Mesenchymal Stem Cell Regulation of Macrophage Phagocytosis; Quantitation and Imaging
Summary
Presented here is a protocol to quantify and produce dynamic images of mesenchymal stem cell (MSC) mediated regulation of macrophage (MΦ) phagocytosis of non-opsonized yeast (zymosan) particles that are conjugated to a pH-sensitive fluorescent molecule.
Abstract
Mesenchymal stem cells (MSC) have traditionally been studied for their regenerative properties, but more recently, their immunoregulatory characteristics have been at the forefront. They interact with and regulate immune cell activity. The focus of this study is the MSC regulation of macrophage phagocytic activity. Macrophage (MΦ) phagocytosis is an important part of the innate immune system response to infection, and the mechanisms through which MSC modulate this response are under active investigation. Presented here is a method to study MΦ phagocytosis of non-opsonized zymosan particles conjugated to a pH-sensitive fluorescent molecule while in co-culture with MSC. As phagocytic activity increases and the labeled zymosan particles are enclosed within the acidic environment of the phagolysosome, the fluorescence intensity of the pH-sensitive molecule increases. With the appropriate excitation and emission wavelengths, phagocytic activity is measured using a fluorescent spectrophotometer and kinetic data is presented as changes in relative fluorescent units over a 70 min period. To support this quantitative data, the change in the phagocytic activity is visualized using dynamic imaging. Results using this method demonstrate that when in co-culture, MSC enhance MΦ phagocytosis of non-opsonized zymosan of both naive and IFN-γ treated MΦ. These data add to the current knowledge of MSC regulation of the innate immune system. This method can be applied in future investigations to fully delineate the underlying cellular and molecular mechanisms.
Introduction
Mesenchymal stem cells (MSC) are progenitor cells that give rise to connective tissue cells. MSC are present in adult mammalian tissues and can be isolated from the bone marrow1. Due to their immunomodulatory properties, these cells are widely studied2. Early studies focused on MSC regulation of T-cells3,4,5,6 but more recently, their regulation of macrophage cells (MΦ), a major cellular component of innate immunity, has received increased attention7,8,9,10,11,12,13,14. The importance of MSC-MΦ interaction in the treatment of inflammatory disease is underscored by the fact that depletion of monocytes/macrophages abrogates the therapeutic effects of MSC in animal models8. Here, the focus is the cell contact interaction of the MSC with MΦ. MSC have the capacity to regulate the phenotype of MΦ by promoting the switch from inflammatory to anti-inflammatory responses, leading to tissue repair activities8,9,10,11, and much has been done to demonstrate these regulatory mechanisms. Under other circumstances, MSC can support or exacerbate a MΦ-driven inflammatory response12,13 and enhance MΦ phagocytic activity14,15. However, there is a critical lack of existing data that identifies the mechanisms whereby and the conditions under which MSC regulate MΦ phagocytic activity.
MΦ have families of receptors that recognize either opsonized (antibody or complement coated) or non-opsonized pathogens leading to phagocytosis16. The activation and activity of the latter is less well studied17. In a non-inflammatory in vitro environment, MSC enhance MΦ phagocytosis of non-opsonized pathogens13. However, recognition of non-opsonized pathogens by MΦ may be reduced after exposure to an inflammatory environment produced by lymphocytes during an adaptive immune response. IFN-γ, released by natural killer cells and effector lymphocytes, has an inhibitory effect on MΦ phagocytosis of non-opsonized particles18. A co-culture model was developed to study the mechanisms of MSC direct contact regulation of MΦ phagocytosis. The goal of the experiment presented here is to determine whether MSC regulate MΦ phagocytosis of non-opsonized pathogens after MΦ have been exposed to IFN-γ (Figure 1).
Protocol
NOTE: All medium preparation and cell culture techniques are carried out under aseptic conditions using a biosafety cabinet with laminar flow. All culture incubation steps described are carried out using an incubator designed to maintain an atmosphere of 37 °C, 5% CO2, and 95% humidity.
1. Cell culture
- Preparation of growth medium
- For MSC and LADMAC, add 50 mL of FBS and 5 mL of 100x antibiotic/antimycotic mix to 500 mL of high glucose DMEM.
- For the MΦ, add 50 mL of FBS, 100 mL of LADMAC condition medium (prepared following the steps of section 1.2), and 5 mL of 100x antibiotic mix to 500 mL of high glucose DMEM.
NOTE: LADMAC cells produce colony-stimulating factor (CSF-1) necessary to support the growth of the MΦ cells.
- Preparation of LADMAC conditioned medium
- To seed LADMAC cells from frozen stock, add 19 mL of growth medium from section 1.1 in each of the five T75 cm2 cell-culture treated flasks and place in the cell culture incubator for 15 min to equilibrate to 37 °C.
- Meanwhile, quick thaw one aliquot of 106 frozen cells by incubating in a 37 °C water bath for 2-3 min or until it gets thawed. Add the cells to 9 mL of fresh MSC growth medium in a 15 mL or 50 mL sterile conical tube and centrifuge at 125 x g in a swinging bucket rotor for 5 min.
- Aspirate or decant the supernatant, add 5 mL of fresh growth medium, and gently pipette up and down to resuspend the cell pellet.
- Add 1 mL of the cell suspension to each of the five T75 cm2 flasks using a sterile serological pipette and place them in the cell culture incubator.
- Every 2-3 days, add an additional 10 mL of medium over a 7-10-day period. Then, collect the cells and the supernatant into sterile 50 mL conical tubes using a sterile serological pipette and centrifuge at 125 x g for 5 min.
- Separate the supernatant from the cell pellet and sterile filter the supernatant using a 0.2 µm sterile single-use vacuum filter unit.
- Remove the aspirator assembly from the package and connect using vacuum tubing to the aspirator pump. Pour the supernatant into the top compartment and replace the cover. Turn on the aspirator pump to filter into the bottom compartment.
- Prepare aliquots by pipetting into 50 mL sterile conical tubes and store at -20 °C.
- To freeze the cell pellet, resuspend in freezing medium at 106 cell/mL, place in a freezing container, and then place them in a -80 °C freezer. After 24 h, transfer to LN2 storage.
- Propagate cells in preparation for co-culture
- To seed MSC and MΦ cells from the frozen stock, equilibrate 9 mL of appropriate growth medium prepared in section 1.1 in each of the four 100 mm cell culture treated dishes for each cell type and place in the cell culture incubator for 15 min.
- Meanwhile, quick thaw the aliquots of 106 frozen cells by incubating them in a 37 °C water bath for 2-3 min or until just thawed. Then, add the thawed MSC cells to 9 mL of fresh MSC growth medium and add the thawed MΦ cells to 9 mL fresh MΦ growth medium in 15 or 50 mL sterile conical tubes and centrifuge at 125 x g in swinging bucket rotor for 5 min.
- Aspirate or decant the supernatant and resuspend each pellet in 4 mL of the appropriate fresh growth medium by gently pipetting up and down. Add 1 mL of the cell suspension to each of the four 100 mm dishes for each cell type that have been equilibrated in step 1.3.1.
- Return the dishes with cells to the incubator. Change the medium every 2-3 days until 70%-80% confluent.
2. Seed MSC in experimental dishes, Day 1
NOTE: Prior to seeding the MSC, design the experimental 96-well plate layout for fluorescent spectrophotometry and the chamber slide for dynamic imaging. For the 96-well plate, flank experimental wells with wells containing cells but do not use them in the assay. Mark at least four wells to be used for the reagent blank (RBL). See Table 1 for an example 96-well template and Table 2 for an example of a 4-well chamber slide template. Black or white 96-well plates with clear bottoms are recommended. A 1.5 mm borosilicate glass chamber slide is optimal, but the number of chambers can be adjusted dependent on the study design. A summary flow chart of the methods that follow is included in Figure 2.
- At 70%-80% confluency, aspirate the growth medium from the cultures in 100 mm dishes and add 5 mL of PBS to isolate the MSC.
- Aspirate the PBS, add 2 mL of 0.05% trypsin/EDTA, and place it in the culture incubator for 3-5 min.
- After 3 min, check the cell detachment using an inverted microscope. If the cells are detached, continue to the next step; if not, continue incubation for another 1-2 min. Do not incubate longer than 5-6 min.
- Add 5 mL of fresh growth medium to the dish with the detached cells. Rinse the dish using the trypsin/medium mixture and collect cells into a clean, sterile 50 mL conical tube using a sterile serological pipette.
- Centrifuge for 5 min at 125 x g. Aspirate the supernatant and resuspend the cell pellet in 10 mL of fresh growth medium by gently pipetting up and down.
- To count the cells, add 10 µL of the cell suspension to 30 µL of 0.4% trypan blue solution in a 1.5 mL microcentrifuge tube. Then, pipette 10 µL of the mixture under the coverslip of a hemocytometer counting chamber.
- Using an inverted or bright field microscope, with the 10x objective, count the unstained (viable) cells in four of the 1 mm2 squares. Calculate the cell number using the formula: cells/mL = cell count/# 1 mm2 squares x dilution factor x 104.
NOTE: For this example, the number of 1 mm2 squares counted is four, and the dilution factor is 4. - Use the equation C1V1 = C2V2 to calculate for and prepare a 1 x 105 cell/mL suspension. Prepare 1 mL of cell/mL suspension for every column of the 96-well plate to be filled and 0.75 mL for every chamber of the 4-well chamber slide to be filled according to the plate design.
NOTE: C1 = cell count, V1 = what is being calculated, C2 = 1 x 105, V2 = 5.50 mL. - Determine V1, subtract it from the desired 5.50 mL of the total volume to determine the volume of the fresh medium needed to prepare the suspension. Add the volume of cells (V1) from the original suspension to the volume of fresh medium for a total of 5.50 mL with 1 x 105 cells/mL.
- Add 100 µL of 1 x 105 cell/mL suspension to each well of the 96-well plate according to the plate design using a multichannel micropipettor and 530 µL to each of the wells of the chamber slide using a micropipette according to the plate design. Incubate overnight.
NOTE: For this experiment, MSC are plated in columns 1, 2, 3, and 6 of the 96-well plate (Table 1) and wells 1 and 2 of the 4-well chamber slide (Table 2). Therefore, at least 5.50 mL of a 1 x 105 cell/mL suspension is prepared. MΦ at 80% confluence are treated with inflammatory mediators the same day the MSC are seeded in the experimental plates.
3. Activate the MΦ with IFN-γ, Day 1
- Prepare the activation medium and IFN-γ stock.
- For 200 mL of activation medium, add 40 mg of BSA to 200 mL of serum-free high glucose DMEM and sterile filter using a 0.2 µm sterile single-use vacuum filter unit.
- To prepare 0.1% BSA/PBS, add 20 mg of BSA to 20 mL of PBS. Vortex to dissolve. Use a 0.2 µm sterile syringe filter to filter into a clean, sterile 50 mL conical tube.
- Add 1 mL of the sterile 0.1% BSA/PBS solution to 100 µg of lyophilized IFN-γ to achieve a 100 µg/mL of stock solution. Store in 50 µL aliquots at -20 °C.
- Activate the MΦ with IFN-γ
- Add 2.5 µL of 100 µg/mL of IFN-γ stock for every 1 mL of the medium needed. For every 100 mm dish to be activated, prepare 10 mL of activation medium containing IFN-γ at a concentration of 250 ng/mL.
- Aspirate the growth medium from the MΦ cultures and replace it with 5 mL of the activation medium without IFN- γ to rinse.
- Aspirate the medium used for rinsing and replace it with 10 mL of IFN- γ supplemented activation medium for the experimental dish and with 10 mL of non-supplemented activation medium for the control. Incubate the cultures for 16-24 h.
4. Isolate the MΦ and prepare the co-cultures, Day 2
- Remove the activation medium from the MΦ cells and replace it with 5 mL of fresh growth medium. Scrape gently with a cell lifter and collect the cells into a 50 mL conical tube.
- Count the cells using trypan blue exclusion and hemocytometry as described for MSC in, steps 2.6-2.7.
- Using the equation in steps 2.8-2.9, prepare two separate suspensions of 2 x 105 cells/mL, one with cells from the control and one with cells from the treated MΦ cultures.
NOTE: Prepare 1 mL of cell/mL suspension for every column of the 96-well plate to be filled and 0.75 mL for every chamber of the 4-well chamber slide to be filled according to the plate design. - Gently aspirate the medium from the experimental wells of the 96-well plate containing MSC. Using a multichannel micropipette, add 100 µL of the treated and the control MΦ cell suspensions in the appropriate experimental wells with and without MSC according to the plate design. Incubate overnight.
- Gently aspirate the medium from the 4-well chamber slide containing MSC, and, using a micropipette, add 530 µL of MΦ cell suspension to the appropriate wells according to the design. Incubate overnight.
5. Phagocytosis assay, kinetic fluorescent 96-well plate read, Day 3
- Set the assay parameters
- On the day of the assay, turn on the fluorescent plate reader and computer-set the temperature on the system to 37 °C.
- Open the system Pro software and check the instrument icon in the upper-left corner to determine whether the instrument is connected to the computer. If the red circle with a line is visible, click on the instrument icon and choose the instrument in the pop-up window to make the connection.
- Select New Experiment to access the Plate Set-Up Helper pop-up window. From the Plate Set-Up Helper menu, select Configure Your Acquisition Settings.
- Select Monochromator from the settings menu for the optical configuration. Select FL (fluorescence) for the read mode and Kinetic for the read type.
- In the same configurations window, under Category, click on Wavelengths, and then set bandwidths to 9 nm for excitation and 15 nm for emission.
- Set the Number of Wavelength Pairs to 1. Set the wavelengths LM1 to 510 nm for excitation and to 540 nm for emission.
- Continue through the categories and select Plate Type next. Select the option that matches the plate type being used.
NOTE: It is important that the selection matches the plate type. The read heights are set by the system according to the selection. - Next, select Read Area and highlight the area of the 96-well plate included in the experimental design.
- Select PMT and Optics, set PMT Gain to High and Flashes per read to 6. Check the box next to Read from Bottom if using a clear-bottom plate.
- Select Timing and set for 10 min intervals over a 70 min period.
- Select Shake, check the Before First Read box, and set for 5 s. Check the Between Reads box and set for 3 s. Set shake intensity for both to Low and Linear.
- Close the window. When the Plate Set-Up Helper window pops up, select Configure Your Plate Layout. Highlight the BL wells of the plate design and click on Plate Blank.
- Highlight each of the experimental rows, click on Add, name the group, and then select a color.
- Under Assignment Options below the plate design, select Series, and then define the series in the window and click on OK. Repeat for each group.
- Prepare the zymosan suspension
- While waiting for the system temperature to reach 37 °C, resuspend 1 mg of zymosan particles in 5 mL of the live-cell imaging medium.
- Add 1 mL of live-cell imaging medium to the vial containing the particles and collect them into a glass culture tube. Rinse the vial with an additional 1 mL of imaging solution and transfer to the same glass tube to ensure that all the particles are transferred. Add 3 mL of additional imaging solution to achieve a 0.2 mg/mL of particle suspension.
- Vortex with quick pulses for 30-60 s. Then, use a probe sonicator to sonicate with 60 quick pulses.
NOTE: It is very important to create a homogenous suspension of particles and not let the suspension sit too long before adding to the experimental wells. Clumping recurs rapidly. The particle per cell density may need to be optimized depending on the source, treatment, and density of MΦ used. In the studies presented, conjugated zymosan particles were used. However, conjugated E. coli and S. aureus are also available.
- While waiting for the system temperature to reach 37 °C, resuspend 1 mg of zymosan particles in 5 mL of the live-cell imaging medium.
- Add zymosan and read the plate
- Aspirate the medium from the experimental wells and rinse 1x with 100 µL of the live-cell imaging solution. Rinse the RBL wells with the live-cell imaging solution as well.
- Aspirate the live-cell imaging solution from experimental and RBL wells and replace it with 100 µL of the zymosan particle suspension prepared in section 5.2.
- Open the plate tray of the fluorescent reader using the touchpad interface. Set the plate, without the lid, in the tray with the A1 well in the upper-left corner. Close the tray using the touchpad and click on the green Read button in the top menu.
- When the read is complete, save the file to the appropriate folder and export the data in a spreadsheet format by clicking on File and selecting Export.
- Calculate the mean ± SEM relative fluorescence for each replicate group at each time point (10 min, 20 min, 30 min, etc.) in the spreadsheet (Supplementary File 1) and transfer the data to graphing software.
- Use a line graph format to present the data and apply two-way ANOVA (Time x Treatment/MSC as factors) followed by Tukey's multiple comparisons test to determine differences between individual groups.
NOTE: While the 96-well plate read is in progress, prepare the dynamic imaging plate for time-lapse image acquisition. Ensure that the dynamic imaging proceeds with the analysis of one experimental well at a time.
6. Phagocytosis assay, dynamic imaging, Day 3
- Turn on the imaging system and computer. Double click to open the software. Select the Pro program mode.
- Check the stage area to ensure no samples are present and nothing is impeding the movement of the stage. Then, click on Calibrate Now.
- Under the Incubation menu on the sidebar on the right, check the box next to H Unit XL and set it to 37 °C.
NOTE: Wait until the incubated stage is almost to the temperature before preparing the samples for imaging. - Rinse the first experimental well with 750 µL of imaging medium and replace it with 400 µL of the zymosan particle suspension prepared in section 5.2. Leave the remaining wells in the growth medium.
NOTE: It may be necessary to vortex and sonicate the particle suspension again briefly if it has settled for more than 15 min. - Place the slide on the incubated stage of the imaging system. Set a timer for 10 min.
- Use the imaging software, select Brightfield under the Locate tab, and then click on the eyepiece icon in the system configuration window below. With the 10x objective in place, use the eyepiece and focusing knob to focus on the cells.
- Select the Acquisition tab, use the Experiment drop-down menu, and then select a set of wavelengths that includes EGFP filter set to accommodate 509 nm excitation 533 nm emission wavelengths of the pH-sensitive dye.
- When there is ~2 min left on the timer, use the software to change the objective to 20x by clicking on the 20x icon in the objective menu.
- Click on the Channels menu, select EGFP filters and use the Exposure menu to set the exposure time to 400 ms to detect the pH-sensitive green fluorophore once it has been enclosed in the phagolysosome.
- Click on Live and adjust the focus using the focusing knob.
- Click on Stop to turn off the light and check the box next to the Time-lapse experimental setting at the top.
- In the Focusing Strategy menu select Software Autofocus. From the drop-down in the Autofocus menu, select Smart and Coarse settings to reduce the time of exposure to light while the autofocus is running.
- In the Time-Lapse menu, set the desired number of acquisitions to 30-60 and the interval time to 1 min.
- After the time-lapse parameters are set and after 10 min of addition of particles to the first well, click on the Start Experiment button to begin acquisition.
- When the acquisition is complete using the first experimental well, repeat steps 6.4-6.12 for each of the remaining experimental wells.
- Save all the experiment files with the date and parameters in the title to the appropriate folder.
- Close the software. Reopen the software in Processing mode and export the files in the MP4 format using the Export menu.
Representative Results
After calculating mean ± SEM for each group at all time points, the data is presented in line graph format with the Y-axis as the Relative Fluorescent Intensity and the X-axis as Time. Supplementary File 1 provides an example of raw data from a kinetic read of the 96-well plate in a spreadsheet format.
In this study, the optimal results presented in Figure 3A, and Table 3 demonstrate that 1) co-culture with MSC enhances the phagocytic activity of the macrophage, 2) IFN-y treatment reduces the activity of the macrophage, and 3) co-culture with MSC partially rescues MΦ phagocytic activity. Optimal cell densities are critical in these studies, and when MΦ are plated at too low of a density, changes in fluorescence intensity cannot be detected (Figure 3B). Figure 3C represents data from a study where the MΦ were plated at too high of a density and fluorescence intensity is elevated rapidly in all groups, and differences cannot be discerned. The dynamic imaging videos confirm that the fluorescent intensity changes result from phagocytosis and not acidification of the medium. They also provide qualitative data and a visual representation of the rate and extent of phagocytic activity (Figure 4).
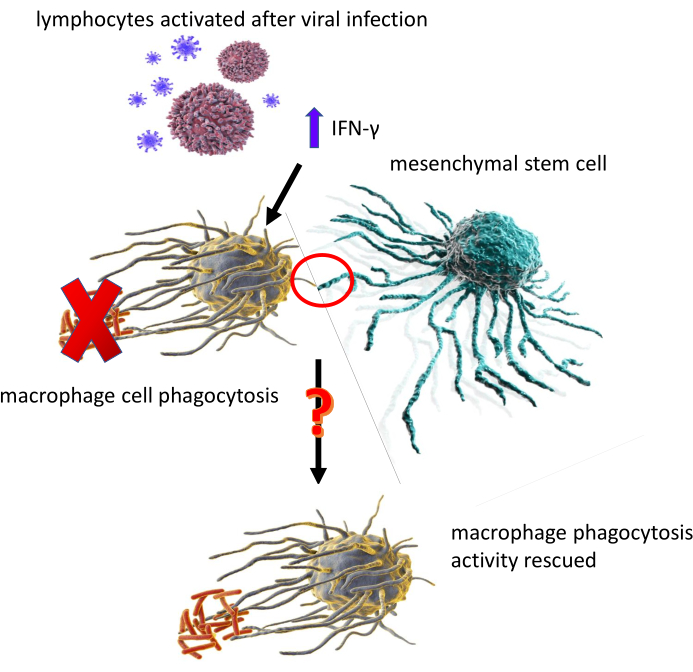
Figure 1: An illustration depicting the central question of the data presented, which is "Can MSC recover the phagocytic activity of IFN-γ treated MΦ?". Please click here to view a larger version of this figure.
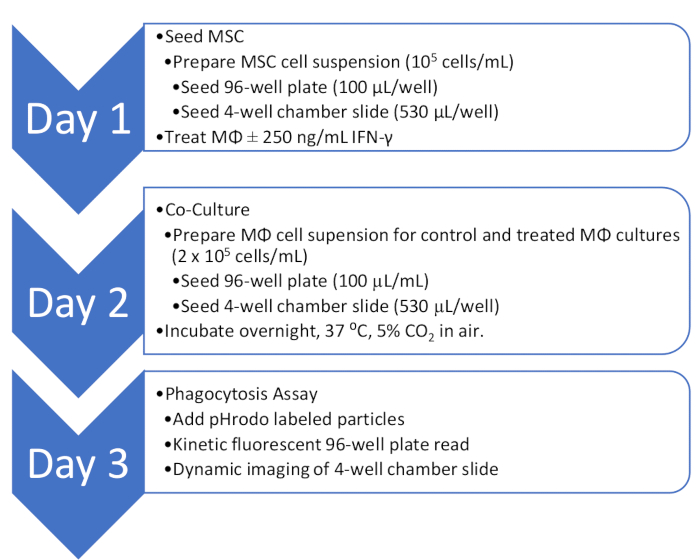
Figure 2: Overview of the co-culture and phagocytosis assay workflow. An outline of the workflow for quantitative and qualitative analysis of MΦ phagocytosis activity in co-culture with MSC. Please click here to view a larger version of this figure.
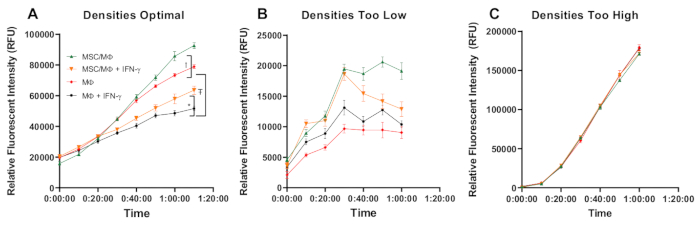
Figure 3: Representative quantitative data from the kinetic readdemonstrating optimal and suboptimal results. In (A), data are representative of an experiment with optimal MΦ cell density, in (B) data are representative of an experiment with suboptimal too low MΦ cell density, and in (C), data are representative of an experiment with suboptimal too high MΦ cell density. The relative fluorescence intensity measured in relative fluorescent units (RFU) is plotted on the Y-axis, while time is plotted on the X-axis. Note the differences in the range of RFU among optimal and sub-optimal experiments. In A, MSC partially rescue MΦ phagocytic activity in the setting of IFN-γ suppression over a 70 min period. The RFU is presented as mean ± the SEM, n = 6. The analysis was performed using Tukey's multiple comparisons test after a significant two-way ANOVA, Interaction effect P = 0.0001, Time effect P = 0.0001, and Treatment/MSC effect P = 0.0001. Symbols denote results of multiple comparison tests. * = significant difference between MSC/MΦ + IFN- γ vs MΦ + IFN-γ, Ŧ = significant difference between MΦ vs MΦ + IFN-γ, and † = significant difference between MSC/MΦ vs MΦ. See Table 3 for detailed results of the multiple comparison tests. Please click here to view a larger version of this figure.
Figure 4: Dynamic imaging videos providing visual confirmation of the cell-specific increase in fluorescence from acidic activation of labeled zymosan particles after incorporation into the MΦ phagolysosome. Time-lapse settings were acquisition every 1 min over a 30 min period using an exposure time of 400 ms and the EGFP filter set. (A) MΦ in monoculture, (B) MΦ in co-culture with MSC, (C) MΦ treated with IFN-γ (250 ng/mL) and (D) MΦ treated with IFN-γ (250 ng/mL) in co-culture with MSC. Please click here to download this File.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| A | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| B | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| C | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| D | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| E | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| F | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| G | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL | |||||
| H | CBL | MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ | CBL | RBL |
Table 1: Example of a 96-well plate design. CBL – Cell blank; MSC – seeded day 1; MΦ+ treated and MΦ untreated seeded day 2; RBL reagent blank added on assay day 3.
| 1 | 2 | 3 | 4 |
| MSC/MΦ | MSC/MΦ+ | MΦ | MΦ+ |
Table 2: Example of a 4-well chamber slide design. MSC – plated day 1; MΦ+ treated and MΦ untreated plated day 2.
| Tukey's multiple comparisons test | Mean Diff. | 95.00% CI of diff. | Below threshold? | Summary | Adjusted P Value |
| 0 minutes | |||||
| MSC/MΦ vs. MΦ | -3871 | -9495 to 1754 | No | ns | 0.2836 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 720.3 | -4904 to 6345 | No | ns | 0.9873 |
| MΦ vs. MΦ + IFN-γ | -77.17 | -5702 to 5548 | No | ns | >0.9999 |
| 10 minutes | |||||
| MSC/MΦ vs. MΦ | -3466 | -9091 to 2159 | No | ns | 0.3817 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 2326 | -3299 to 7950 | No | ns | 0.7062 |
| MΦ vs. MΦ + IFN-γ | 992 | -4633 to 6617 | No | ns | 0.968 |
| 20 minutes | |||||
| MSC/MΦ vs. MΦ | -1311 | -6936 to 4314 | No | ns | 0.9303 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 3315 | -2310 to 8940 | No | ns | 0.422 |
| MΦ vs. MΦ + IFN-γ | 3146 | -2478 to 8771 | No | ns | 0.4689 |
| 30 minutes | |||||
| MSC/MΦ vs. MΦ | 384.8 | -5240 to 6010 | No | ns | 0.998 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 2313 | -3312 to 7937 | No | ns | 0.7098 |
| MΦ vs. MΦ + IFN-γ | 8726 | 3101 to 14350 | Yes | *** | 0.0005 |
| 40 minutes | |||||
| MSC/MΦ vs. MΦ | 2247 | -3377 to 7872 | No | ns | 0.7278 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 4913 | -712.2 to 10537 | No | ns | 0.1101 |
| MΦ vs. MΦ + IFN-γ | 16521 | 10896 to 22145 | Yes | **** | <0.0001 |
| 50 minutes | |||||
| MSC/MΦ vs. MΦ | 5657 | 32.12 to 11282 | Yes | * | 0.0481 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 4932 | -692.9 to 10557 | No | ns | 0.1079 |
| MΦ vs. MΦ + IFN-γ | 19083 | 13458 to 24708 | Yes | **** | <0.0001 |
| 60 minutes | |||||
| MSC/MΦ vs. MΦ | 12376 | 6752 to 18001 | Yes | **** | <0.0001 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 9361 | 3736 to 14986 | Yes | *** | 0.0002 |
| MΦ vs. MΦ + IFN-γ | 24748 | 19123 to 30373 | Yes | **** | <0.0001 |
| 70 minutes | |||||
| MSC/MΦ vs. MΦ | 13770 | 8145 to 19395 | Yes | **** | <0.0001 |
| MSC/MΦ + IFN-γ vs. MΦ + IFN-γ | 11987 | 6362 to 17612 | Yes | **** | <0.0001 |
| MΦ vs. MΦ + IFN-γ | 27264 | 21639 to 32888 | Yes | **** | <0.0001 |
Table 3: Detailed statistical analysis of the data presented in Figure 3A. Results of Tukey's multiple comparison tests after a significant two-way ANOVA of data presented in Figure 3A.
Supplementary File 1: A representative spreadsheet file of raw kinetic data from an optimal experiment. Please click here to download this File.
Discussion
Analysis of phagocytosis using bioparticles conjugated to a pH-sensitive dye is a relatively new tool that has proven advantageous over traditional fluorescently labeled particles12,19,20. With traditional fluorescent-labeled particles, only end-point analysis is feasible. Detection is carried out with fluorescent microscopy and/or spectrofluorometry after washing or quenching particles that have not been taken up by the phagocyte. Quantitative data derived from spectrofluorometry has the potential to detect non-engulfed particles, and image analysis to quantify only intracellular particles is tedious and time-consuming using traditional fluorescent microscopy systems14. Bioparticles conjugated to pH-sensitive dyes fluoresce only in an acidic environment such as the phagolysosome, and, therefore, the tedious washing and quenching steps are unnecessary14. Additionally, pH-sensitive labeled bioparticles provide the advantage of providing kinetic data that would not be acquired easily with FITC or other fluorophore-conjugated bioparticles.
Studies using this new tool have employed flow cytometry and/or imaging platforms to generate a quantitative and kinetic measurement of phagocyte activity19,20,21. Flow cytometry is advantageous if there is a limited phagocyte population or if one is interested in sorting for downstream analyses such as genetic screens19. MSC are known to regulate the MΦ phenotype through both direct contact and through soluble factors. Preliminary studies have shown that conditioned medium from MSC suppressed MΦ phagocytosis, and therefore, this protocol was designed to determine the changes in MΦ phagocytic activity while in direct co-culture with MSC. Under these conditions, spectrofluorometry to quantify and dynamic imaging to visualize and confirm phagocytic activity are most appropriate.
Critical to the success of these methods is the optimization of cell densities. The MSC need to be plated at a density that allows for optimal cell contact with the MΦ cells. MΦ need to be seeded in mono and co-cultures at a density that not only allows for optimal contact but also allows for optimal fluorescence detection. Too low of a density will result in low incorporation into the phagolysosome and small or flat changes in relative fluorescent units (Figure 3B). If MΦ are seeded at too high of a density, phagocytosis increases rapidly, and detection of differences between groups is masked (Figure 3C). Optimizing the concentration of pH-sensitive dye labeled zymosan particles per cell number is also critical. Preliminary experiments should be performed to optimize cell densities of MSC and MΦ, and the number of particles per MΦ cell. Additionally, these optimization steps should be taken if other labeled bioparticles are used, such as E. coli or S. aureus.
The appropriate imaging medium is also critical. The medium for both methods should be a buffered medium and should not contain phenol red. Phenol red will mute the detection of the fluorescent emission. Also, any additive or condition that can acidify the medium will prematurely activate the labeled particles and introduce elevated background. The reagent blank is critical for identifying the potential acidification of the medium.
Not only can these protocols be used to investigate MSC regulation of MΦ phagocytosis of a variety of pH-sensitive bioparticles, but the method can also be applied to a variety of co-culture models that include neutrophils and other phagocytes. Additionally, by using this model, molecules and pathways suspected of being involved in cell contact-mediated regulation of macrophage phagocytic activity can be probed via siRNA or CRISPR mediated down-regulation to validate or refute suspected molecular targets. Potential targets include adhesion molecules, phagocytic receptors, and integrin molecules.
Work using these methods will uncover new information regarding the signaling mechanisms underlying the increased phagocytic responses of MΦ following cell contact interactions with MSC, thereby furthering our understanding of the role MSC play in regulating innate immunity. Studies such as these address an understudied area and will yield a complete picture of the influence MSC have on macrophage activity, which is necessary for a full understanding of their role in immunity.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the NSF Major Research Instrument mechanism under grant numbers 1626093 and 1919583.
Materials
| 96 Well Black Polystyrene Microplate | MilliporeSigma | CLS3603-48EA | |
| 0.4% trypan blue solution | MilliporeSigma | T8154-20ML | |
| 15 mL and 50 mL Conical Sterile Polypropylene Centrifuge Tubes | ThermoFisher | 339653 | |
| 4-well Chambered Coverglass w/ non-removable wells | ThermoFisher | 155382PK | |
| Antibiotic-Antimycotic (100X) Gibco | ThermoFisher | 15240096 | |
| Axiobserver 7 Imaging System | Zeiss | ||
| Bovine Serum Albumin (BSA) | MilliporeSigma | A8806-1G | |
| Cell lifter | MilliporeSigma | CLS3008-100EA | |
| Culture flasks, tissue culture treated, surface area 75 cm2, canted neck, with cap, filtered | MilliporeSigma | C7231-120EA | |
| D1 ORL UVA [D1] | ATCC | CRL-12424 | Mouse MSC Cell Line |
| DMEM, High Glucose | ThermoFisher | 11965092 | |
| Fetal Bovine Serum, qualified, heat inactivated | ThermoFisher | 16140071 | |
| Hemocytometer | FisherScientific | 02-671-51B | |
| I-11.15 | ATCC | CRL-2470 | Mouse MΦ Cell Line |
| LADMAC Cell Line | ATCC | CRL-2420 | LADMAC cells secrete the growth factor colony stimulating factor 1 (CSF-1). |
| Live-Cell Imaging solution | ThermoFisher | A14291DJ | |
| PBS, pH 7.4 | ThermoFisher | 10010031 | |
| pHrodo Green Zymosan Bioparticles Conjugate | ThermoFisher | P35365 | |
| Recombinant Murine IFN-γ | Preprotech | 315-05 | |
| Spectramax i3X | Molecular Devices | ||
| Sterile Single Use Vacuum Filter Units, 250 mL, 0.2 µm | ThermoFisher | 568-0020 | |
| Sterile syringe filters, 0.2 micrometer | ThermoFisher | 723-2520 | |
| Tissue-culture treated culture dishes, 100 mm x 20 mm | MilliporeSigma | CLS430167-100EA | |
| Trypsin-EDTA (0.05%), phenol red | ThermoFisher | 25300054 |
Referências
- Phinney, D. G., Prockop, D. J. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 25 (11), 2896-2902 (2007).
- Bernardo, M. E., Fibbe, W. E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 13 (4), 392-402 (2013).
- Tobin, L. M., Healy, M. E., English, K., Mahon, B. P. Human mesenchymal stem cells suppress donor CD4(+) T cell proliferation and reduce pathology in a humanized mouse model of acute graft-versus-host disease. Clinical and Experimental Immunology. 172 (2), 333-348 (2013).
- Giuliani, M., et al. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS One. 6 (5), 19988 (2011).
- Plumas, J., Chaperot, L., Richard, M. J., Molens, J. P., Bensa, J. C., Favrot, M. C. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 19 (9), 1597-1604 (2005).
- Luz-Crawford, P., et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Research & Therapy. 4 (3), 65 (2013).
- Waterman, R. S., Tomchuck, S. L., Henkle, S. L., Betancourt, A. M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 5 (4), 10088 (2010).
- Nemeth, K., et al. marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their Interleukin-10 production. Nature Medicine. 15 (1), 42-49 (2009).
- Maggini, J., et al. Mouse Bone Marrow-Derived Mesenchymal Stromal Cells Turn Activated Macrophages into a Regulatory-Like Profile. PLoS One. 5 (2), 9252 (2010).
- Takizawa, N., et al. marrow-derived mesenchymal stem cells propagate immunosuppressive/anti-inflammatory macrophages in cell-to-cell contact-independent and-dependent manners under hypoxic culture. Experimental Cell Research. 358 (2), 411-420 (2017).
- Kim, J., Hematti, P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental Hematology. 37 (12), 1445-1453 (2009).
- Evans, J. F., Salvador, V., George, S., Trevino-Gutierrez, C., Nunez, C. Mouse aorta-derived mesenchymal progenitor cells contribute to and enhance the immune response of macrophage cells under inflammatory conditions. Stem Cell Research & Therapy. 6 (1), 56 (2015).
- Cho, D. I., et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Experimental Molecular Medicine. 46, 70 (2014).
- Fernandez, N., et al. Mouse mesenchymal progenitor cells expressing adipogenic and osteogenic transcription factors suppress the macrophage inflammatory response. Stem Cells International. 2017, 5846257 (2017).
- Jackson, M. V., et al. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 34 (8), 2210-2223 (2016).
- Chaplin, D. D. Overview of the immune response. The Journal of Allergy and Clinical Immunology. 125 (2), 3-23 (2010).
- Lim, J., et al. Characterizing the mechanisms of nonopsonic uptake of cryptococci by macrophages. Journal of Immunology. 200 (10), 3539-3546 (2018).
- Wang, Z., et al. Interferon-γ inhibits nonopsonized phagocytosis of macrophages via an mTORC1-c/EBP pathway. Journal of Innate Immunity. 7 (2), 165-176 (2015).
- Lindner, B., Burkard, T., Schuler, M. Phagocytosis assays with different PH-sensitive fluorescent particles and various readouts. Biotechniques. 68, 245-250 (2020).
- Kapellos, T. S., et al. A novel real time imaging platform to quantify macrophage pahgocytosis. Biochemical Pharmacology. 116, 107-119 (2016).
- Takahashi, D., et al. Flow cytometric quantitation of platelet phagocytosis by monocytes using a pH-sensitive dye, pHrodo-SE. Journal of Immunological Methods. 447, 57-64 (2017).

