Acquisition of Resting-State Functional Magnetic Resonance Imaging Data in the Rat
Summary
This protocol describes a method for obtaining stable resting-state functional magnetic resonance imaging (rs-fMRI) data from a rat using low dose isoflurane in combination with low dose dexmedetomidine.
Abstract
Resting-state functional magnetic resonance imaging (rs-fMRI) has become an increasingly popular method to study brain function in a resting, non-task state. This protocol describes a preclinical survival method for obtaining rs-fMRI data. Combining low dose isoflurane with continuous infusion of the α2 adrenergic receptor agonist dexmedetomidine provides a robust option for stable, high-quality data acquisition while preserving brain network function. Furthermore, this procedure allows for spontaneous breathing and near-normal physiology in the rat. Additional imaging sequences can be combined with resting-state acquisition creating experimental protocols with anesthetic stability of up to 5 h using this method. This protocol describes the setup of equipment, monitoring of rat physiology during four distinct phases of anesthesia, acquisition of resting-state scans, quality assessment of data, recovery of the animal, and a brief discussion of post-processing data analysis. This protocol can be used across a wide variety of preclinical rodent models to help reveal the resulting brain network changes that occur at rest.
Introduction
Resting-state functional magnetic resonance imaging (rs-fMRI) is a measure of the blood-oxygen-level-dependent (BOLD) signal when the brain is at rest and not engaged in any particular task. These signals can be used to measure correlations between brain regions to determine the functional connectivity within neural networks. rs-fMRI is widely used in clinical studies due to its non-invasiveness and the low amount of effort required of patients (as compared to task-based fMRI) making it optimal for diverse patient populations1.
Technological advances have allowed rs-fMRI to be adapted for use in rodent models to uncover mechanisms underlying disease states (see reference2 for review). Preclinical animal models, including disease or knockout models, allow a wide range of experimental manipulations not applicable in humans, and studies can also make use of post-mortem samples to further enhance experiments2. Nevertheless, due to the difficulty in both limiting motion and mitigating stress, MRI acquisition in rodents is traditionally performed under anesthesia. Anesthetic agents, depending on their pharmacokinetics, pharmacodynamics, and molecular targets, influence brain blood flow, brain metabolism, and potentially affect neurovascular coupling pathways.
There have been numerous efforts to develop anesthetic protocols that preserve neurovascular coupling and brain network function3,4,5,6,7,8. We previously reported an anesthetic regime that applied a low dose of isoflurane along with a low dose of the α2 adrenergic receptor agonist dexmedetomidine9. Rats under this method of anesthesia exhibited robust BOLD responses to whisker stimulation in regions consistent with established projection pathways (ventrolateral and ventromedial thalamic nuclei, primary and secondary somatosensory cortex); large-scale resting-state brain networks, including the default mode network10,11 and salience network12 have also been consistently detected. Furthermore, this anesthetic protocol allows for repeated imaging on the same animal, which is important for monitoring the disease progression and the effect of experimental manipulations longitudinally.
In the present study, we detail the experimental setup, animal preparation, and physiological monitoring procedures involved. In particular, we describe the specific anesthetic phases and acquisition of scans during each phase. Data quality is assessed following each resting-state scan. A brief summary of post-scan analysis is also included in the discussion. Laboratories interested in uncovering the potential of using rs-fMRI in rats will find this protocol useful.
Protocol
All experiments were performed on a 9.4 T MRI scanner, and were approved by the Institutional Animal Care and Use Committee at Dartmouth College. Additional approval was obtained to record and show the animals used in the video and figures below.
1. Preparations before scanning
- Subcutaneous infusion line
- Partially remove a 23 G needle from its package so that the needle point remains sterile.
- Securely hold the hub of the needle and use a razor blade to score the needle shaft where it meets the hub.
- Clamp a needle holder around the shaft directly below the scoring and gently break the shaft from the hub.
- Insert 1/3 of the needle shaft (blunt end) into previously sterilized PE50 line with enough line length to extend from the drug pump to the animal inside the magnet bore.
- Dilution of dexmedetomidine and atipamezole
- Prepare a solution of diluted dexmedetomidine hydrochloride using 0.5 mL of 0.5 mg/mL stock mixed with 9.5 mL of sterile saline in a clear, sterile glass bottle (diluted concentration = 0.025 mg/mL).
- Prepare a solution of diluted atipamezole using 0.1 mL of 5 mg/mL stock mixed with 9.9 mL of sterile saline in a clear, sterile glass bottle (diluted concentration = 0.05 mg/mL).
- Scanning parameters
- Use the parameters presented in Table 1 to prepare scanning sequences.
2. Phase 1 anesthesia: Animal induction and preparation
- Setup
- Ensure that all equipment is on and working properly including the oxygen and air mixer, heating pad, and active scavenging system (see Figure 1).
- Set the heating system's temperature set point to 37.5 °C.
- Animal induction
- Place the animal (90-day old, male Sprague Dawley rat) in the induction chamber and induce anesthesia with 2.5% isoflurane in 30% oxygen-enriched air.
NOTE: A wide range of animal ages and both sexes can be used. - Once the animal is anesthetized, remove it from the chamber, weigh the animal, and place it in the nose cone (at 2.5% isoflurane) on the heating pad in the preparation space.
- Place the animal (90-day old, male Sprague Dawley rat) in the induction chamber and induce anesthesia with 2.5% isoflurane in 30% oxygen-enriched air.
- Animal preparation
- Apply ophthalmic lubricating ointment to each eye to prevent drying.
- Confirm the depth of anesthesia by a lack of toe pinch response.
- Use clippers to shave a 2" by 2" square area on the lower lumbar region of the animal's back (i.e., directly above the tail).
- Administer 0.015 mg/kg of the dexmedetomidine solution with an intraperitoneal (i.p.) injection (e.g., a 300 g rat would receive 0.18 mL) into the lower right quadrant of the abdomen using a 25 G needle.
- Switch isoflurane flow from the preparation space to the animal cradle.
- Move the animal into the animal cradle. Place the rat's front teeth securely over and into the bite bar. Push the nose cone over the nose to ensure a tight fit.
NOTE: If the nose cone does not cover the lower jaw, use a paraffin film to gently hold the jaw closed while also sealing around the nose cone. - Position the respiration pad under the rat's abdomen below the rib cage and re-position it until the respiration waveform shows a deep trough centered on each breath (see respiration waveform in Figure 2).
- Monitor the animal's breathing using the physiology monitoring software. Move to the next phase of anesthesia when respiration is less than 40 breaths/min (bpm; approximately 5 min after dexmedetomidine injection).
3. Phase 2 anesthesia: Animal setup
- Insert ear bars into the ear canal to stabilize the rat's head in the animal cradle. Once positioned, pull forward on the bite bar and confirm the head does not move. Re-adjust the nose cone and paraffin film as needed (see Figure 3a).
- Insert the temperature probe into a pre-lubricated, disposable probe cover. Gently insert the temperature probe approximately ½" into the rectum, and tape it to the base of the tail with medical tape.
- Place the pulse oximeter clip onto the metatarsal area of the hind foot, ensuring the light source is on the bottom of the foot (palm).
NOTE: Rotation of the clip can affect the signal; thus, creating a holder to keep the paw and clip upright will lead to greater stability. Also note that until the rat is at normal body temperature, the oxygen saturation may be low (<95%). - Use the rat's weight to calculate the infusion rate to eject 0.015 mg/kg/h of dexmedetomidine (a 300 g rat receives 0.18 mL/h).
- Set the drug pump to eject the calculated infusion rate.
- Fill a 3 mL syringe with the sterile, diluted dexmedetomidine solution and insert the tip of the needle into the open end of the sterilized infusion line (extending from the drug pump to the animal cradle with the subcutaneous needle previously attached). Fill the line and secure the syringe in the syringe holder of the drug pump.
- Move the pusher block forward until it touches the plunger, and the drug is expelled at the needle, ensuring the infusion line is completely filled.
- Using an alcohol wipe, clean the shaved area to remove any stray hair.
- Pinch the skin approximately two finger widths above the base of the tail. Insert 1/3 of the infusion line needle into the tented skin.
- Secure the needle to the skin with a 3" piece of wide medical tape. Place a second piece of wide medical tape over the first, across the rat, and attached to both sides of the animal cradle (see Figure 4).
NOTE: It is critically important that the ferromagnetic needle is well secured to prevent movement during the scan. - Begin the infusion of subcutaneous dexmedetomidine.
- Place a piece of gauze on the bridge of the rat's nose to create a level surface for the coil. Use paper tape, which does not interfere with the MRI signal, to secure the coil to the rat's head, centering it over the brain (see Figure 3b,c).
- Secure all lines and cables within the animal cradle with lab tape and check whether all the physiology signals are stable (see Figure 2).
- Place paper towels over the animal, securing them to the animal cradle with laboratory tape. If using an air heating system, wrap a plastic sheet around the entire cradle to contain the warm air.
- Move the animal into the bore and tune the magnet.
4. Phase 3 anesthesia: Anatomical scan acquisition
- Reduce isoflurane to 1.5%, resulting in a steady increase in respiration to approximately 45-50 bpm. Remain at this level for the duration of the anatomical scanning.
- Use the FLASH localizer scan to ensure the brain is aligned with the magnet isocenter (Figure 5a). Reposition the animal and repeat if necessary.
- Run the higher resolution RARE localizer scan and use this scan output to align 15 sagittal slices centered across the brain (left to right, Figure 5b).
- Using the middle sagittal slice, align the center axial slice to the decussation of the anterior commissure, which appears as a dark spot (Figure 5c). Note the slice offset to use later in the resting-state scans.
- Acquire 23 slices using both the FLASH and RARE axial protocols to aid in registration to a common space during post-scan analysis.
- Shim across the whole brain using the PRESS sequence.
5. Phase 4: Resting-state scan acquisition
- After completing anatomical scans, reduce isoflurane to 0.5% to 0.75%, adjusting so that the animal's respiration is 60-65 breaths per minute. Remain at this level for at least 10 min before beginning resting-state scanning to ensure stability.
- When physiology is stable (respiration range is 60-75 bpm with no gasping or irregularities, core body temperature is 37.5 ± 1.0 °C, and oxygen saturation is 95% or greater), acquire a 15 slice EPI scan using the same slice offset as the anatomical axial series.
- After each resting-state scan is complete, check the quality using an independent component analysis (ICA) to decompose the data into spatial and temporal components.
- Obtain at least three high-quality resting-state scans.
6. Post-scan recovery
- When scanning is complete, increase isoflurane to 2% and stop the subcutaneous dexmedetomidine infusion.
- Remove the animal cradle from the magnet bore, unwrap the animal, and remove ear bars, temperature probe, pulse oximeter clip, and the dexmedetomidine needle.
- Inject 0.015 mg/kg of the diluted atipamezole solution into the rat's hind leg muscle using a 1 mL syringe with a 25 G needle (i.e., a 300 g rat would receive 0.09 mL).
- Place the rat back in the home cage on top of a heating pad and monitor until the animal is ambulatory.
Representative Results
Following each resting-state scan, stability is assessed using an independent component analysis (ICA; example script included in Supplementary Files). Figure 6 shows examples of component outputs from resting-state scans. Figure 6a shows a signal component from a scan with high stability. Note that spatially, the component has high regionality. Within the time course below the spatial component, the signal is stable and not predictable, indicative of true brain activity. The power spectrum at the bottom shows predominantly low frequencies. Figure 6b shows a component from the same scan as Figure 6a that represents noise. Note the non-regionality in the spatial component, high-frequency time course, and high frequency peak in the power spectrum. Finally, Figure 6c shows a component from a scan with unstable anesthesia. The time course is variable and irregular. When this occurs, improvements are needed to the anesthetic protocol, commonly to the sealing of the nose cone and the scavenging of waste gases.
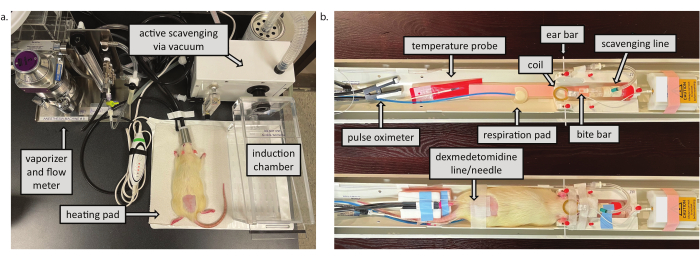
Figure 1: Preparation space and MRI animal cradle. a) Preparation space. The vacuum scavenges waste gases from both the induction chamber and the nose cone at the animal cradle. The heating pad helps to maintain animal temperature during both Phase 1 and recovery. b) MRI animal cradle. The top indicates components of the animal setup in Phase 2. The bottom shows a rat fully set-up and ready for scanning. Please click here to view a larger version of this figure.
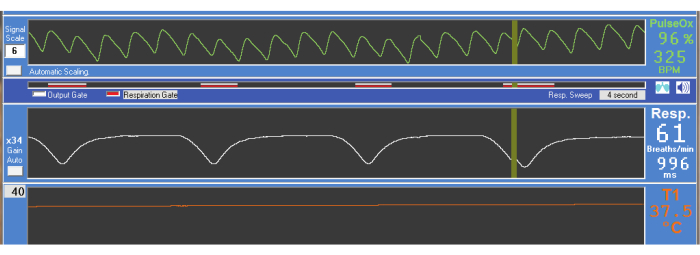
Figure 2: Physiologic scan output. Oxygen saturation (PulseOx, 96%), heart rate (325 BPM [beats per minute]), respiration rate (61 breaths/min), and core body temperature (T1, 37.5 °C) are constantly monitored throughout the scanning session. Please click here to view a larger version of this figure.
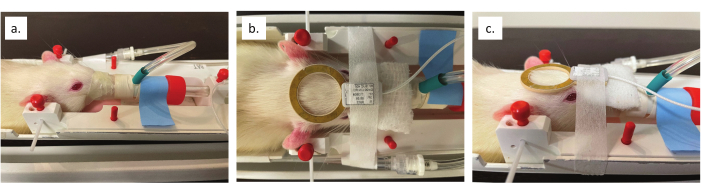
Figure 3: Nose cone and coil placement. (a) Close up view of the nose cone sealed around the animal's nose and lower jaw. (b) Overhead view of the alignment of surface coil to the brain. (c) Side view of coil alignment with the midpoint of the animal's eye. Please click here to view a larger version of this figure.
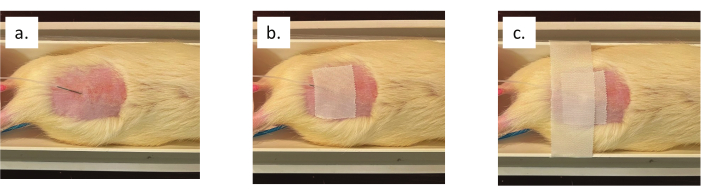
Figure 4: Subcutaneous dexmedetomidine infusion line and needle placement. (a) Needle insertion into the lower lumbar region of the animal's back. (b) Tape securing the needle to the animal's skin. (c) Tape across the animal cradle to prevent movement of the ferromagnetic needle. Please click here to view a larger version of this figure.
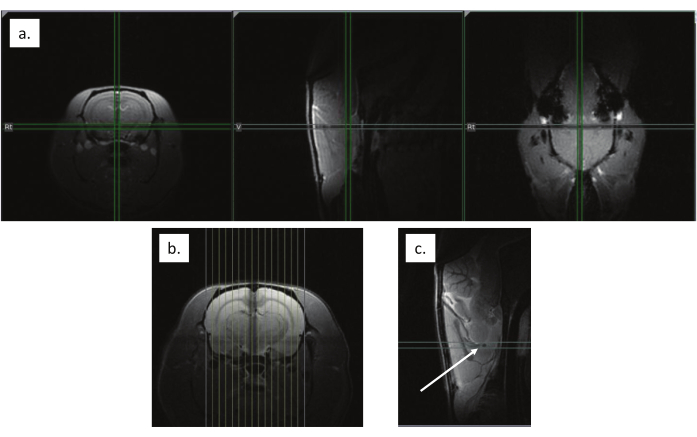
Figure 5: Anatomical scan alignment. (a) Localizer scan to align the animal's brain to the magnet isocenter, noted with crosshairs. (b) Sagittal slices aligned across the brain from left to right. (c) Alignment to the decussation of the anterior commissure, indicated by the white arrow. Please click here to view a larger version of this figure.
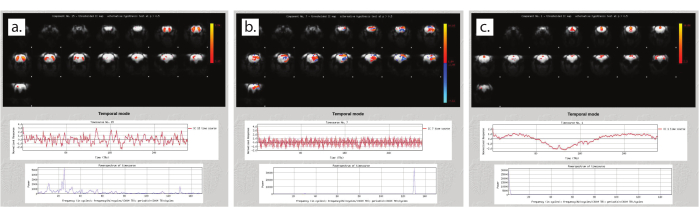
Figure 6: Quality assessment using independent component analysis. (a) Signal component during steady anesthesia. (b) Noise component during steady anesthesia. (c) Unsteady anesthesia. Please click here to view a larger version of this figure.
| Scan | Sequence | Orientation | FOV (mm x mm) | Matrix | Slices | Slice Thickness (mm) | TE (ms) | TR (ms) | Averages | Echo Spacing (ms) | Rare Factor | Repetitions | Scan Time |
| Localizer | FLASH | All planes | 50 | 256 | 1/dir | 1 | 2.5 | 100 | 1 | 1 | 12.8 s | ||
| Localizer | RARE | All planes | 35 | 192 | 1/dir | 0.75 | 28 | 2500 | 1 | 7 | 8 | 1 | 1 min |
| Anat | RARE | Sagittal | 35 | 192 | 15 | 1 | 28 | 2500 | 1 | 7 | 8 | 1 | 1 min |
| Anat | FLASH | Axial | 35 | 192 | 23 | 1 | 5 | 250 | 2 | 1 | 1 min 36 s | ||
| Anat | RARE | Axial | 35 | 192 | 23 | 1 | 28 | 2500 | 4 | 7 | 8 | 1 | 4 min |
| Shim | PRESS | All planes | 16.223 | 2500 | 1 | 1 | 2.5 s | ||||||
| Resting-State | EPI | Axial | 35 | 64 | 15 | 1 | 15 | 1200 | 1 | 300 | 6 min each |
Table 1: Reference table of scan parameters. Parameters for the sequences outlined in the protocol. FLASH = Fast Low Angle Shot, RARE = Rapid Acquisition with Relaxation Enhancement, PRESS = Point RESolved Spectroscopy, EPI = Echo Planar Imaging.
Supplementary Files: Example script for ICA quality assessment. Please click here to download this File.
Discussion
Stability of the animal, both physically and physiologically, is key to obtaining high-quality resting-state data. This protocol achieves stability by moving through four distinct phases of anesthesia. It is imperative that the animal has met the set physiological thresholds before moving to the next phase of anesthesia; since this method relies on physiological autoregulatory mechanisms, individual animals may require slightly different amounts of time at each anesthesia phase. It is our experience that taking more time at each phase is more efficient than hurrying through earlier stages without giving the rat's physiology sufficient time to settle. The key components that allow for stability are the fit of the nose cone and proper waste gas scavenging.
A properly sealed nose cone and scavenging allow the animal to remain stable with regularly spaced breathing and steady oxygen saturation levels. If gasping, irregular spacing, holding of the breath, or decreasing oxygen saturation levels occur, one should work to improve the nose cone sealing and scavenging. The nose cone should fit closely but should not push into the bridge of the nose. A custom nose cone may need to be fabricated. The original nose cone from our manufacturer had an air outtake valve that was too small, so a falcon tube was fitted with a larger sealed vacuum line closer to the animal. This resulted in better clearance of expired CO2 and steady oxygen saturation. As mentioned in the protocol, paraffin film may be wrapped around the lower jaw and edge of the nose cone, but if wrapped too tightly, it can restrict breathing and lead to instability. Additionally, improper placement of ear bars and bite bar not only affect the necessary stability of the head for imaging but can also affect breathing; continued blinking or audible noise from the animal is a likely indication of improper ear bar placement. The front teeth should sit securely on the bite bar and be pulled forward after ear bar placement to ensure a tight fit. The rat's tongue may need to be pulled forward if it sits too far back in the mouth and restricts proper breathing.
As each system is unique, fine tuning the vacuum level is required to achieve optimal scavenging. As a practical guide, it should be possible to feel a small amount of suction either by placing a finger over the vacuum line opening inside the nose cone, or by sealing the entire nose cone opening with the palm. Matching flow rate for anesthesia input (0.8 L/min was used here) is a good starting point. Oxygen saturation in the animal should remain above 95% throughout the scan. If oxygen saturation shows a decreasing trend, this may be an indication that CO2 is building up in the nose cone and scavenging needs to be increased. Another possibility is that the pressure of the pulse oximeter clip on the foot needs to be adjusted, either loosened to improve blood flow or tightened to ensure a strong, stable signal. If respiration of the animal is higher than the thresholds outlined, this may indicate that scavenging is set too high and is removing too much isoflurane. In rare circumstances, it may be necessary to increase the dose of subcutaneous dexmedetomidine to 0.02 mg/kg/hr, but we have found that 0.015 mg/kg has worked well across a wide range of rat ages and both sexes, and is supported in pharmacological studies4.
The scan duration necessary for fMRI activation is a function of effect size, spatial signal-to-noise ratio (SNR) and temporal SNR, as shown previously by Murphy et al.13. The use of a small surface coil (2 cm) and high magnetic field (9.4 T) substantially enhances SNR and BOLD sensitivity. With our imaging setup, we have found that a single 6 min scan is sufficient to detect a robust resting-state functional connectivity network, consistent with our previous report10. Nevertheless, we typically repeat the scan 3 to 4 times, and average the results to derive functional brain networks for individual animals. Alternatively, one can scan a single time with a longer duration (10 min or more) to derive functional connectivity networks14.
After collecting high quality rs-fMRI using this protocol, preprocess the data as has been previously published15,16. With the use of both ear bars and a bite bar, motion artifacts in the fMRI time course are minimal, and the use of motion correction has not had a noticeable effect on our data. Individual resting-state EPI scans need to be skull-stripped and registered to a common space (we use a single representative rat brain)16,17. Remove the beginning volumes from each EPI so those included are all acquired when the magnet is at steady-state (we remove 5 time points). Denoise individual scans (see Representative Results for examples of signal and noise components). Apply slice timing correction, as well as linear and quadratic trend removal, band pass filtering (0.005-0.1 Hz) and spatial smoothing (0.6 mm FWHM [full width at half maximum]). Additionally, remove the average signal time course from the white matter and ventricles through linear regression. After these standard preprocessing steps, further group level analysis can be performed including seed-based functional connectivity11,15,18,19,20,21,22, independent components analyses10,20,22, and modularity analyses12,19.
There are two main advantages of the current protocol: 1) it allows for spontaneous brain activity; and 2) it keeps the animal at near-normal physiology. Alternative anesthetic methods (such as propofol21, α-chloralose15, and pancuronium bromide in combination with another anesthetic21,23) have also been used to acquire resting-state data. However, using a combination of low dose isoflurane with low dose dexmedetomidine, as described in this protocol, has been shown to only minimally disrupt brain network functions while also providing the physiologic stability needed to obtain quality resting-state functional connectivity data9,10,18,24. Furthermore, BOLD responses from somatosensory stimulation9 and mechanical whisker deflection11 can be seen at or after a period of 90 min when using this protocol, suggesting a consistent arousal level. Interestingly, using dexmedetomidine in isolation can elicit epileptic activity; however, this activity was abolished with supplemented isoflurane8. Another advantage to the current protocol is that it eliminates the need for artificial ventilation. Although mechanical ventilation may lead to a narrower range of partial carbon dioxide and oxygen saturation across animals, in longitudinal studies, maintaining physiological parameters without the need for intubation may result in fewer complications and unwanted side effects.
Interest in resting-state fMRI has grown considerably in the past 10 years, and with it a need to acquire high-quality, preclinical resting-state scans from rodents. This survival protocol achieves stable anesthesia for up to 5 h with near-normal physiology during resting-state acquisition. As the protocol is highly stable, additional sequences (structural, stimulation, pharmacological MRI, etc.) can easily be added to achieve the desired experimental design. The combination of low-dose isoflurane with dexmedetomidine utilized in this protocol allows for a wide variety of preclinical studies for investigators interested in studying the rodent brain in its resting state.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by funding from the National Institute of Health (NIH)'s National Institute on Drug Abuse (NIDA) [DJW, EDKS, and EMB were supported by Grant R21DA044501 awarded to Alan I. Green and DJW was supported by Grant T32DA037202 to Alan J. Budney] and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) [Grant F31AA028413 to Emily D. K. Sullivan]. Additional support was provided through Alan I. Green's endowed fund as the Raymond Sobel Professor of Psychiatry at Dartmouth.
Hanbing Lu is supported by the National Institute on Drug Abuse Intramural Research Program, NIH.
The authors wish to acknowledge and thank the late Alan I. Green. His unwavering dedication to the field of co-occurring disorders helped to establish collaboration among the authors. We thank him for his mentorship and guidance, which will be greatly missed.
Materials
| 9.4T MRI | Varian/Bruker | Varian upgraded with Bruker console running Paravision 6.0.1 software | |
| Air-Oxygen Mixer | Sechrist | Model 3500CP-G | |
| Analysis of Functional NeuroImages (AFNI) | NIMH/NIH | Version AFNI_18.3.03 | Freely available at: https://afni.nimh.nih.gov/ |
| Animal Cradle | RAPID Biomedical | LHRXGS-00563 | rat holder with bite bar, nose cone and ear bars |
| Animal Physiology Monitoring & Gating System | SAII | Model 1025 | MR-compatible system including oxygen saturation, temperature, respiration and fiber optic pulse oximetry add-on |
| Antisedan (atipamezole hydrochloride) | Patterson Veterinary | 07-867-7097 | Zoetis, Manufacturer Item #10000449 |
| Ceramic MRI-Safe Scissors | MRIequip.com | MT-6003 | |
| Clippers | Patterson Veterinary | 07-882-1032 | Wahl touch-up trimmer combo kit, Manufacturer Item #09990-1201 |
| Dexmedesed (dexmedetomidine hydrochloride) | Patterson Veterinary | 07-893-1801 | Dechra Veterinary Products, Manufacturer Item#17033-005-10 |
| Digital Rectal Thermometer Covers | Medline | MDS9608 | |
| FMRIB Software Library | FMRIB | MELODIC Version 3.15 | Freely available at: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki |
| Heating Pad | Cara Inc. | Model 50 | |
| Hemostat forceps, straight | Kent Scientific | INS750451-2 | |
| Isoflurane | Patterson Veterinary | 07-893-1389 | Patterson Private Label, Manufacturer Item #14043-0704-06 |
| Isoflurane Vaporizer | VetEquip Inc. | 911103 | |
| Lab Tape, 3/4" | VWR International | 89097-990 | |
| Needles, 23 gauge | BD | 305145 | plastic hub removed |
| Parafilm Laboratory Film | Patterson Veterinary | 07-893-0260 | Medline Industries Inc., Manufacturer Item #HSFHS234526A |
| Planar Surface Coil | Bruker | T12609 | 2cm |
| Polyethylene Tubing | Braintree Scientific | PE50 50FT | 0.023" (inner diameter), 0.038" (outer diameter) |
| Puralube Ophthalmic Ointment | Patterson Veterinary | 07-888-2572 | Dechra Veterinary Products, Manufacturer Item #211-38 |
| Sprague Dawley Rats | Charles River | 400 SAS SD | |
| Sterile 0.9% Saline Solution | Patterson Veterinary | 07-892-4348 | Aspen Vet, Manufacturer Item #14208186 |
| Sterile Alcohol Prep Pads | Medline | MDS090735 | |
| Surgical Tape, 1" (3M Durapore) | Medline | MMM15381Z | 3M Healthcare, "wide medical tape" |
| Surgical White Paper Tape, 1/2" (3M Micropore) | Medline | MMM15300 | 3M Healthcare |
| Syringes, 1 mL w/ 25 gauge needle | BD | 309626 | |
| Syringes, 3 mL | BD | 309657 | |
| Vented induction and scavenging system | VetEquip Inc. | 942102 | 2 liter induction chamber with active scavenging |
| 411724 | omega flowmeter | ||
| 931600 | scavenging cube, "vacuum" | ||
| 921616 | nose cone, non-rebreathing |
Referências
- Smitha, K. A., et al. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. The Neuroradiology Journal. 30 (4), 305-317 (2017).
- Gorges, M., et al. Functional connectivity mapping in the animal model: Principles and applications of resting-state fMRI. Frontiers in Neurology. 8, (2017).
- Paasonen, J., Stenroos, P., Salo, R. A., Kiviniemi, V., Gröhn, O. Functional connectivity under six anesthesia protocols and the awake condition in rat brain. NeuroImage. 172, 9-20 (2018).
- Pawela, C. P., et al. A protocol for use of medetomidine anesthesia in rats for extended studies using task-induced BOLD contrast and resting-state functional connectivity. NeuroImage. 46 (4), 1137-1147 (2009).
- Jonckers, E., et al. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magnetic Resonance in Medicine. 72 (4), 1103-1112 (2014).
- Williams, K. A., et al. Comparison of alpha-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat. Magnetic Resonance Imaging. 28 (7), 995-1003 (2010).
- Zhurakovskaya, E., et al. Global functional connectivity differences between sleep-like states in urethane anesthetized rats measured by fMRI. PloS One. 11 (5), 0155343 (2016).
- Fukuda, M., Vazquez, A. L., Zong, X., Kim, S. -. G. Effects of the α2-adrenergic receptor agonist dexmedetomidine on neural, vascular and BOLD fMRI responses in the somatosensory cortex. The European Journal of Neuroscience. 37 (1), 80-95 (2013).
- Brynildsen, J. K., et al. Physiological characterization of a robust survival rodent fMRI method. Magnetic Resonance Imaging. 35, 54-60 (2017).
- Lu, H., et al. Rat brains also have a default mode network. Proceedings of the National Academy of Sciences of the United States of America. 109 (10), 3979-3984 (2012).
- Lu, H., et al. Low- but not high-frequency LFP correlates with spontaneous BOLD fluctuations in rat whisker barrel cortex. Cerebral Cortex. 26 (2), 683-694 (2016).
- Tsai, P. -. J., et al. Converging structural and functional evidence for a rat salience network. Biological Psychiatry. 88 (11), 867-878 (2020).
- Murphy, K., Bodurka, J., Bandettini, P. A. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage. 34 (2), 565-574 (2007).
- Birn, R. M., et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 83, 550-558 (2013).
- Lu, H., et al. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proceedings of the National Academy of Sciences of the United States of America. 104 (46), 18265-18269 (2007).
- Lu, H., et al. Registering and analyzing rat fMRI data in the stereotaxic framework by exploiting intrinsic anatomical features. Magnetic Resonance Imaging. 28 (1), 146-152 (2010).
- Cox, R. W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 29 (3), 162-173 (1996).
- Ash, J. A., et al. Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats. Proceedings of the National Academy of Sciences of the United States of America. 113 (43), 12286-12291 (2016).
- Hsu, L. -. M., et al. Intrinsic insular-frontal networks predict future nicotine dependence severity. The Journal of Neuroscience. 39 (25), 5028-5037 (2019).
- Li, Q., et al. Resting-state functional MRI reveals altered brain connectivity and its correlation with motor dysfunction in a mouse model of Huntington’s disease. Scientific Reports. 7, (2017).
- Lu, H., et al. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain Connectivity. 4 (7), 499-510 (2014).
- Seewoo, B. J., Joos, A. C., Feindel, K. W. An analytical workflow for seed-based correlation and independent component analysis in interventional resting-state fMRI studies. Neuroscience Research. 165, 26-37 (2021).
- Broadwater, M. A., et al. Adolescent alcohol exposure decreases frontostriatal resting-state functional connectivity in adulthood. Addiction Biology. 23 (2), 810-823 (2018).
- Jaime, S., Cavazos, J. E., Yang, Y., Lu, H. Longitudinal observations using simultaneous fMRI, multiple channel electrophysiology recording, and chemical microiontophoresis in the rat brain. Journal of Neuroscience Methods. 306, 68-76 (2018).

