouse Lung and Lymph Nodes. Methods in Molecular Biology. 1809, 33-44 (2018).
- Vines, D. C., Green, D. E., Kudo, G., Keller, H. Evaluation of mouse tail-vein injections both qualitatively and quantitatively on small-animal PET tail scans. Journal of Nuclear Medicine Technology. 39, 264-270 (2011).
- Tighe, R. M., et al. Improving the Quality and Reproducibility of Flow Cytometry in the Lung. An Official American Thoracic Society Workshop Report. American Journal of Respiratory Cell and Molecular Biology. 61, 150-161 (2019).
- Anderson, K. G., et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. Journal of Immunology. 189, 2702-2706 (2012).
- Gibbings, S. L., et al. Three Unique Interstitial Macrophages in the Murine Lung at Steady State. American Journal of Respiratory Cell and Molecular Biology. 57, 66-76 (2017).
- Steel, C. D., Stephens, A. L., Hahto, S. M., Singletary, S. J., Ciavarra, R. P. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Animals (NY). 37, 26-32 (2008).
- Ho, D., et al. Heart Rate and Electrocardiography Monitoring in Mice. Current Protocols in Mouse Biology. 1, 123-139 (2011).

.
