Gene Knock-in by CRISPR/Cas9 and Cell Sorting in Macrophage and T Cell Lines
Summary
This protocol uses fluorescent reporters and cell sorting to simplify knock-in experiments in macrophage and T cell lines. Two plasmids are used for these simplified knock-in experiments, namely a CRISPR/Cas9- and DsRed2-expressing plasmid and a homologous recombination donor plasmid expressing EBFP2, which is permanently integrated at the Rosa26 locus in immune cells.
Abstract
Functional genomics studies of the immune system require genetic manipulations that involve both deletion of target genes and addition of elements to proteins of interest. Identification of gene functions in cell line models is important for gene discovery and exploration of cell-intrinsic mechanisms. However, genetic manipulations of immune cells such as T cells and macrophage cell lines using CRISPR/Cas9-mediated knock-in are difficult because of the low transfection efficiency of these cells, especially in a quiescent state. To modify genes in immune cells, drug-resistance selection and viral vectors are typically used to enrich for cells expressing the CRIPSR/Cas9 system, which inevitably results in undesirable intervention of the cells. In a previous study, we designed dual fluorescent reporters coupled to CRISPR/Cas9 that were transiently expressed after electroporation. This technical solution leads to rapid gene deletion in immune cells; however, gene knock-in in immune cells such as T cells and macrophages without the use of drug-resistance selection or viral vectors is even more challenging. In this article, we show that by using cell sorting to aid selection of cells transiently expressing CRISPR/Cas9 constructs targeting the Rosa26 locus in combination with a donor plasmid, gene knock-in can be achieved in both T cells and macrophages without drug-resistance enrichment. As an example, we show how to express human ACE2, a receptor of SARS-Cov-2, which is responsible for the current Covid-19 pandemic, in RAW264.7 macrophages by performing knock-in experiments. Such gene knock-in cells can be widely used for mechanistic studies.
Introduction
Immune cells are critical for defense against pathogens. Both innate and adaptive immunity are required for clearance of infectants and maintenance of tissue homeostasis1,2. Cell line models are essential tools for understanding the molecular fundamentals of the mammalian immune system; they are used in in vitro functional assays, such as those modeling human T cell activation, and in determining the function of genetic factors in activating or dampening immune responses3,4. It is important to note that the mammalian immune system is enormously heterogeneous and, equally important, a huge number of molecules control the differentiation, migration, and function of a given cell type5,6.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)/Cas9 genome editing tools allow for genetic manipulation of specific cell types, which facilitates functional annotation of genes in a precise manner7,8. Several published protocols have described the delivery of CRISPR/Cas9 in the form of Cas9-guide RNA complexes known as a ribonucleoproteins (RNPs) in HEK293 cells, Jurkat cell lines, primary T cells9,10, macrophages11,12,13, stem cells14, and others15,16. In these protocols, gene tagging is usually achieved by fusing a fluorescent tag to endogenous proteins17,18. However few attempts have been made to use dual fluorescent reporters, which are compatible with single cell sorting, to facilitate knock-in experiments19,20, particularly in immune cells.
In-depth mechanistic analyses aimed at understanding the functions of a novel genetic factor in immune cells generally require cell-type specific deletion of a gene, genetic rescue experiments, and ideally identification of its interactors. Even though methods for optimization of genetic deletion of genes in immune cells have been published9,15,21, far fewer methods have been reported for introducing knock-in alleles with versatile functions to understand the immune response. Therefore, in this protocol we aim to describe in detail an efficient and highly reproducible protocol to express a protein of interest (POI) at the safe harbor locus Rosa26 in both human and murine immune cell lines. We designed a two-color reporter system to enrich for cells transfected with plasmids expressing CRISPR/Cas9 (DsRed2) and a recombinant DNA template (EBFP2), which can be isolated by cell sorting. Following this protocol, we obtained multiple knock-in lines of the human T cell line Jurkat and murine macrophage RAW264.7 for functional analyses of poorly studied proteins.
As an example, we show in this protocol how to obtain knock-in RAW264.7 macrophages stably expressing human ACE2 (a receptor of SARS-Cov-2)22. Because innate immune cells are involved in the pathogenesis of Covid-1923,24 and human ACE2 is regarded as a major receptor required for viral entry into cells before replication, macrophages with knock-in of human ACE2 can serve as a useful tool for mechanistic studies of viral multiplication inside macrophages. In parallel, we also present an example of knock-in of a gene at the human ROSA26 locus to express the RASGRP1 protein, which was fused at its amino terminus with an affinity Twin-Strep-tag (OST). T cells are key target cells in immune therapies, and an increasing number of studies have focused on manipulation of their responsiveness to cancer25,26. As Rasgrp1 is known to be a key signaling molecule downstream of the T cell receptor and its interactors are not well elucidated27, the OST-RASGRP1 knock-in model provides the foundation for identifying interactors regulating the response of T cells to tumors and infection. Taken together, these tools can be used for Covid-19 studies and the discovery of novel molecules interacting with Rasgrp1.
Protocol
1. Design and Plasmid Construction of sgRNAs Targeting Rosa26 Locus
- Design guide RNAs around the desired insertion site
- Ensure that the insertion site for mouse Rosa26 (hereafter designated as mRosa26) knock-in experiments is located in the first intron of mRosa26; this site has been used in previous studies28,29. For knock-in experiments in human cells, ensure that the insertion site resides in the human ROSA26 locus (hereafter referred as hROSA26), which has been identified as the human homolog of mRosa2630.
- Use the genomic sequences of mouse and human species obtained from Mouse Genome Informatics (http://www.informatics.jax.org/) and Ensembl genome browser (http://www.ensembl.org/index.html), respectively. Copy 50 base pairs (bp) of the genomic sequence of the mRosa26 or hROSA26 locus on each side flanking the desired insertion site.
- Design guide RNAs using the online web tool CRISPOR (http://crispor.tefor.net/)31. Paste the 100 bp of input sequence from 1.1.2. Select two guide RNAs with a high specificity score, a high efficiency score, and low off-target activity. In addition, choose RNA guides with higher Doench scores and avoid those labeled as “Inefficient”32.
NOTE: Alternative CRISPR design tools are also valuable and publicly available, for instance the Benchling CRISPR design tool (https://benchling.com/crispr). To increase the frequency of CRISPR/Cas9-mediated knock-in mutated cells, select two guide RNAs close to the desired insertion site when possible33. - For the mRosa26 locus, use two adjacent guide RNAs designated as mR26-sg1 (5'- CTCCAGTCTTTCTAGAAGAT -3') and mR26-sg2 (5'- CGCCCATCTTCTAGAAAGAC -3') (Figure 1A). For the hROSA26 locus, use two adjacent guide RNAs, namely hR26-sg1 (5'- GGCGATGACGAGATCACGCG -3') and hR26-sg2 (5'- AATCGAGAAGCGACTCGACA -3') (Figure 1B).
NOTE: Genome editing activities of mR26-sg1 and mR26-sg2 and those of hR26-sg1 and hR26-sg2 were evaluated in RAW264.7 cells and Jurkat cells, respectively (See Supplementary File, Figure S1).
- Cloning of CRISPR expression vectors containing sgRNA targeting the Rosa26 Locus
- Synthesize forward and reverse oligos for one guide RNA (See Supplementary File).
NOTE: It is preferable to add a 'G' nucleotide to the start of the guide sequence, which helps with expression driven by the U6 promoter. For example, to clone the aforementioned mR26-sg1, the forward oligo is CACCGCTCCAGTCTTTCTAGAAGAT and the reverse oligo is AAACATCTTCTAGAAAGACTGGAGC. The additional G in the forward oligo and its complement in the reverse oligo are indicated in bold. - Clone synthetic oligos corresponding to the guide RNAs into the all-in-one CRISPR expression vector pX458-DsRed2 generated in our previous work21 (Figure 1C) using the general protocol for gRNA cloning developed by Zhang's Lab (https://www.addgene.org/crispr/zhang/); however, skip the gel purification step and purify the digested vector using a PCR purification kit (see Table of Materials).
NOTE: In previous protocols, gel purification of the BbsI-digested vector was performed; however, this step is time-consuming. In the present protocol, the digested product is purified using a PCR purification kit and used directly for the downstream ligation reaction, which generally produces positive colonies with comparable efficiency. - Transform 10 µL of the ligation product (step 1.2.2) into 50 µLof Escherichia coli DH5α competent cells according to the manufacturer's instructions. Randomly pick three colonies from an LB agar plate with ampicillin (100 µg/mL) and inoculate each into 3 mL of LB medium with ampicillin for culturing overnight.
- Use 1 mL of the overnight bacterial culture for Sanger sequencing and keep the rest at 4 °C until the construct is confirmed to be correct: pDsR-mR26-sg1 and pDsR-mR26-sg2 for mRosa26 locus knock-in, and pDsR-hR26-sg1 and pDsR-hR26-sg2 for the hROSA26 locus.
- Prepare a high concentration of plasmid DNA (approximately 2 µg/µL) with a maxiprep kit for purification of transfection-grade plasmid (see Table of Materials).
- Synthesize forward and reverse oligos for one guide RNA (See Supplementary File).
2. Design and Construction of Targeting Vectors as Homologous Recombination Templates
- Design a homology-directed repair template
- Design a targeting vector to constitutively express the POI using an expression cassette optimized from a previous study29, which includes a CAG hybrid promoter, an AscI restriction site permitting cloning of the cDNA of the POI, an IRES-EBFP2 reporter, and a bovine growth hormone polyadenylation (bGH-polyA) signal (Figure 1B and Supplementary File).
- Design homology arms (HAs) that share homology with the genomic sequences of the mRosa26 or hROSA26 locus. Include ~1 kb of the 5' HA corresponding to the upstream genomic sequence from the desired insertion site to the left of the expression cassette. Similarly, include ~1 kb of the 3' HA corresponding to the downstream genomic sequence from the insertion site to the right of the expression cassette.
NOTE: The expression cassette is inserted as close as possible to the CRISPR/Cas9 cleavage sites34. To avoid re-cutting of the targeted allele by CRISPR/Cas9, incorporate nucleotide changes into the protospacer-adjacent motif (PAM) sequence (5'-NGG-3') in the targeting vector34,35. - To allow linearization of the targeting vector, insert a unique EcoRI site just upstream of the 5' HA and a unique BamHI site just downstream of the 3' HA.
- Have a commercial vendor synthesize the targeting vectors and designate them as pKR26-iBFP and pKhR26-iBFP for mRosa26 and hROSA26 knock-in, respectively.
- Construct a targeting vector as a homology-directed repair template
- To express the POI, obtain the cDNA sequence (coding sequence) from the Ensembl genome browser and choose the transcript possessing the longest protein sequence. For example, the cDNA of the human ACE2 gene is ACE2-202 ENST00000427411.2 and the cDNA of human RASGRP1 gene is RASGRP1 ENSG00000172575.
- Synthesize the cDNA of the POI with the help of a commercial vendor. It is also possible to clone the cDNA via PCR amplification (not described here). Place the sequence GGCGCGCCACC (5' – 3'), which includes an AscI restriction site (italics and bold) and Kozak consensus translation initiation site (underlined), immediately before the ATG initiation codon of the cDNA and another AscI restriction site (5'- GGCGCGCC -3') after the stop codon of the cDNA.
- Digest the synthesized sequence with AscI and purify using a PCR purification kit designed for purifying DNA fragments after restriction digestion, following the manufacturers' instructions (see Table of Materials).
- Ligate the purified cDNA insert into the AscI-linearized backbone vector pKR26-iBFP or pKhR26-iBFP using T4 DNA ligase following the manufacturer's instructions.
- Verify the final targeting vector, pKR26-POI-iBFP or pKhR26-POI-iBFP, by sequencing. Use maxiprep to purify the verified constructs according to the manufacturer's protocol.
- Digest the targeting vector using either EcoRI or BamHI and purify the digestion product using a PCR purification kit according to the manufacturer's instructions. Store the linearized targeting vector (~0.8 µg/µL) at -20 °C until electroporation.
3. Electroporation of Macrophage and T Cell Lines
- Prepare cell cultures for electroporation
- Prepare Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% fetal bovine serum (FBS) as complete growth medium for Jurkat T cells. Prepare Dulbecco's Modified Eagle Medium (DMEM) with 10% FBS for culturing RAW264.7 macrophages. Supplement all complete growth media with 100 U/mL penicillin and 100 µg/mL streptomycin (Pen/Strep) except for media used for post-transfection incubation before cell sorting.
- Perform subculturing of RAW264.7 and Jurkat T cells according to the supplier's instructions (See Table of Materials). When subculturing RAW264.7 cells, use trypsin-EDTA solution (0.25%) to detach cells. Use FBS supplemented with 10% (v/v) DMSO as a cryopreservation medium for RAW264.7 and Jurkat cells.
- Collect cells at 250 × g for 5 min for RAW264.7 macrophages and 90 × g for 8 min for Jurkat T cells. Then wash with 5-10 mL of 1× DPBS (without Ca2+ or Mg2+ ions). Remove the DPBS.
- Resuspend the cell pellet using 2 mL of 1× DPBS. Use 10 µL of cells and mix with an equal volume of 0.2% trypan blue to estimate the cell count and viability.
NOTE: Ensure that the cell culture has >90% viability on the day of transfection. - For a single knock-in experiment, perform electroporation with 10 µL nucleofection tips with five repetitions. Calculate the volume needed for 2.0 × 106 cells and pellet the cells by centrifugation. Wash the cell pellet again with 1× DPBS as described in step 3.1.3.
NOTE: When using 10 µL nucleofection tips, 4.0 × 105 cells are needed per electroporation. Accordingly, prepare at least 2.0 × 106 cells for one knock-in experiment. - Prepare a 24-well plate with 0.5 mL of complete growth medium (prepared in step 3.1.1) per well without Pen/Strep and prewarm in a 37 °C incubator.
- Electroporation of CRISPR/Cas9 components and the targeting vector
- Turn on the electroporation system. Use electroporation parameters optimized in a previous study21: 1,400 V/20 ms/2 pulses for RAW264.7 macrophages and 1350 V/20 ms/2 pulses for Jurkat T cells.
- Accounting for sample loss due to pipetting, prepare a 55 µL electroporation mixture in a sterile 1.5 mL microcentrifuge tube containing 2.5 µg of each CRISPR/Cas9 vector, 2.4 µg of the linearized targeting vector, and the Resuspension Buffer R.
NOTE: To save time, the electroporation mixture can be prepared during centrifugation (step 3.1.5). - Resuspend 2.0 × 106 cells (prepared in step 3.1.5) in the 55 µL electroporation mixture from step 3.2.2.
- Aspirate the cell/electroporation mixture from step 3.2.3 using a 10 µL nucleofection tip with a pipette.
NOTE: During pipetting, avoid introducing air bubbles, which may cause electroporation failure. - Add the sample to a tube filled with 3 mL of Buffer E from the electroporation kit.
- Apply the electroporation parameters for the two cell types as described in step 3.2.1.
- Transfer the sample into one well of the 24-well plate with prewarmed medium from step 3.1.6.
- Repeat steps 3.2.4-3.2.7 for the other four repetitions as well as for the targeting vector only and CRISPR expression vector only controls.
NOTE: Change the nucleofection tip and tube when switching to a different cell type/plasmid DNA. - Culture the transfected cells for 48-72 h to allow for recovery after electroporation and expression of CRISPR/Cas9 components prior to flow cytometry analysis or fluorescence-activated cell sorting (FACS). Examine the expression of DsRed2 using a fluorescent microscope equipped with a red channel at 24 h post-transfection.
NOTE: A benefit of monitoring DsRed2 fluorescent cells is that the efficiency of electroporation can be estimated and the suitability for further cell sorting can be predicted.
4. Cell Sorting to Isolate Putative Knock-in Cells
- Before FACS sorting, wash the transfected cells once with complete growth medium. Pellet cells by centrifugation as described in step 3.1.3 and resuspend the pellet in 500 µL of fresh medium.
- Set the cell sorter (located in a biosafety cabinet) with an 85 µm nozzle and low flow rate. Select the "single cell" mode for sorting and test the single cell sorting efficiency and precision by sorting 20-30 cells onto the cover of 96-well microplate. One droplet localized at the center of each well indicates proper setup of the instrument.
- Transfer the cell suspension from step 4.1 into sterile FACS tubes and add SYTOX Red Dead Cell Stain to a final concentration of 1 nM.
- Analyze samples on the cell sorter. SYTOX Red and EBFP2 are excited by a 405 nm laser and detected by the V450/BV421 and APC channels, respectively. DsRed2 is excited by a 488 nm laser and detected by the PE channel. Use non-transfected cells and EBFP2- and DsRed2-single positive cells as controls for spectral compensation.
- Sort 10 single cells into each well of a 96-well microplate containing 150 µL per well of pre-warmed complete growth medium. Use round bottom microplates for culturing suspension cell lines such as Jurkat T cells and flat bottom microplates for adherent RAW264.7 macrophages.
NOTE: Sorting of 10 cells per well is an optimized strategy for improving cell survival and minimizing the number of 96-well microplates that need to be seeded and the number of samples that need to be screened by flow cytometry and genotyping; if sorting only one cell per well, seeding of more microplates is required to increase the chance of obtaining correctly edited cells.
5. Screening and Validation of Positive Knock-in Cells
- Screening for candidate knock-in cells by flow cytometry
- Incubate the cells from step 4.5 for 10-15 days and add complete growth medium every 3 days to replace liquid lost through evaporation. For Jurkat T cells, transfer cells grown in the round bottom 96-well plate to a flat bottom plate to optimize cell proliferation.
- Transfer the expanded sorted cells to 48-well plates for further expansion.
- When cells are close to confluence, use half of the culture to screen for EBFP2 expression by flow cytometry (Figure 3). Normally, half of the culture in a 48-well plate after confluent growth equates to 0.5-1.0 × 105 cells, which is adequate for analysis of one sample by flow cytometry.
- Transfer the remaining cells to 24-well plates for further proliferation.
- Keep the candidate cell populations possessing a high percentage of EBFP2-positive cells for further genotyping experiments.
NOTE: Because 10 cells are sorted into each well of the 96-well microplate, it is possible that the knock-in cells will grow with cells that do not express the knock-in gene. Therefore, it is normal to perform an additional round of cell sorting to separate the EBFP2-positive cells from the negative cells.
- Screening for candidate knock-in cells by PCR and sequencing
- Collect 2 × 105 candidate cells. Extract the genomic DNA using a DNA prep kit (See Table of Materials).
- To verify that precise homology-directed repair (HDR) has occurred at the mRosa26 locus rather than at random sites in the genome of the candidate knock-in cells, perform PCR with primers spanning each side of the HAs (Figure 4). Choose one primer located in the genomic region outside of the targeting vector (external oligo) and another primer located inside the targeting vector (internal oligo).
NOTE: A similar design is used to verify HDR at the hROSA26 locus. PCR primers can also be easily designed for detecting the insertion of the POI sequence. In addition, the wild-type and knock-in allele genotypes can be simultaneously detected using three-primer PCR (See Supplementary File, Figure S2). - Clone the positive PCR products using a fast-cloning kit (see Table of Materials). Transform the reaction mixture into DH5α competent cells.
- Randomly pick 8-10 individual bacterial colonies per transformation and sequence the PCR products from step 5.2.3 by Sanger sequencing.
- Validation of positive knock-in cells by immunoblot analysis and affinity purification
NOTE: Candidate cells are subjected to immunoblot analysis for validation of insertion of the POI, or affinity purification (AP) for studies of protein-protein interaction.- Perform immunoblot analysis according to antibody manufacturer's instructions. See Table of Materials for information regarding the use of primary antibodies and secondary antibodies.
- Subject the lysates of the knock-in cells expressing the OST-tagged POI to AP using Strep-Tactin Sepharose beads following the manufacturer's instructions (see Table of Materials).
- Detect chemiluminescence using an imager.
Representative Results
Following the protocol described above to perform knock-in experiments at the mRosa26 locus using murine RAW264.7 macrophages, we designed a targeting vector to express human ACE2, a receptor for the SARS-Cov-2 virus (Figure 2A). Using a similar design, we generated human Jurkat T cells with knock-in of the OST-tagged RASGRP1 fusion protein (Figure 2C). After transfection of three plasmids, two of which were used for expression of CRISPR/Cas9 (DsRed2; pDsR-mR26-sg1 and pDsR-mR26-sg2 for mRosa26 knock-in; pDsR-hR26-sg1 and pDsR-hR26-sg2 for the hROSA26 knock-in) and another used as a DNA template for homologous recombination (EBFP2), double positive cells expressing two fluorescent reporters were sorted into a 96-well plate. Of the RAW264.7 cells sorted using the single cell sorting mode, 0.91% were DsRed2+ EBFP2+, but 10 cells were collected into each well (Figure 2B). The Jurkat T cells had a higher transfection efficiency, and the percentage of double positive cells was 7.91% in this representative experiment, demonstrating successful knock-in of the OST-RASGRP1 fusion protein (Figure 2D).
In the next step, flow cytometry was used to screen for the candidate wells with EBFP2-positive cells. Representative histograms showed that there were obvious EBFP2-positive populations (Figure 3). It is notable that the knock-in cells did not express DsRed2 when flow cytometry was performed two weeks after cell sorting.
For discrimination of precise knock-ins and random insertions, genomic DNA from the EBFP2-positive cells was further tested by performing PCR with primers recognizing genomic sequence external to the HAs of the targeting vector and the specific region inside the expression cassette (Figure 4A and 4B). Sequence analysis of these PCR products confirmed the occurrence of HDR at the mRosa26 target site (Figure 4C). The same genotyping strategy can be applied to validate the precise insertion of the expression cassette inthe hROSA26 locus of Jurkat T cells.
To obtain a pure population of hACE2-expressing RAW264.7 cells, we further sorted the cells and validated the presence of the fluorescent reporters following expansion using wild-type cells as controls (Figure 5A). The expanded cells were EBFP2 positive, and the hACE2 protein was readily detectable in murine RAW264.7 macrophages (Figure 5B and 5C). Similarly, we validated the expression of fluorescent reporters and the OST-RASGRP1 protein in human Jurkat T cells after screening and a second round of cell sorting (Figure 6A and 6B). In addition, affinity purification of the OST-RASGRP1 protein was performed using commercially available beads. We found a higher amount of RASGRP1 protein in the total cell lysates of knock-in Jurkat cells than in those of wild-type cells; RASGRP1 knockout Jurkat cells were used as controls (Figure 6C). After purification using the OST tag, only the knock-in samples had detectable RASGRP1 (Figure 6D).
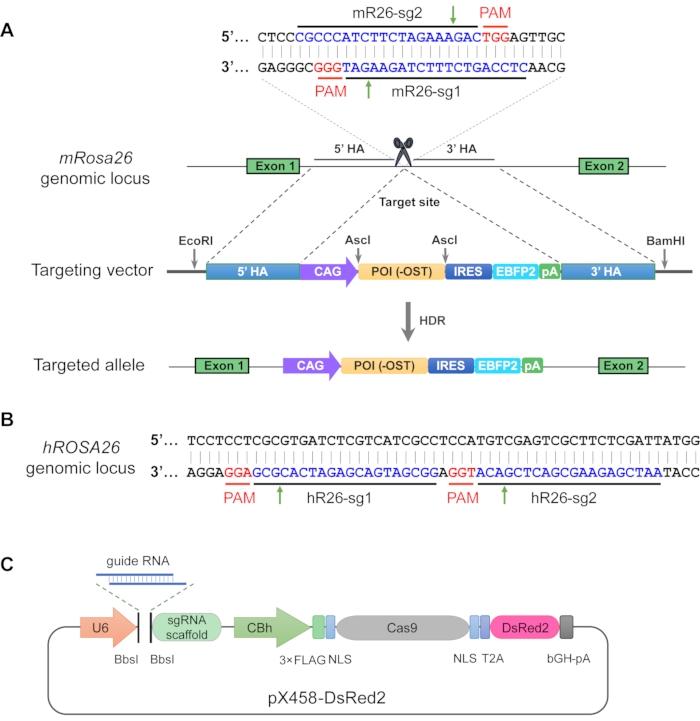
Figure 1. Gene targeting strategy for generating mRosa26/hROSA26 knock-in cell lines overexpressing a protein of interest. (A) mRosa26-specific guide RNA targeting sequences and the protospacer adjacent motifs (PAMs) in the desired insertion site are indicated in blue and red letters, respectively. Cas9 normally cleaves 3-4 bp upstream of the PAM sequence, which is indicated by green arrows. The targeting vector is used as a template for homology-directed repair (HDR) leading to the precise insertion of the expression cassette in the mouse or human genome. Each of the 1 kb sequences upstream and downstream of the desired target site are used as 5' and 3' homology arms (HAs) in the targeting vector. The HAs are separated by an expression cassette consisting of a CAG promoter, the cDNA sequence of the protein of interest (POI) with an OST-tag, an IRES-EBFP2 reporter, and a bGH-polyA signal (pA). The restriction site AscI is used for cloning of the POI, and EcoRI and BamHI are used for linearization of the targeting vector. The strategy for CRISPR/Cas9-mediated knock-in at the hROSA26 locus is similar. (B) Diagram of the human ROSA26 locus. The hR26-sg1 and hR26-sg2 targeting sequences are indicated in blue letters and the corresponding PAM sequences in red. (C) Schematic for the all-in-one CRISPR expression vector pX458-DsRed2, which contains a human U6-driven sgRNA expression cassette and Cas9-T2A-DsRed2 fluorescent reporter cassette. Two BbsI restriction sites allow for the cloning of the guide RNA. U6, human U6 RNA polymerase III promoter; sgRNA, a chimeric single-guide RNA; CBh, a chicken β-actin hybrid promoter; NLS, nuclear localization signal; T2A, Thosea asigna virus 2A self-splicing peptide; bGH-pA, bovine growth hormone polyadenylation signal. Please click here to view a larger version of this figure.
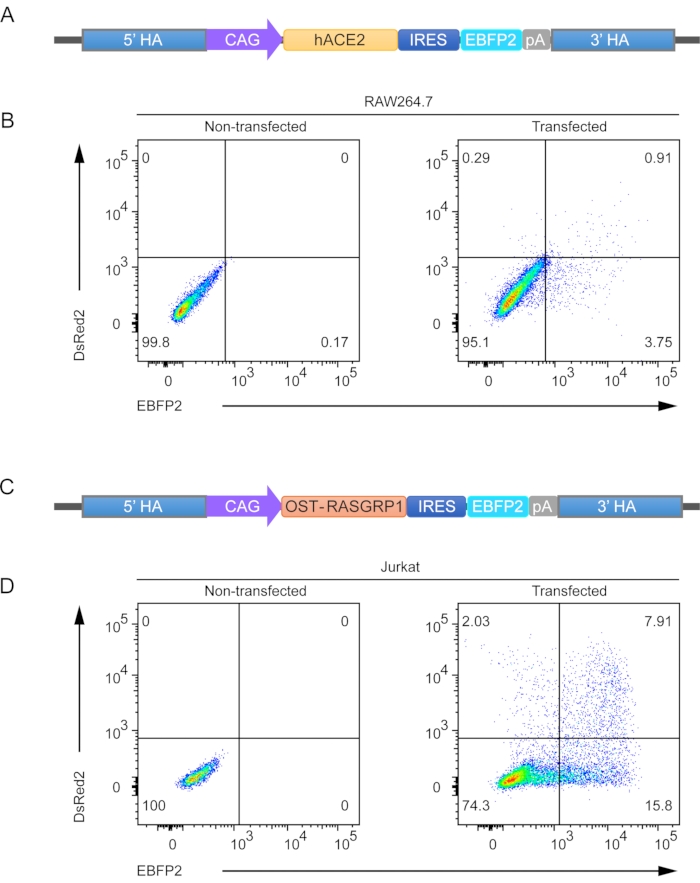
Figure 2. Single cell sorting of RAW264.7 and Jurkat cells transfected with CRISPR/Cas9 vectors and targeting vectors for homologous recombination. Targeting vectors used for stable gene expression in RAW264.7 (A) and Jurkat (C) cells. Representative flow cytometric plots of mouse RAW264.7 macrophages (B) and human Jurkat T cells (D) transfected with CRISPR/Cas9 vectors expressing the DsRed2 reporter and the targeting vector expressing the EBFP2 reporter. Cells co-expressing DsRed2 and EBFP2 were subjected to cell sorting and cultured for expansion; numbers adjacent to outlined areas indicate the percentage of cells in each gate, and non-transfected cells were used as a negative control. Please click here to view a larger version of this figure.
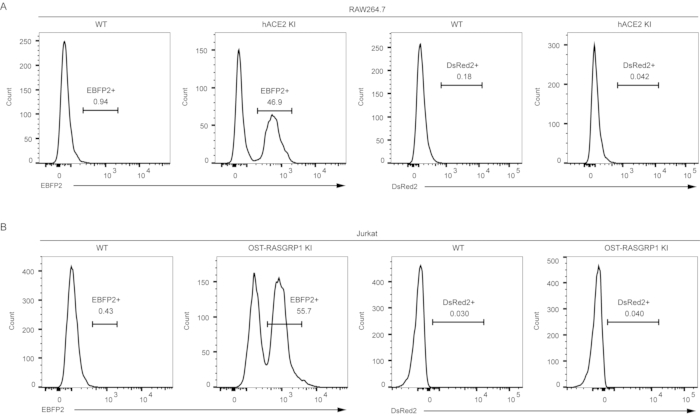
Figure 3. Screening of knock-in cells by detection of EBFP2 expression. Flow cytometric analysis of EBFP2+ and DsRed2+ RAW264.7 macrophages (A) and Jurkat T cells (B) 14 days after electroporation. In the histograms, fluorescence intensity (FI) of either EBFP2+ or DsRed2+ cells is displayed on the X-axis and the count of events in each fluorescence channel is displayed on the Y-axis. (A) The expression of EBFP2 and hACE2 was driven by the same promoter at the Rosa26 locus in RAW264.7 murine macrophages. (B) In Jurkat cells, OST-RASGRP1 expression is linked to EBFP2 expression at the human ROSA26 locus. At 14 days post-electroporation, flow cytometric analysis revealed nearly zero DsRed2+ cells among the sorted cells. Wild-type (WT) cells were used as a negative control, and KI stands for knock-in cells. Please click here to view a larger version of this figure.
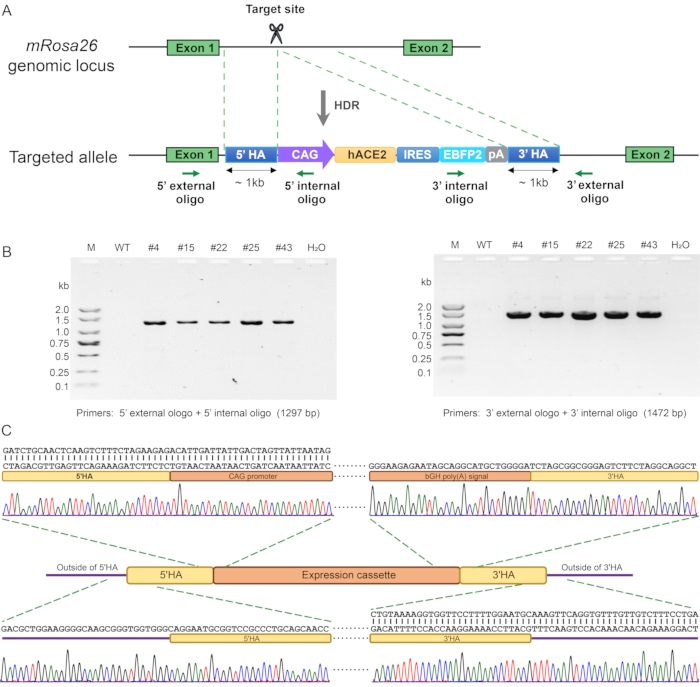
Figure 4. Screening for candidate knock-in cells by PCR and sequencing. (A) Strategy for generating hACE2 knock-in cells. The positions of PCR primers used to distinguish precise HDR and random insertion are indicated by green arrows. (B) PCR genotyping of five candidate cells (#4, #15, #22, #25, and #43) that were identified by flow cytometry screening for EBFP2 expression as exemplified in Figure 3, showed that both the 5' junction (1472 bp) and 3' junction (1472 bp) spanning the homology arms were correct. M, DNA ladder; WT, the wild-type RAW264.7 control; H2O, negative control. (C) Sanger sequencing of the PCR products from B revealed successful knock-in of the hACE2-expression cassette into the mRosa26 locus without mutations. Please click here to view a larger version of this figure.
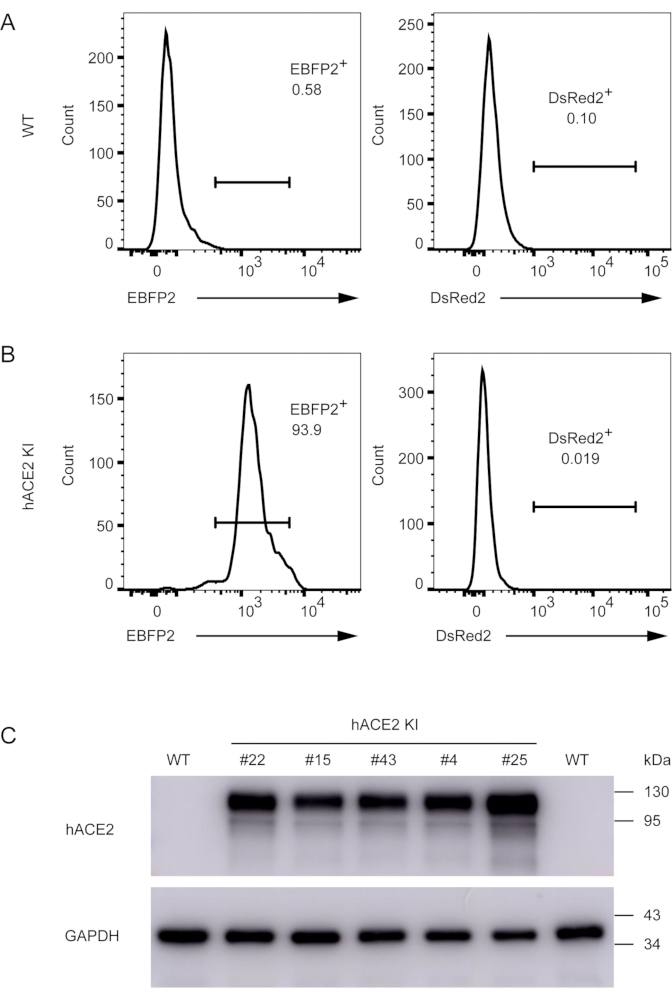
Figure 5. Validation of successful knock-in of the hACE2 expression cassette into the mRosa26 locus in RAW264.7 macrophages. (A) WT RAW264.7 macrophages were used as a negative control for flow cytometric analysis. (B) To separate the EBFP2-positive cells from the negative cells, an additional round of cell sorting was performed to obtain a population consisting of nearly 100% EBFP2+ knock-in cells, designated as hACE2 KI cells. DsRed2 expression was also examined to ensure that the CRISPR/Cas9 plasmid was not integrated into the genome of RAW264.7 macrophages. In the histograms, fluorescence intensity (FI) of either EBFP2+ or DsRed2+ cells is displayed on the X-axis and the number of events in each fluorescence channel is displayed on the Y-axis. (C) Detection of hACE2 expression by immunoblot analysis using rabbit anti-human ACE2 monoclonal antibody. Expression of hACE2 was observed in cells from multiple wells (#4, #15, #22, #25, and #43). WT RAW264.7 macrophages were used as a negative control and GAPDH was used as the loading control. Please click here to view a larger version of this figure.
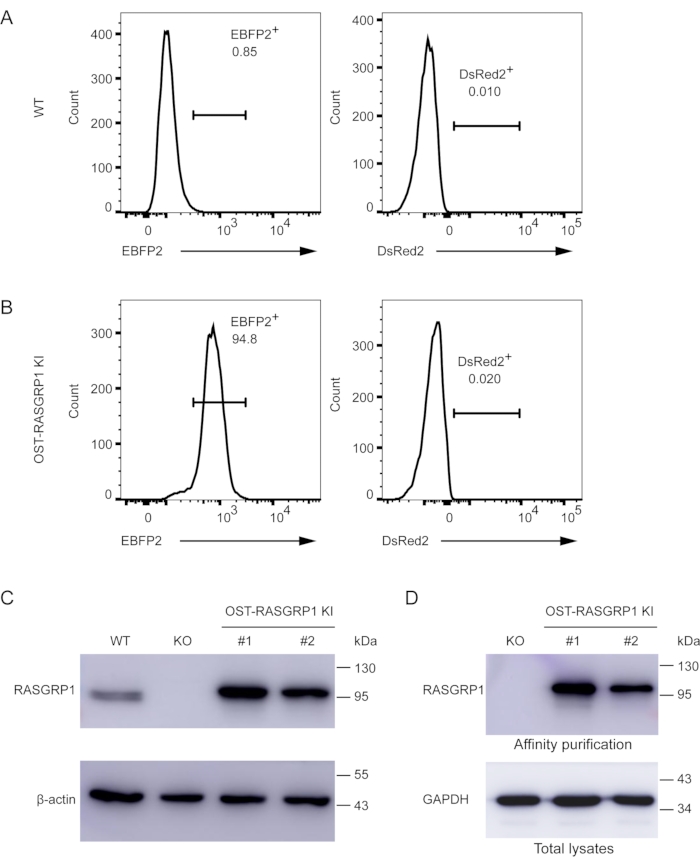
Figure 6. Validation of successful knock-in of the OST-RASGRP1 expression cassette into the hROSA26 locus in Jurkat T cells. (A) WT Jurkat T cells were used as a negative control for flow cytometric analysis. (B) EBPF2+ subpopulations of Jurkat cells were enriched by additional rounds of cell sorting and expanded; these cells were designated as OST-RASGRP1 KI cells. The knock-in cells were analyzed by flow cytometry and did not keep the DsRed2-expressing vector. In the histograms, the fluorescence intensity (FI) of either EBFP2+ or DsRed2+ cells is displayed on the X-axis and the count of events in each fluorescence channel is displayed on the Y-axis. (C) Detection of OST-RASGRP1 expression in two independent knock-in cells (#1 and #2) by immunoblot analysis using anti-RASGRP1 antibody. WT Jurkat and RASGRP1-knockout Jurkat cells were used as controls and β-actin was used as the loading control. (D) OST-mediated affinity purification was used to validate the expression of OST-RASGRP1 using RASGRP1 knockout cells as a negative control. Immunoblot analysis of equal amounts of proteins from cell lysates that were either subjected to affinity purification on Strep-Tactin Sepharose beads (Affinity purification) or directly analyzed (Total lysates) and probed with RASGRP1 or GAPDH (loading control) antibodies. Please click here to view a larger version of this figure.
Supplemental File: Supporting figures, table, and sequences Please click here to download this File.
Discussion
In our experiments, we demonstrated how to perform knock-in editing in immune cells from construct design to knock-in cell screening and validation using human Jurkat T cells and murine RAW264.7 macrophages as examples. Both T cell and macrophage cell lines are resistant to transfection36,37; however, the problem of low efficiency of CRISPR/Cas9 delivery can be overcome with the aid of fluorescent reporters coupled with cell sorting. This protocol is suitable for gene rescue experiments and protein-protein interaction experiments, but it cannot be applied to the study of regulatory DNA sequences such as binding sites of transcription factors because the protocol was developed for knock-in modification of the Rosa26 locus.
Dual fluorescent reporters aided CRISPR/Cas9 knock-in editing
We successfully applied a dual fluorescent reporter system to transiently express independent sets of CRISPR/Cas9 vectors in immune cells, which resulted in deletion of large fragments of DNA in previous studies21. We designed a CRISPR/Cas9 targeting tool using the DsRed2 fluorescent protein as a reporter and an additional spectrally distinct fluorescent protein to track delivery of the DNA template for knock-in modification. To make knock-in allele modification feasible, we used the EBFP2 fluorescent protein reporter, which has no spectral spillover with the RFP (DsRed2 in our case), to monitor transfection of the donor DNA serving as the template during homologous recombination. To optimize the cassette expressing the POI and fluorescent reporter, an IRES sequence was introduced to obtain independent proteins. In our previous study, we noted that the residual amino acids from the P2A or T2A linker remaining after post-transcriptional cleavage affected protein localization on the cell surface. The IRES sequence does not leave such residual amino acids. As described in a previous study, the IRES gave rise to higher levels of the fluorescent reporter, following expression of the Cas9 protein driven by CAG promoter38.
All-in-one CRISPR/Cas9 plasmid
Various formats and combinations of the CRISPR/Cas9 system have been described in previous studies, such as Cas9 transfection as an mRNA or protein together with chemically synthesized sgRNAs39. CRISPR/Cas9 RNP complexes have also been delivered into mammalian cells; this strategy offers the advantages of earlier onset of nuclease activity and a shorter half-life; however, labeling the RNPs is less cost effective compared with constructing all-in-one plasmid. In our previous studies, we found that using single cell sorting to isolate those rare cells expressing CRISPR/Cas9 (DsRed2 positive) and the knock-in protein (EBFP2 positive) is far less complicated than using RNP delivery, and it is easy to prepare the plasmids. It is true that this protocol relies on single cell sorting. But our protocol is easy to perform and yields successful knock-in modifications with high reproducibility.
Expression of a POI from the Rosa26 locus
There are multiple reports in the literature describing methods for tagging endogenous proteins with fluorescent reporters using CRISPR/Cas9 editing40,41,42. The advantages of endogenous tagging are that it is feasible to determine subcellular localization and perform in vivo tracking of the endogenous protein. However, problems may be encountered if it is not possible to design an appropriate CRIPSR guide RNA at the endogenous locus. Here we developed an alternative knock-in method by incorporating POI-IRES-EBFP2 into the genomic safe harbor locus Rosa26, which overcomes the limitations of finding appropriate guides for positioning endogenous tags.
We summarize a few key points that need to be considered during the experiments to ensure technical reproducibility. First, the cell sorter needs to have a 405 nm violet laser for excitation of EBFP2 and a 488 nm blue laser or alternatively a 561 nm yellow laser for excitation of DsRed2. With such configurations, EBFP2 and DsRed2 can be detected with no spectral spillover, which may lead to false positive results. In our experiments, the proportion of DsRed2+ EBFP2+ double positive cells was as low as 0.9%; therefore, gating of the proper population was essential for the success of the experiments. A second round of sorting was performed to gate the EBFP2+ positive cells, followed by PCR validation. In addition, for knock-in protein detection, it is preferable to introduce a protein tag such as OST or another type of tag. Knockout of the gene prior to Rosa26 locus knock-in experiments provides a good opportunity to assess whether the antibody has desirable specificity. When the antibody specificity is not sufficient, detection of the knock-in protein should be performed following pulldown via the protein tag. Finally, during FACS screening of the cells expressing the knock-in allele, the intensity of EBFP2 can be used to assess whether two copies or one copy of the knock-in allele is present.
Applications
In this protocol we described the knock-in modification of RASGRP1, a key molecule involved in T cell activation27. We first obtained RASGRP1-knockout Jurkat cells which can be used for loss-of-function studies, and we generated additional Jurkat cells expressing OST-RASGRP1 at the hROSA26 locus. Jurkat cells are the most used human cell line for studying T cell biology43. Because of the success of immunotherapy in preventing T cell exhaustion in cancer patients, immunologists and cancer biologists have great interest in modifying Jurkat cells for functional studies of candidate molecules. It is also noteworthy that the Jurkat T cell line is commonly used for dissection of signaling pathways, but there are limitations to using this cell line, as Jurkat cells are poor producers of IFN-γ upon stimulation44. Previous studies used both endogenous locus modification45 and genetic engineering at the hROSA26 locus46 to perform knock-in experiments in human Jurkat cells. Both strategies have their own advantages; by modifying the endogenous locus the protein is presumably expressed at a "physiological" level. Knock-in modification at the hROSA26 locus produces predictable results because alternative splicing of mRNA is avoided, and the abundance of the modified protein is also readily detectable. Other genomic safe harbors, such as the adeno-associated virus site 1 (AAVS1) and chemokine (CC motif) receptor 5 (CCR5)47,48 deserve more exploration.
In our previous study, when Vav1-OST was expressed at higher levels at the mouse Rosa26 locus in RAW264.7 cells, which are very frequently used macrophage cells, we were able to detect its interaction with lowly expressed Vav3 molecules because of the high levels of bait protein and high efficiency of OST affinity purification13. We also described knock-in experiments to establish a macrophage cell line stably expressing hACE2, a receptor for SARS-CoV-2, in which abundant expression is guaranteed. In the single cell RNA sequencing database, murine Ace2 is expressed in lung macrophages, and the genetic cellular model expressing hACE2 we developed could be useful for studies of macrophages during SARS-CoV-2 infection.
Other considerations
This protocol is designed to identify knock-in cells that express a POI with aid of flow cytometric analyses of a fluorescent reporter, in our case the EBFP2 reporter. However, when surface labeling antibodies detectable by FACS are available for a surface protein49, it is not necessary to use the reporter system50. The T cell and macrophage cell lines, as well as the examples of OST-tagged proteins used for interactome studies, are mainly used for signaling studies, and the majority of these signaling molecules are localized in the cytosol or nuclei of cells. Thus, a fluorescent reporter may be needed for the identification of desirable knock-in cells.
It is important to point out that this protocol was developed for cell lines, and application to primary immune cells such as T cells, monocytes/macrophages was not validated. Because of the limited capacity of the cells to proliferate, we do not recommend this protocol for use with primary immune cells. As the fluorescent reporter EBFP2 was expressed under the same promoter as the knock-in gene or POI using an IRES element, we did not observe cells that expressed the fluorescent reporter in the absence of the knock-in gene. We suspect that the recovery of the fluorescent protein-expressing cells is highly dependent on the success of homologous recombination. As reported in a previous study, it is tedious to sort, expand, and identify the correct knock-in cells by single cell sorting when the knock-in efficiency is very low42, which also explains why we needed to sort the cells in bulk to improve the success rate.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank the flow cytometry core facility of Xinxiang Medical University. Development of such technology has been supported by NSFC grants 81601360 to LZ, 81471595 and 32070898 to YL. The work is also supported by Foundation of Henan Educational Committee No. 21IRTSTHN030.
Materials
| Amersham Imager 600 | Ge Healthcare | imaging of chemiluminescence | |
| Ampicillin, sodium salt | MP Biomedicals | 194526 | |
| Anti-rabbit IgG, HRP-linked Antibody | Cell Signaling Technology | 7074 | at 1/5000 dilution |
| Anti-RasGRP1 antibody, clone 10.1 | Merck | MABS146 | 1.0 μg/mL of working concentration |
| AscI | New England BioLabs | R0558S | |
| β-Actin (D6A8) Rabbit mAb | Cell Signaling Technology | 8457 | at 1/1000 dilution |
| BamHI-HF | New England BioLabs | R3136S | |
| BbsI-HF | New England BioLabs | R3539S | |
| Cellometer Mini Automated Cell Counter | Nexcelom Bioscience | ||
| E.coli DH5α Competent Cells | Takara | 9057 | |
| DMSO (Dimethyl Sulfoxide) | MP Biomedicals | 196055 | |
| DNeasy Blood & Tissue Kits | Qiagen | 69506 | cell culture reagent |
| DPBS (10X), no calcium, no magnesium | ThermoFisher Scientific | 14200075 | |
| Dulbecco's Modified Eagle Medium (DMEM) with high glucose | HyClone | SH30022.01 | |
| EcoRI-HF | New England BioLabs | R3101S | |
| FACSAria™ Fusion | BD Biosciences | equipped with biosafety cabinet | |
| FACS Canto flow cytometer | BD Biosciences | ||
| Falcon 5 ml polystyrene round bottom test tube | BD Biosciences | 352003 | |
| Fetal bovine serum (FBS) | ThermoFisher Scientific | 10099141 | |
| FlowJo version 10.7 | BD Biosciences | ||
| GAPDH (D16H11) XP Rabbit mAb | Cell Signaling Technology | 5174 | at 1/1000 dilution |
| Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP | ThermoFisher Scientific | 31430 | at 1/5000 dilution |
| Immobilon ECL Ultra Western HRP Substrate | Millipore | WBKLS0500 | |
| Immobilon-PSQ PVDF Membrane | Millipore | ISEQ00010 | |
| Jurkat | ATCC | TIB-152 | https://www.atcc.org/ |
| Kanamycin sulfate | MP Biomedicals | 194531 | |
| LB agar powder | ThermoFisher Scientific | 22700041 | |
| Multi-channel Pipette (30-300 μL) | Eppendorf, or similar | ||
| Neon Transfection System | ThermoFisher Scientific | MPK5000 | |
| Neon Transfection System, 10 μL kit | ThermoFisher Scientific | MPK1096 | |
| Nunc 15 mL Conical Sterile Centrifuge Tubes | ThermoFisher Scientific | 339651 | |
| OneTaq® Hot Start Quick-Load® 2X Master Mix | New England BioLabs | (M0489) | for high GC% template |
| PageRuler Prestained Protein Ladder, 10 to 180 kDa | ThermoFisher Scientific | 26616 | |
| Pipette tip 0.1-20µl | Eppendorf, or similar | 0030 075.005 | |
| Pipette tip 2-200µl | Eppendorf, or similar | 0030 075.021 | |
| Pipette tip 50-1000µl | Eppendorf, or similar | 0030 075.064 | |
| Plasmid Maxi Kit | Qiagen | 12163 | |
| pX458-DsRed2 | Addgene | 112219 | |
| QIAquick PCR Purification Kit | Qiagen | 28104 | purify plasmid from restriction digestion |
| Q5 Hot Start High-Fidelity 2X Master Mix | New England BioLabs | M0494S | |
| RAW264.7 | ATCC | TIB-71 | https://www.atcc.org/ |
| Recombinant Anti-ACE2 antibody [EPR4435(2)] | Abcam | ab108252 | at 1/1000 dilution |
| RPMI 1640 Medium | HyClone | SH30027.01 | |
| Strep-Tactin Sepharose beads | IBA Lifesciences | 2-1201-010 | |
| Penicillin-Streptomycin | ThermoFisher Scientific | 15140122 | |
| SYTOX™ Red Dead Cell Stain, for 633 or 635 nm excitation | ThermoFisher Scientific | S34859 | |
| T4 DNA ligase | New England BioLabs | M0202S | |
| T4 Polynucleotide Kinase | New England BioLabs | M0201S | |
| Trypan Blue Solution, 0.4% | ThermoFisher Scientific | 15250061 | |
| Trypsin-EDTA solution (0.25%), with phenol red | ThermoFisher Scientific | 25200056 | |
| ZOE Fluorescent Cell Imager | Bio-Rad | ||
| 1.5 mL microtubes, PCR-clean | Eppendorf, or similar | 0030 125.215 | |
| 24-well Clear TC-treated Multiple Well Plates | Corning | 3524 | |
| 96-well Clear Flat Bottom Polystyrene TC-treated Microplates | Corning | 3599 | |
| 96-well Clear Round Bottom TC-treated Microplate | Corning | 3799 |
Referências
- Cronkite, D. A., Strutt, T. M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. Journal of Immunology Research. 2018, 1467538 (2018).
- Iwasaki, A., Medzhitov, R. Control of adaptive immunity by the innate immune system. Nature Immunology. 16 (4), 343-353 (2015).
- Chicaybam, L., et al. An Efficient Electroporation Protocol for the Genetic Modification of Mammalian Cells. Frontiers in Bioengineering and Biotechnology. 4, 99 (2016).
- Manguso, R. T., et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 547 (7664), 413-418 (2017).
- Siggs, O. M. Dissecting mammalian immunity through mutation. Immunology and Cell Biology. 92 (5), 392-399 (2014).
- Satija, R., Shalek, A. K. Heterogeneity in immune responses: from populations to single cells. Trends in Immunology. 35 (5), 219-229 (2014).
- Shui, B., Hernandez Matias, L., Guo, Y., Peng, Y. The Rise of CRISPR/Cas for Genome Editing in Stem Cells. Stem Cells International. 2016, 8140168 (2016).
- Komor, A. C., Badran, A. H., Liu, D. R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell. 168 (1-2), 20-36 (2017).
- Seki, A., Rutz, S. Optimized RNP transfection for highly efficient CRISPR/Cas9-mediated gene knockout in primary T cells. Journal of Experimental Medicine. 215 (3), 985-997 (2018).
- Oh, S. A., Seki, A., Rutz, S. Ribonucleoprotein Transfection for CRISPR/Cas9-Mediated Gene Knockout in Primary T Cells. Current Protocols in Immunology. 124 (1), 69 (2019).
- Lee, Y. W., et al. In Vivo Editing of Macrophages through Systemic Delivery of CRISPR-Cas9-Ribonucleoprotein-Nanoparticle Nanoassemblies. Advances in Therapy (Weinh). 2 (10), (2019).
- Freund, E. C., et al. Efficient gene knockout in primary human and murine myeloid cells by non-viral delivery of CRISPR-Cas9. Journal of Experimental Medicine. 217 (7), (2020).
- Huang, R., et al. The three members of the Vav family proteins form complexes that concur to foam cell formation and atherosclerosis. Journal of Lipid Research. 60 (12), 2006-2019 (2019).
- Scharenberg, S. G., et al. Engineering monocyte/macrophage-specific glucocerebrosidase expression in human hematopoietic stem cells using genome editing. Nature Communications. 11 (1), 3327 (2020).
- Jacobi, A. M., et al. Simplified CRISPR tools for efficient genome editing and streamlined protocols for their delivery into mammalian cells and mouse zygotes. Methods. 121-122, 16-28 (2017).
- Liang, X., Potter, J., Kumar, S., Ravinder, N., Chesnut, J. D. Enhanced CRISPR/Cas9-mediated precise genome editing by improved design and delivery of gRNA, Cas9 nuclease, and donor DNA. Journal of Biotechnology. 241, 136-146 (2017).
- Haupt, A., Grancharova, T., Arakaki, J., Fuqua, M. A., Roberts, B., Gunawardane, R. N. Endogenous Protein Tagging in Human Induced Pluripotent Stem Cells Using CRISPR/Cas9. Journal of Visualized Experiments. (138), (2018).
- Li, S., Xue, H., Long, B., Sun, L., Truong, T., Liu, Y. Efficient generation of hiPSC neural lineage specific knockin reporters using the CRISPR/Cas9 and Cas9 double nickase system. Journal of Visualized Experiments. (99), e52539 (2015).
- Schumann, K., et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proceedings of the National Academy of Sciences of the United States of America. 112 (33), 10437-10442 (2015).
- Hamilton, J. R., et al. Targeted delivery of CRISPR-Cas9 and transgenes enables complex immune cell engineering. Cell Reports. 35 (9), 109207 (2021).
- Luo, J., et al. Speed genome editing by transient CRISPR/Cas9 targeting and large DNA fragment deletion. Journal of Biotechnology. 281, 11-20 (2018).
- Bourgonje, A. R., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease. Journal of Pathology. 251 (3), 228-248 (2020).
- Schultze, J. L., Aschenbrenner, A. C. COVID-19 and the human innate immune system. Cell. 184 (7), 1671-1692 (2021).
- Taefehshokr, N., Taefehshokr, S., Hemmat, N., Heit, B. Covid-19: Perspectives on Innate Immune Evasion. Frontiers in Immunology. 11 (2549), (2020).
- Waldman, A. D., Fritz, J. M., Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews: Immunology. 20 (11), 651-668 (2020).
- Chandran, S. S., Klebanoff, C. A. T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunological Reviews. 290 (1), 127-147 (2019).
- Dower, N. A., et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nature Immunology. 1 (4), 317-321 (2000).
- Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 21 (1), 70-71 (1999).
- Chu, V. T., et al. Efficient generation of Rosa26 knock-in mice using CRISPR/Cas9 in C57BL/6 zygotes. BMC Biotechnology. 16, 4 (2016).
- Irion, S., Luche, H., Gadue, P., Fehling, H. J., Kennedy, M., Keller, G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nature Biotechnology. 25 (12), 1477-1482 (2007).
- Concordet, J. -. P., Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Research. 46, 242-245 (2018).
- Haeussler, M., et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biology. 17 (1), 148 (2016).
- Jang, D. E., et al. Multiple sgRNAs with overlapping sequences enhance CRISPR/Cas9-mediated knock-in efficiency. Experimental and Molecular Medicine. 50 (4), 1-9 (2018).
- Miura, H., Quadros, R. M., Gurumurthy, C. B., Ohtsuka, M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nature Protocols. 13 (1), 195-215 (2018).
- Paix, A., Rasoloson, D., Folkmann, A., Seydoux, G. Rapid Tagging of Human Proteins with Fluorescent Reporters by Genome Engineering using Double-Stranded DNA Donors. Current Protocols in Molecular Biology. 129 (1), 102 (2019).
- Ebert, O., et al. Lymphocyte apoptosis: induction by gene transfer techniques. Gene Therapy. 4 (4), 296-302 (1997).
- Zhang, X., Edwards, J. P., Mosser, D. M. The expression of exogenous genes in macrophages: obstacles and opportunities. Methods in Molecular Biology. 531, 123-143 (2009).
- Chu, V. T., et al. Efficient CRISPR-mediated mutagenesis in primary immune cells using CrispRGold and a C57BL/6 Cas9 transgenic mouse line. Proceedings of the National Academy of Sciences of the United States of America. 113 (44), 12514-12519 (2016).
- Banan, M. Recent advances in CRISPR/Cas9-mediated knock-ins in mammalian cells. Journal of Biotechnology. 308, 1-9 (2020).
- Roberts, B., et al. Systematic gene tagging using CRISPR/Cas9 in human stem cells to illuminate cell organization. Molecular Biology of the Cell. 28 (21), 2854-2874 (2017).
- Bukhari, H., Müller, T. Endogenous Fluorescence Tagging by CRISPR. Trends in Cell Biology. 29 (11), 912-928 (2019).
- Koch, B., Nijmeijer, B., Kueblbeck, M., Cai, Y., Walther, N., Ellenberg, J. Generation and validation of homozygous fluorescent knock-in cells using CRISPR-Cas9 genome editing. Nature Protocols. 13 (6), 1465-1487 (2018).
- Abraham, R. T., Weiss, A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nature Reviews Immunology. 4 (4), 301-308 (2004).
- Freen-van Heeren, J. J. -., Popović, B., Guislain, A., Wolkers, M. C. Human T cells employ conserved AU-rich elements to fine-tune IFN-γ production. European Journal of Immunology. 50 (7), 949-958 (2020).
- Roncagalli, R., et al. The scaffolding function of the RLTPR protein explains its essential role for CD28 co-stimulation in mouse and human T cells. Journal of Experimental Medicine. 213 (11), 2437-2457 (2016).
- He, L., et al. ARHGAP45 controls naïve T- and B-cell entry into lymph nodes and T-cell progenitor thymus seeding. EMBO Reports. 22 (4), 52196 (2021).
- Sadelain, M., Papapetrou, E. P., Bushman, F. D. Safe harbours for the integration of new DNA in the human genome. Nature Reviews: Cancer. 12 (1), 51-58 (2011).
- Papapetrou, E. P., Gene Schambach, A. Insertion Into Genomic Safe Harbors for Human Gene Therapy. Molecular Therapy. 24 (4), 678-684 (2016).
- Wang, X., et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 118 (5), 1255-1263 (2011).
- Eyquem, J., et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 543 (7643), 113-117 (2017).

