Doxycycline Loaded Collagen-Chitosan Composite Scaffold for the Accelerated Healing of Diabetic Wounds
Summary
The prepared DOX-CL scaffold satisfied the prerequisites of an ideal DW dressing in mechanical strength, porosity, water absorption, degradation rate, sustained release, anti-bacterial, biocompatibility, and anti-inflammatory properties, which are considered to be essential for the recovery of damaged tissue in DWs.
Abstract
One major complication of diabetes mellitus is diabetic wounds (DW). The prolonged phase of inflammation in diabetes obstructs the further stages of an injury leading to delayed wound healing. We selected doxycycline (DOX), as a potential drug of choice, due to its anti-bacterial properties along with its reported anti-inflammatory properties. The current study aims to formulate DOX loaded collagen-chitosan non-crosslinked (NCL) & crosslinked (CL) scaffolds and evaluate their healing ability in diabetic conditions. The characterization result of scaffolds reveals that the DOX-CL scaffold holds ideal porosity, a low swelling & degradation rate, and a sustained release of DOX compared to the DOX-NCL scaffold. The in vitro studies reveal that the DOX-CL scaffold was biocompatible and enhanced cell growth compared with CL scaffold treated and control groups. The anti-bacterial studies have shown that the DOX-CL scaffold was more effective than the CL scaffold against the most common bacteria found in DW. Using the streptozotocin and high-fat diet-induced DW model, a significantly (p≤0.05) faster rate of wound contraction in the DOX-CL scaffold treated group was observed compared to those in CL scaffold treated and control groups. The use of the DOX-CL scaffold can prove to be a promising approach for local treatment for DWs.
Introduction
Diabetes mellitus (DM) is a condition where the body's failure to deliver insulin or react to its outcomes in abnormal digestion of straightforward sugars brings about an upsurge of blood glucose 1. The most consecutive and crushing entanglement of DM is the diabetic wound (DW). Roughly 25% of patients with DM have the opportunity to build up a DW in their lifetime 1. The hindered healing of DW is accredited to a triopathy of DM: immunopathy, vasculopathy, and neuropathy. Whenever DW is left untreated, it may result in gangrene development, therefore prompting the removal of the concerned organ 2.
Plenty of treatments, such as instructing the patients (inspect wound daily, cleanse the wound, avoid activities that creates pressure on the wound, periodic glucose monitoring, etc.), controlling their blood glucose, wound debridement, pressure offloading, medical procedure, hyperbaric oxygen therapy, and advanced therapies are in practice 3,4. The majority of these medications fail to address all prerequisites vital for DW care in light of the multifactorial pathophysiological conditions and unexpected expenses related to these medicines 5. Even though the DW pathogenesis is multifactorial, the persistent inflammation with inappropriate tissue management is stated to be the actual reason for delayed healing in DWs 5,6.
Augmented levels of inflammatory and pro-inflammatory mediators in DW result in diminished growth factors responsible for delayed wound healing 2,6. Improper extracellular matrix (ECM) formation in DWs is accredited to increased levels of matrix metalloproteinases (MMPs) accountable for the rapid degradation of formed ECM. In MMPs, MMP-9 is reported as a major intermediary responsible for prolonged inflammation and rapid ECM degradation 7. It is stated that local treatment with an anti-inflammatory drug that decreases the elevated levels of MMP-9 re-establishes cutaneous homeostasis, framework arrangement and prompts better healing of DWs 8,9.
Doxycycline (DOX), an MMP-9 inhibitor, was chosen to suppress the elevated levels of MMP-9, a major inflammatory mediator responsible for persistent inflammation in DWs 10,11,12. In addition, DOX possess antioxidant (produce free hydroxy and phenoxy radicals capable of binding with reactive oxygen species) 13 and anti-apoptotic (inhibit caspase expression and mitochondrial stabilization) 14 activities that are essential for the treatment of DW. The arrangement of frameworks containing DOX, collagen (COL), and chitosan (CS) was chosen. The choice of COL depends on the way that it helps in providing the necessary framework responsible for mechanical strength and tissue regeneration 15. On the other hand, CS is structurally homologous to glycosaminoglycan, associated with several wound healing phases. It is also reported that CS holds significant anti-bacterial property 15. Hence, the COL/CS scaffold of DOX is formulated to suppress the prolonged inflammation, followed by supporting the matrix formation for successful wound healing in DM conditions.
Protocol
All the animal procedures performed were approved by the institutional animal ethical committee of JSS College of Pharmacy, Ooty, India.
1. Preparation of DOX loaded porous scaffolds by freeze-drying method
- Add 1.2 g of COL to 100 mL of water (e.g., Millipore) and keep aside for swelling.
- Stir the swollen COL dispersion at 2000 rpm overnight to ensure complete dissolution of COL.
- Prepare CS solution by dissolving approximately 0.8 g of CS in 100 mL of 1% acetic acid.
- Stir the CS solution overnight at 2000 rpm to ensure uniform dispersion.
- Mix DOX (1% w/v), followed by CS solution, to the COL solution, and stir for 30 min.
- Filter the obtained physical mixture using a muslin cloth to remove the particulate matter.
- Deep freeze the obtained filtrate at -85 °C ± 4 °C for about 24 h.
- Lyophilize the deep freeze mixture at -85 °C ± 4 °C for 72 h.
- Store the obtained scaffolds in a desiccator for further analysis 16,17.
2. Crosslinking of scaffold
- Dissolve MES (0.488 g) in 50 mL of water.
- Soak 50 mg of the DOX loaded scaffold in 20 mL of the MES buffer for 30 min.
- Mix 19.5 mL of MES buffer with 0.1264 g of EDC and 0.014 g of NHS in a separate beaker.
- Immerse the scaffold in the buffer mixture for 4 h to achieve crosslinking 16.
- Store the DOX loaded crosslinked (CL) and non-crosslinked scaffolds (NCL) for further evaluation.
3. Characterization of scaffolds
- Morphological examination using a scanning electron microscopy (SEM)
- Characterize the scaffolds for morphological analysis using SEM (1 cm × 1 cm × 0.5 cm).
- Stain the cross-section and exterior surface of the scaffold with the delicate layer of gold (~150 Å).
- Capture the photographic image at the excitation voltage of 5 kV and 10 kV.
- Place the samples in aluminum stubs and enclose them with the gold at approximately 9 V.
- Measure the scaffold using SEM with the increased resolution at 10 kV.
- Porosity determination
- Measure the porosity of the scaffolds using the liquid displacement method (ethanol) 18.
- Calculate the porosity of the scaffolds using the below formulae.
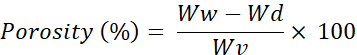
Ww = Wet weight of the scaffold
Wd = Dry weight of the scaffold
Wv = Volume of the scaffold
- Determining the water absorption capacity
- Measure the dry weight of the scaffold.
- Incubate the weighed scaffold at 37 °C for 24 h in phosphate buffer saline (PBS) pH 7.4.
- Remove the excess PBS over the scaffold using filter paper.
- Measure the water absorption capacity using the below formulae 17.
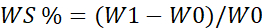
WS = Percentage of water absorption
W1=Wet weight of the scaffold
W0= Dry weight of the scaffold
- Scaffold degradation
- Incubate the scaffold (1cm x 1cm) at 37 °C for 7 days in a PBS of pH 7.4 containing lysozymes.
- Wash the scaffold to remove any adhered ions on the surface.
- Freeze dry the washed scaffold 17.
- Calculate the rate of degradation using formulae.
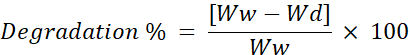
Ww = Initial weight of the scaffold
Wd = Weight of the scaffold after freeze-drying
- In vitro release studies
- Determine the release behavior of the DOX from the scaffold using the dialysis sack method.
- Disperse the scaffold in a few milliliters of simulated wound fluid (pH 7.4) and transfer it into a dialysis bag.
- Tightly close the ends of the membrane bag and immerse in the 500 mL of simulated wound fluid solution.
- Stir the wound fluid solution containing the dialysis bag at 200-250 rpm.
- Collect the supernatant solution and replace it with an equal quantity of fresh buffer solution at definite time intervals.
- Determine the percentage of DOX release from the scaffolds in the supernatant solution using a UV-visible spectrometer at 240 nm.
4. In vitro anti-bacterial studies
- Determine the minimum inhibitory concentration (MIC) of the CL and DOX-CL scaffolds against the S. aureus, S. epidermis, E. coli, P. aeruginosa using the micro-broth dilution method.
- Prepare the bacterial cultures using Mueller-Hinton broth at a ratio of 1:1000 to obtain 0.5 McFarland turbidity.
- Add D-glucose (800 mg/dL) to the bacterial cultures for hyperglycation 19,20.
- Mince and solubilize the CL and DOX-CL in DMSO (negative control).
- Serially dilute the hyperglycated bacterial suspension (100 µL) and test samples (100 µL of scaffolds solution) in 96 well plate.
- Incubate the plate at 37 °C for 20-24 h.
- Record the absorbance at a wavelength of 600 nm 21.
5. In vitro biocompatibility studies
- Evaluate the biocompatibility of the prepared scaffolds using MTT [(3-(4, 5 dimethyl thiazole-2 yl) -2, 5-diphenyl tetrazolium bromide)] assay.
- Sterilize the scaffolds of standard dimension and place them in 24 well plates.
- Add 3T3-L1 cells to the 24 well plate and incubated for 72 h.
6. In vivo animal studies
- Induction of DM and excision wound
- Feed the animal with a high-fat diet for two weeks and administer a single dose of streptozotocin (STZ) (50 mg/kg body weight) in citrate buffer solution intraperitoneally to Wistar albino rats (180-200 g) for the induction of type-2 diabetes.
- Choose the animals with a constant blood glucose of 250 mg/dL for the study.
- Randomize the selected animals for the induction of excision wounds.
- Anesthetize the diabetic rats using diethyl ether (5 mL was added to the priorly saturated anesthesia chamber) and confirm using the toe pinch method and mucous membrane color.
- Shave the dorsal area (Dorsal thoracic, lumbar region) using an aseptic trimmer and blades (A40).
- Sterilize the shaved area with an alcoholic swab.
- Excise the skin (2 x 2 cm2 and a depth of 1 mm) with an aseptic surgical A40 blade on the shaved area to create an open wound.
- Divide the animals into three groups (Group 1- Disease control (Control), Group 2- CL scaffold (Placebo), Group 3- DOX CL scaffold), each group consisting of 6 rats.
- Affix the CL and DOX CL scaffolds using surgical tape and cover the control group with sterile gauze for 21 days.
- Trace the wound area on a sterile OHP sheet and measure the percentage reduction of the wound using the grid method on days 0, 7, 14, and 21 for all groups.
- Calculate the percentage wound reduction using the below formulae.

7. Histopathological studies
- Isolate the healed wound area on days 7, 14, and 21, store in formalin solution (10%).
- Section the tissues using a microtome to obtain a thickness of 6 µm.
- Mount the sections on a glass slide and stain using Hematoxylin and eosin 17.
- Capture the images under 40x magnification using a digital microscope.
8. Hydroxyproline estimation
- Isolate the healed wound area on days 0, 7, 14, and 21 for evaluation.
- Estimate the hydroxyproline content using the procedure described by Reddy G et al., 1996 22.
9. Elisa test
- Estimate the MMP-9 levels using the Elisa kit as per the manufacturer's instructions.
- Isolate the tissue samples from the healed wound area on day 21 and mince using a tissue homogenizer.
- Centrifuge the obtained homogenate and collect the supernatant.
- Dilute the supernatant at 100-fold using assay buffer.
- Scan the plate using a multiple plate reader.
10. Statistical analysis
- Represent the obtained outcomes as Mean ± SD.
- Perform the statistical analysis using Graph pad prism v5.01.
- Attain the statistical significance using One Way Analysis of Variance (ANOVA) and Dunnet's post hoc test.
- Consider the values with p≤0.05 as significant.
Representative Results
Characterization of the DOX loaded NCL and CL scaffold
On visual examination, the NCL and CL scaffold was found to be cream in color. Besides, both the scaffolds appear to be like a sponge, stiff and inelastic when examined physically. SEM images of the NCL and CL scaffolds are shown in Figure 1. From the figure, it was clear that there was a decrease in pore size after crosslinking by forming intermolecular linkages. Also, the NCL and CL scaffolds porosity were found to be 92.3±4.21 and 71.35±2.65, respectively. The percentage of water absorption of the NCL and CL scaffolds was 750±11.4% and 492±8.66% at 24 h time intervals.
In addition, biodegradation studies were performed for seven days in the simulated wound fluid of pH 7.4, comprising lysozymes. The NCL scaffold exhibited a faster rate of degradation initially in the first three days and decreased slowly for four consecutive days. On the other side, the CL scaffold showed a prolonged rate of degradation. Crosslinking of the scaffold enhanced mechanical properties and network strength that, in turn, resulted in decreased degradation rate, indicating improved resistance to the degradation (Figure 2).
Further, the in vitro release of DOX from NCL and CL scaffold was performed for 120 h (Figure 3). In the initial 1 h, 27.92±3.45% DOX was released from the NCL scaffold, whereas only 16.54±2.21% DOX was released from the CL scaffold. After 6 h, the DOX release from the scaffolds increased by 63.15±3.78% in the NCL scaffold and 44.43±3.57% in CL scaffolds. After 24 h, there was a release of 70% DOX from the NCL scaffold, whereas CL scaffold took more than 72 h to release 70% of the DOX. Based on the results obtained, the DOX loaded CL scaffold was selected for further evaluation and represented as DOX-CL scaffold. Whereas CL scaffold without DOX (placebo) is designated as CL Scaffold.
In vitro anti-bacterial studies
Patients with DW usually experience infections that result in prolonged wound healing. Thus, prepared scaffolds were examined for their anti-bacterial activity using the MIC against a selected panel of bacteria (Table 1). From the results, it is clear that DOX exhibited inhibitory activity with a MIC of <4 µg/mL against both S. aureus and S. epidermis. A MIC of <8 µg/mL and <16 µg/mL was observed against E. coli and P. aeruginosa. Alone chitosan and CL scaffold extract exhibited minimal activity against selected organisms such as S. aureus (<64 µg/mL), S. epidermis (<64 µg/mL), E. coli (<128 µg/mL), and P. aeruginosa (<128 µg/mL). DOX-CL scaffold has shown similar inhibitory activity with a MIC of <2 µg/mL against both S. aureus and S. epidermis. Further, the DOX-CL scaffold exhibited moderate activities with a MIC of <8 µg/mL against E. coli and P. aeruginosa.
In vitro biocompatibility study
MTT assay was performed to determine the cellular viability of 3T3-L1 cells in the presence of CL and DOX-CL scaffolds. The results exemplified that the CL and DOX-CL scaffolds did not stimulate any cytotoxicity. Furthermore, the cellular viability was comparatively higher in the scaffold treated groups than in control, representing the enhanced growth of fibroblasts in the presence of scaffolds (Figure 4).
In vivo wound healing studies
The mean wound area decreased in all the groups was determined using the graphical method on days 0, 7, 14, and 21 (Figure 5). On visual examination, the wounds in the CL and DOX-CL scaffold treated groups were free from oozing on day seventh. At the same time, the wounds in the control group were oozing. On day 14, a dry scab was observed in all groups; however, a faster rate of wound contraction was observed in the DOX-CL scaffold (89.663%). On day 21, 99.9% of the wound got healed, and a scar was formed in the DOX-CL scaffold, whereas partial healing was observed in control and CL scaffold treated groups.
Histopathology study
Histopathological observation of wound healing on day seventh postinjury exhibited disruption of all skin layers at the edges of the wound in all groups. There was no visibility of the epidermis; however, predominant neutrophils with intermittent monocytes and lymphocytes were observed in the control group. In CL scaffold treated group, moderate visibility of epidermis was noticed along with few neutrophils and macrophages. Whereas, in the DOX-CL scaffold treated group, the wound was covered with a slim layer of the epidermis, representing the phase of reepithelization. Mild neutrophils and macrophages, along with granulation tissue, were also seen more often in the DOX-CL scaffold treated group.
On day 14 postinjury, wounds in all the groups were found to be covered with epidermis. In the control group, the epidermis layer formed was observed to be a very slim layer along with prevailed neutrophils. In the CL scaffold treated group, the epidermis layer formed was thicker than that in the control group, along with mild multinucleated massive cells. In the DOX-CL scaffold treated group, the epidermis developed was thicker compared to other groups, along with an abundant number of histiocytes and giant multinucleated cells. The dense zone of fibroblasts was also observed in the DOX-CL scaffold treated group.
On day 21 postinjury, in the control group, a dominant number of neutrophils were decreased along with recurrence of macrophages and histiocytes representing the decreased inflammation. Whereas, in the CL scaffold treated group, a moderate number of histocytes and lymphocytes were observed. The epidermis formed was observed to be substantially thicker in the DOX-CL scaffold treated group than those in the control group, along with a rich number of histiocytes, lymphocytes, and massive multinucleated cells. In the DOX-CL scaffold treated group, the neutrophils count was decreased on day 7. Also, the gathering of macrophages and their morphological variants such as multinucleated massive cells and histiocytes were noticed on day 14 and 21 (Figure 6).
Hydroxyproline estimation
Hydroxyproline estimation is an indirect measure of the amount of collagen present in healing wounds. Higher hydroxyproline concentration designates a rapid percentage of wound healing. The biochemical examination revealed a higher amount of hydroxyproline in the DOX-CL scaffold treated group followed by the CL scaffold treated group than those in the control group (Figure 7).
MMP-9 estimation using the Elisa kit
MMP-9 content in the DOX-CL scaffold treated group was significantly decreased compared to those in control, and CL scaffold treated group. Whereas the MMP-9 content in the CL scaffold treated group was marginally less compared with that of the DOX-CL scaffold treated group, as shown in Figure 8.
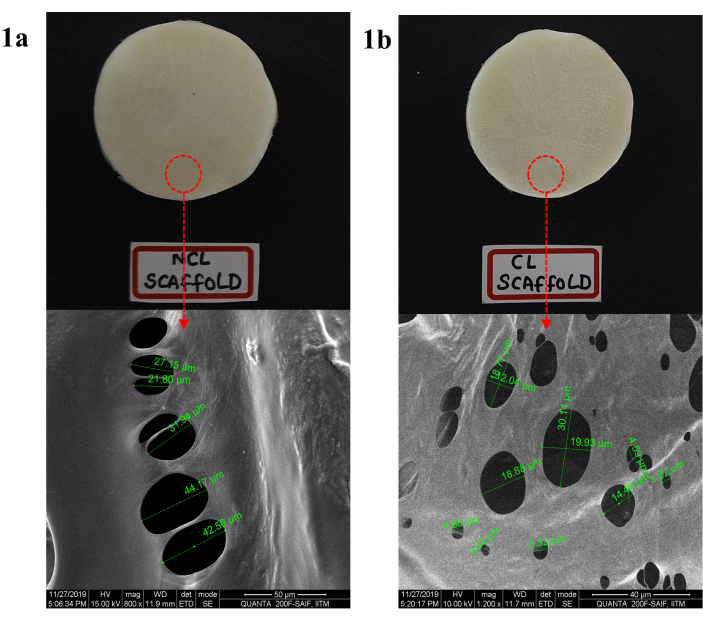
Figure 1: Morphology of DOX loaded COL-CS scaffold a) before CL and b) after CL determination by SEM at a scale range of 50 µm. Please click here to view a larger version of this figure.
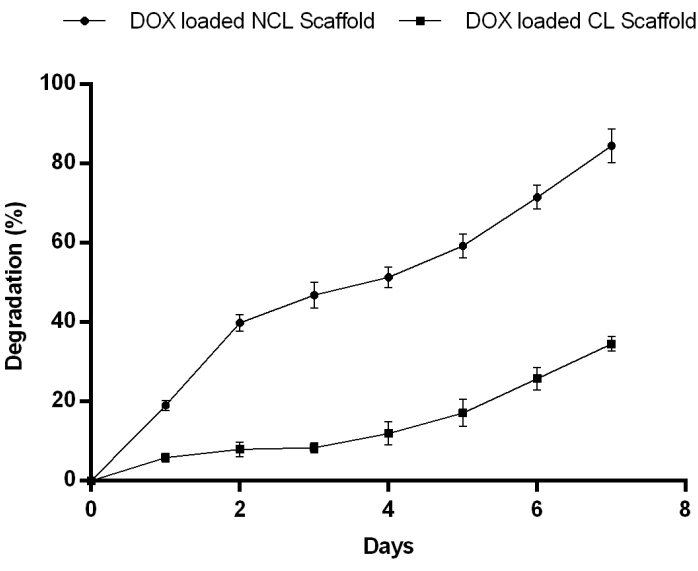
Figure 2: Matrix degradation of DOX loaded in NCL and CL scaffolds from day 1 to 7 in PBS pH 7.4 at 37 °C showing NCL degraded gradually for 7 days. Contrary to this, the CL scaffold degradation rate reduced considerably indicates enhanced resistance to enzymatic degradation. Please click here to view a larger version of this figure.
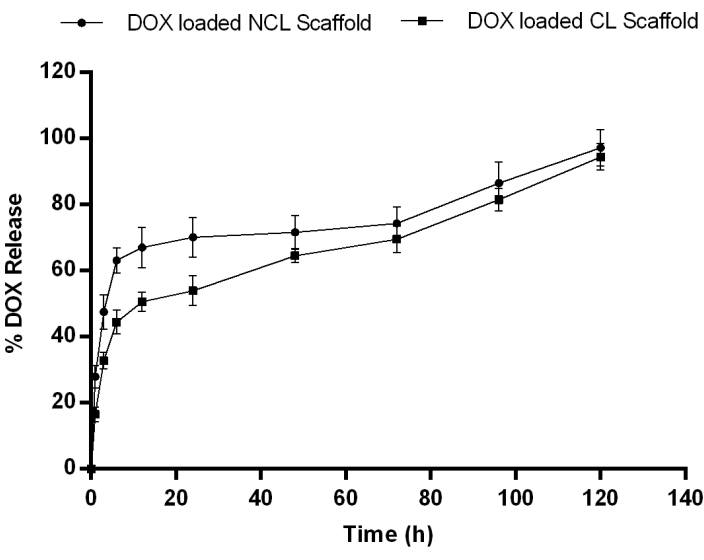
Figure 3: In vitro drug release profile of DOX from NCL and CL scaffolds in PBS pH 7.4 at 37° C showing a slow release of drug in all formulation followed by a sustained release. Data expressed as mean ± SD (n=3). Please click here to view a larger version of this figure.
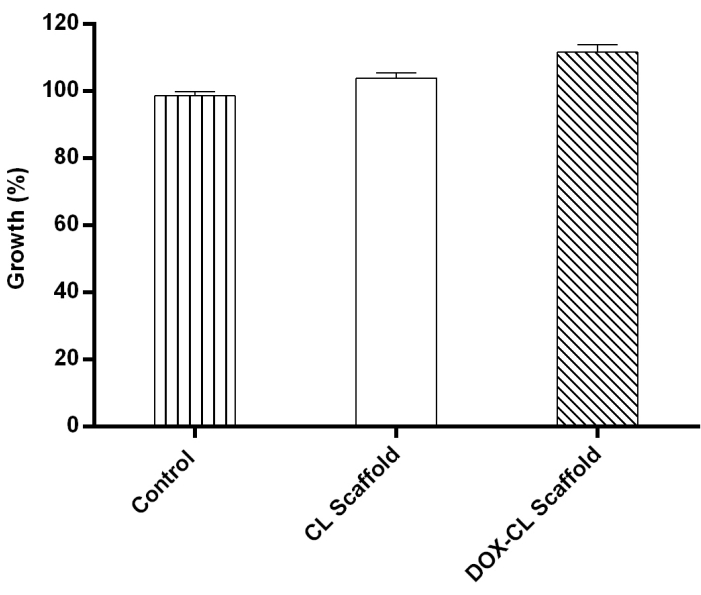
Figure 4: 3T3-L1 cells cultured in the presence of CL and DOX-CL scaffolds showing percentage cell growth more in DOX-CL scaffold treated group followed by CL scaffold treated and control. Please click here to view a larger version of this figure.
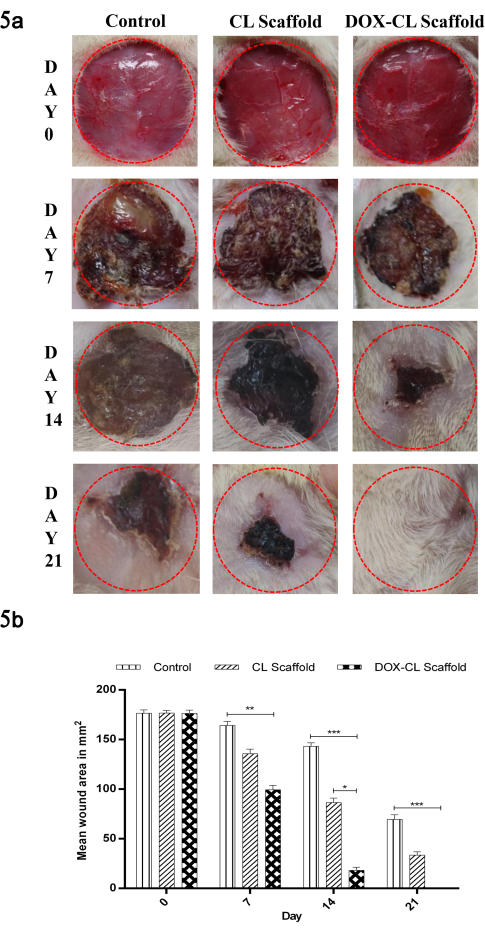
Figure 5: (a) Images representing the reduction in mean wound area in the control, CL scaffold and DOX-CL scaffold treated groups on day 0, 7, 14 and 21 post wounding. (b) Graphical representation of reduction in mean wound area in the control, CL scaffold and DOX-CL scaffold treated groups on day 0, 7, 14 and 21 post wounding. Data is expressed as mean ± SD (n=6 wounds/ group). Statistical significance was obtained by One Way Analysis of Variance (ANOVA) followed by Dunnet's post hoc test. Please click here to view a larger version of this figure.
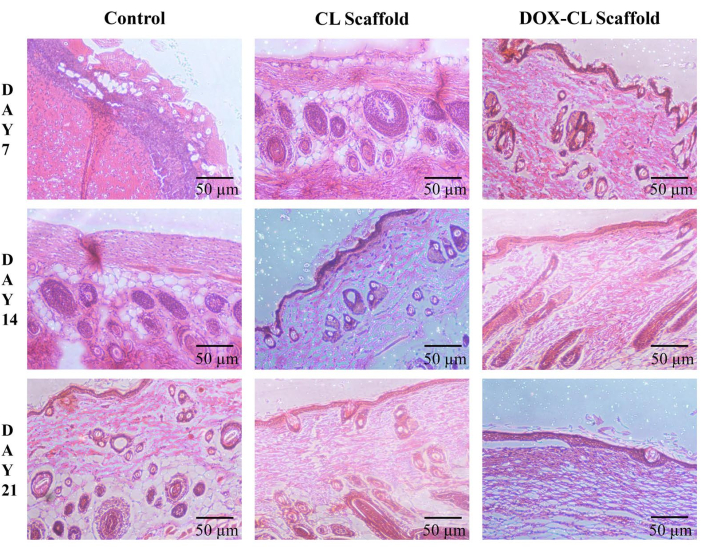
Figure 6: Histological changes during the wound healing process in STZ and high-fat diet-induced induced diabetes in Wistar albino rat skin on days 7, 14, and 21 without (control) and with treatment (CL and DOX-CL scaffolds) in a full-thickness excision wound model. Please click here to view a larger version of this figure.
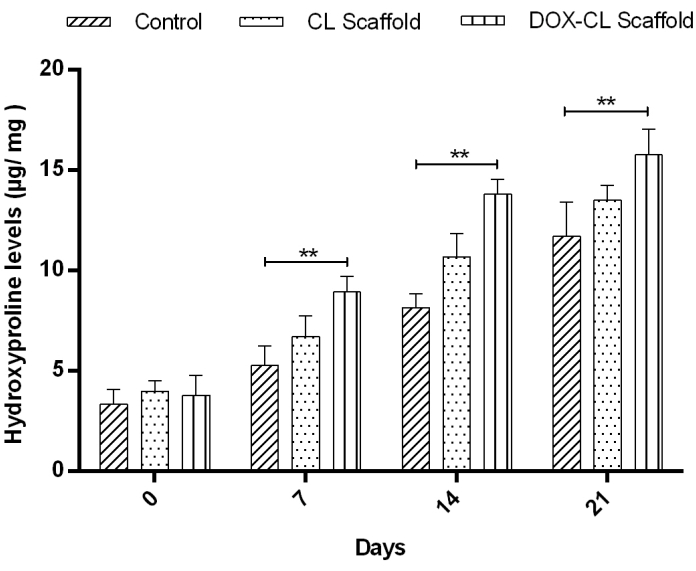
Figure 7: Result representing the hydroxyproline content in wounds on days 0, 7, 14, and 21 as an indicator of indirect collagen level estimation. The results are expressed in µg hydroxyproline/ mg of dry wound tissue. The data represents the Mean±SD (n=6 wounds/group). Statistical significance was obtained by One Way Analysis of Variance (ANOVA) followed by Dunnet's post hoc test. Please click here to view a larger version of this figure.
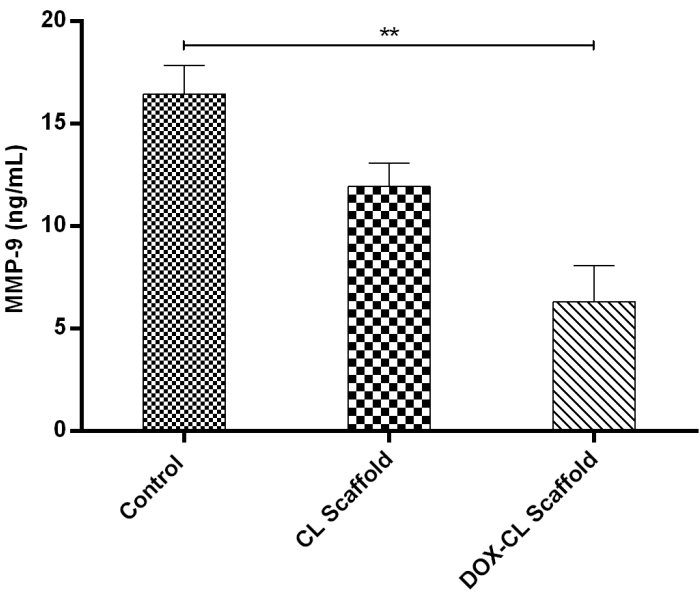
Figure 8: Graph representing the MMP-9 levels in homogenates obtained from healed wounds in STZ and high-fat diet-induced induced diabetic rat model on day 21. Levels of MMP-9 were determined in 100-fold aliquots of wound fluids using ELISA analysis. The data represent Mean±SD (n=3). Please click here to view a larger version of this figure.
| MIC (µg/mL) | ||||
| Test sample | S. aureus | S. epidermis | E. coli | P. aeruginosa |
| DOX | <4 | <4 | <8 | <16 |
| CS | <64 | <64 | <128 | <128 |
| CL scaffold extract | <64 | <64 | <128 | <128 |
| DOX-CL scaffold extract | <2 | <2 | <4 | <4 |
Table 1: Minimum inhibitory concentration of CS, DOX, CL, and DOX-CL Scaffolds against a selected panel of bacteria.
Discussion
The main objective of this study was to determine the effect of DOX loaded COL-CS scaffold on DW healing in rats. CL and NCL were prepared and evaluated in terms of morphology, swelling index, in vitro release kinetics, and biocompatibility.
Characterization of the DOX loaded NCL and CL scaffold
The prepared scaffolds were found to be porous with interconnected pores. These interconnected pores assure the porous, spongy nature that helps in the proper diffusion of oxygen and nutrients to the cells for proliferation and migration, leading to accelerated healing. Here, the pore size was mainly dependent on the crosslinking. Crosslinking the scaffold resulted in decreased pore size by forming strong intramolecular interactions by EDC/NHS with CS and COL, increasing the scaffold mechanical strength.
The porosity index relies on relative density, which is a function of the mass of sponge struts and volume. Generally, shrinkage of scaffold volume occurs if a crosslinking agent alters the struts position by dragging them closer to each other, leading to scaffold densification. However, in the case of carbodiimide chemistry, an increase in the mass of scaffold is not anticipated due to its unique mechanism, i.e., the introduction of amide linkage (O=C-NH) using carboxylic (>COOH) and amine (-NH) group of adjacent peptide chain without entering into the scaffold. Simultaneously, during crosslinking, minor mass loss may occur due to the dissolution and migration of unattached polymer chains. Nevertheless, in case of scaffold treatment with EDC/NHS did not produce any noticeable effect on scaffold morphology.
The ability of the scaffold to absorb biological fluid is crucial in evaluating its suitability as a drug carrier. The water absorption property not only impacts the shape of the scaffold but also affects cell growth. It was observed that the CL scaffold had shown a lower water absorption rate than the NCL scaffold, which may be attributed to the crosslinking of the scaffold. Moreover, the hydrophilicity of scaffold material was significantly reduced after crosslinking due to (i) loss of hydrophilic groups (>COOH & -NH) in the process of amide linkage formation; (ii) inhibition of swelling by the formation of new bonds (O=C-NH). These results are in good agreement with the previously published reports 23,24.
An enzymatic biodegradation study was performed by monitoring the percentage of a residual mass after seven days of incubation in the simulated wound fluid of pH 7.4, comprising lysozymes. The NCL scaffold showed a faster rate of degradation initially in the first three days and decreased slowly for four consecutive days. On the other side, the CL scaffold displayed a prolonged rate of degradation. Crosslinking of the scaffold enhanced mechanical properties and network strength that, in turn, resulted in decreased degradation rate, demonstrating improved resistance to the degradation.
Drug release studies were performed for CL and NCL scaffolds for 120 h. In general, the scaffold's drug release coefficient decreases as the crosslinking density increases due to enhanced interconnections between the pores and decreased space between polypeptide chains. The drug release coefficient is also influenced by the porosity index, water content, swelling index, and degree of crosslinking. In our study, 27.92±3.45% and 63.15±3.78% of DOX from the NCL scaffold, whereas 16.54±2.21% and 44.43±3.57% of DOX from the CL scaffold was released at 1 h and 6 h, respectively. After 24 h, there was a release of 70% of DOX from the NCL scaffold, whereas; CL scaffold took more than 72 h to release 70% of the DOX. The results showed that the scaffold crosslinking inversely influenced DOX release by decreasing the swelling ratio and water content.
In vitro anti-bacterial studies
DOX-CL scaffold extract exhibited higher inhibitory activity (MIC) against gram-positive organisms. The higher activity of the DOX-CL scaffold extract might be because of the increased hydrophobicity of the sample. However, DOX-CL scaffold extract exhibited minimal activity against gram-negative organisms, probably due to cell wall penetration failure. Further, the MIC of DOX-CL was comparatively higher than CS, CL scaffold, and DOX samples, which may be attributed to the synergistic activity of DOX and CS in the DOX-CL extract.
In vitro biocompatibility study
The in vitro biocompatibility study was performed on fibroblast cells (3T3-L1). Fibroblasts are responsible for the production of ECM and proteins. Further, they play a crucial role in maintaining structural integrity by enhancing the formation of the framework required for wound healing. The results show that the DOX-CL scaffold and CL scaffold did not produce any cell cytotoxicity. However, both the scaffold groups were observed with the cell growth and differentiation of fibroblasts in the scaffold pores, indicating that the prepared scaffolds were biocompatible. Increased cell growth and differentiation in both the groups were mainly due to the presence of CS and COL as an extracellular matrix in the scaffold structure.
Moreover, comparatively higher cell growth was observed with the DOX-CL scaffold group than that of the CL scaffold due to activation of the PI3K-AKT signal by DOX, responsible for cell growth and survival 25.
In vivo wound healing studies
The various complications of DM are responsible for the prolonged healing of DW. To mimic the diabetic condition in rats, a high-fat diet was given, followed by an intraperitoneal injection of STZ. After 2-3 days of diabetic confirmation, open wounds were created by the excision of skin at the dorsal thoracic region. The treatment was carried out for 21 days with respective scaffolds. Reduction in the wound area was determined for all the groups on days 0, 7, 14, and 21. Diabetic rats treated with the DOX-CL scaffold exhibited a significant degree of wound contraction compared to CL scaffold groups and untreated groups.
The inflammatory phase of wound healing is responsible for functional barrier formation. In the next stage, the proliferative phase of wound healing grabs attention in wound care, in which 43% of wound area reduction was observed with the DOX-CL scaffold group. In comparison, only 23% and 7% were observed with CL scaffold and untreated groups on day 7, respectively. From the images (Figure 5), the contraction of the wound was initiated after the inflammatory and proliferative phase completes. DOX-CL scaffold group exhibited significant wound contraction due to the presence of DOX, which is responsible for the anti-inflammatory and anti-infective effects. Moreover, COL and CS in the scaffold helped cell proliferation, differentiation, and migration resulting in 99% of DW healing in 21 days.
Untreated excisional wounds showed neutrophils, lymphocytes, and monocytes on day 7 postinjury. As stated earlier, neutrophils aid in wound debridement in the early stage of wound healing. Besides, the overabundance of neutrophils may negatively influence the healing process by damaging the normal tissue. As reported by Dovi et al., a minimal number of neutrophils accelerates the re-epithelialization process 26.
Further, increased macrophages, histiocytes, and massive multinucleated cells in the DOX-CL scaffold treated group favored the environment for accelerated wound healing by repairing the damaged cells and promoting the COL synthesis. Moreover, COL amalgamation with tissue was determined using the hydroxyproline levels in all the groups. However, comparatively higher hydroxyproline levels were observed with the DOX-CL scaffold treated group than the untreated and CL scaffold treated group. As stated in the literature, the prolonged inflammatory phase in the diabetic condition hinders the other phases of healing. The faster rate of wound contraction in the DOX-CL scaffold may be attributed to DOX's anti-inflammatory property, resulting in the further stages of the wound occurring in the proper time phase leading to accelerated wound healing.
The biochemical estimation of MMP-9 levels using the ELISA test reveals that the DOX-CL scaffold treated group exhibited decreased levels of MMP-9 in comparison with those in CL scaffold treated and untreated groups. According to the literature, increased levels of MMP-9 responsible for matrix degradation, which, in turn, prolongs the wound healing process. DOX, an anti-inflammatory agent, inhibited the MMP-9 levels, which is considered the reason to prevent the COL breakdown, resulting in accelerated DW healing. These results are in agreement with earlier published studies by Lindeman et al., 2009 and Zhang et al., 2014 27,28.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Ashish D Wadhwani. (Assistant Professor and Head, Department of Pharmaceutical Biotechnology, JSS College of Pharmacy, Ooty, India) for assisting in In vitro cell viability studies.
The authors would like to thank the Department of Science and Technology – Fund for Improvement of Science and Technology Infrastructure in Universities and Higher Educational Institutions (DST-FIST), New Delhi, for supporting our department.
The authors also like to thank Mr. Sanju. S and Mr. Sriram. Narukulla M. Pharm students for their support in the video shoot.
This research was supported by the JSS Academy of Higher Education & Research (JSSAHER).
Materials
| 1-ethyl-(3-3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC) | Merck Millipore, Mumbai, India | E7750 | |
| 2-(N-morpholino) ethane sulfonic acid (MES) | Merck Millipore, Mumbai, India | 137074 | |
| 3-(4, 5 dimethyl thiazole-2 yl) -2, 5-diphenyl tetrazolium bromide (MTT) | Thermo Fisher, Mumbai, India | M6494 | |
| Deep freezer verticle | Labline Instruments, Kochi, India | ||
| Dialysis sack | Merck Millipore, Mumbai, India | D6191-Avg. flat width 25 mm (1.0 in.), MWCO 12,000 Da | |
| Doxycycline | Sigma chemicals Co. Ltd, Mumbai, India | D9891 | |
| Elisa kit | R&D Systems | RMP900 | |
| Escherichia coli (E. coli) | National Collection of Industrial Microorganisms, Pune, India | NCIM 2567 | |
| Ethanol | Merck Millipore, Mumbai, India | 100983 | |
| Lyophilizer-SZ042 | Sub-Zero lab instruments, Chennai, India | ||
| Mechanical Stirrer-RQ-122/D | Remi laboratory instruments, Mumbai, India | ||
| Medium molecular weight Chitosan | Sisco Research Laboratories Pvt. Ltd., Mumbai, India | 18824 | |
| Microtome-RM2135 | Leica, U.K | ||
| Mouse embryonic fibroblast cells (3T3-L1) | National Centre for Cell Sciences, Pune, India | ||
| Multiple plate reader -Inifinte M200 Pro | Tecan Instruments, Switzerland | ||
| N-hydroxy succinimide (NHS) | Sigma chemicals Co. Ltd, Mumbai, India | 130672 | |
| Pseudomonas aeruginosa (P. aeruginosa) | National Collection of Industrial Microorganisms, Pune, India | NCIM 2036 | |
| Scanning Electron Microscopy (SEM)-S-4800 | Hitachi, India | ||
| Sodium hydroxide (NaOH) pellets | Qualigen fine chemicals, Mumbai, India | Q27815 | |
| Staphylococcus aureus (S. aureus) | National Collection of Industrial Microorganisms, Pune, India | NCIM 5022 | |
| Staphylococcus epidermis (S. epidermis) | National Collection of Industrial Microorganisms, Pune, India | NCIM 5270 | |
| Streptozotocin (STZ) | Sisco Research Laboratories Pvt. Ltd., Mumbai, India | 14653 | |
| Type-1 rat Collagen | Sigma chemicals Co. Ltd, Mumbai, India | C7661 | |
| Ultraviolet–visible spectroscopy-1700 | Shimadzu |
Referências
- . IDF Diabetes Atlas, 9th edn Available from: https://www.diabetesatlas.org (2019)
- Falanga, V. Wound healing and its impairment in the diabetic foot. The Lancet. 366 (9498), 1736-1743 (2005).
- Frykberg, R. G., Banks, J. Challenges in the treatment of chronic wounds. Advances in Wound Care. 4 (9), 560-582 (2015).
- Alexiadou, K., Doupis, J. Management of diabetic foot ulcers. Diabetes Therapy. 3 (1), 1-15 (2012).
- Karri, V. V. S. R., et al. Current and emerging therapies in the management of diabetic foot ulcers. Current Medical Research and Opinion. 32 (3), 519-542 (2016).
- Sanapalli, B. K., et al. Human beta defensins may be a multifactorial modulator in the management of diabetic wound. Wound Repair and Regeneration. 28 (3), 416-421 (2020).
- Caley, M. P., Martins, V. L., O’Toole, E. A. Metalloproteinases and wound healing. Advances in Wound Care. 4 (4), 225-234 (2015).
- Reiss, M. J., et al. Matrix metalloproteinase-9 delays wound healing in a murine wound model. Surgery. 147 (2), 295-302 (2010).
- Gill, S. E., Parks, W. C. Metalloproteinases and their inhibitors: regulators of wound healing. The International Journal of Biochemistry & Cell Biology. 40 (6-7), 1334-1347 (2008).
- Stechmiller, J., Cowan, L., Schultz, G. The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biological Research for Nursing. 11 (4), 336-344 (2010).
- Griffin, M. O., Fricovsky, E., Ceballos, G., Villarreal, F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. American Journal of Physiology-Cell Physiology. 299 (3), 539-548 (2010).
- Burns, F., Stack, M., Gray, R., Paterson, C. Inhibition of purified collagenase from alkali-burned rabbit corneas. Investigative Ophthalmology & Visual Science. 30 (7), 1569-1575 (1989).
- Kraus, R. L., et al. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. Journal of Neurochemistry. 94 (3), 819-827 (2005).
- Yrjänheikki, J., Keinänen, R., Pellikka, M., Hökfelt, T., Koistinaho, J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proceedings of the National Academy of Sciences. 95 (26), 15769-15774 (1998).
- Moura, L. I., Dias, A. M., Carvalho, E., de Sousa, H. C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment-a review. Acta Biomaterialia. 9 (7), 7093-7114 (2013).
- Natarajan, J., et al. Nanostructured Lipid Carriers of Pioglitazone Loaded Collagen/Chitosan Composite Scaffold for Diabetic Wound Healing. Advances in Wound Care. 8 (10), 499-513 (2019).
- Karri, V. V. S. R., et al. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. International Journal Of Biological Macromolecules. 93, 1519-1529 (2016).
- Hsieh, W. -. C., Chang, C. -. P., Lin, S. -. M. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids and Surfaces B: Biointerfaces. 57 (2), 250-255 (2007).
- Xie, Y., Chen, J., Xiao, A., Liu, L. Antibacterial activity of polyphenols: structure-activity relationship and influence of hyperglycemic condition. Molecules. 22 (1913), 1-11 (2017).
- Geerlings, S. E., Brouwer, E. C., Gaastra, W., Verhoef, J., Hoepelman, A. I. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichia coli: studies with urine from diabetic and non-diabetic individuals. Journal of Medical Microbiology. 48 (6), 535-539 (1999).
- Eloff, J. N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica. 64 (8), 711-713 (1998).
- Reddy, G. K., Enwemeka, C. S. A simplified method for the analysis of hydroxyproline in biological tissues. Clinical Biochemistry. 29 (3), 225-229 (1996).
- Charulatha, V., Rajaram, A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 24 (5), 759-767 (2003).
- Rehakova, M., Bakoš, D., Vizarova, K., Soldán, M., Jurícková, M. Properties of collagen and hyaluronic acid composite materials and their modification by chemical crosslinking. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials. 30 (3), 369-372 (1996).
- Chang, M. -. Y., et al. Doxycycline enhances survival and self-renewal of human pluripotent stem cells. Stem Cell Reports. 3 (2), 353-364 (2014).
- Dovi, J. V., He, L. K., DiPietro, L. A. Accelerated wound closure in neutrophil-depleted mice. Journal of Leukocyte Biology. 73 (4), 448-455 (2003).
- Lindeman, J. H., Abdul-Hussien, H., van Bockel, J. H., Wolterbeek, R., Kleemann, R. Clinical Perspective. Circulation. 119 (16), 2209-2216 (2009).
- Zhang, C., Gong, W., Liu, H., Guo, Z., Ge, S. Inhibition of matrix metalloproteinase-9 with low-dose doxycycline reduces acute lung injury induced by cardiopulmonary bypass. International Journal Of Clinical And Experimental Medicine. 7 (12), 4975-4982 (2014).

