Employing Transcranial Magnetic Stimulation in a Resource Limited Environment to Establish Brain-Behavior Relationships
Summary
Transcranial Magnetic Stimulation (TMS) and low-frequency TMS (lfTMS) have been demonstrated to be major contributors to brain literature. Here we highlight the methods for investigating the cortical correlates of self-deception using TMS.
Abstract
Neuroimaging is typically perceived as a resource demanding discipline. While this is the case in certain circumstances, institutions with limited resources have historically contributed significantly to the field of neuroscience, including neuroimaging. In the study of self-deception, we have successfully employed single-pulse TMS to determine the brain correlates of abilities including overclaiming and self-enhancement. Even without the use of neuro-navigation, methods provided here lead to successful outcomes. For example, it was discovered that decreases in self-deceptive responding lead to a decrease in affect. These methods provide data that are reliable and valid, and such methods provide research opportunities otherwise unavailable. Through the use of these methods, the overall knowledge base in the field of neuroscience is expanded, providing research opportunities to students such as those at our institution (Montclair State University is a Hispanic-Serving Institute) who are often denied such research experiences.
Introduction
There are a number of challenges to investigating brain-behavior correlates at research institutions with limited resources (often referred to as 'teaching universities'). According to data provided by the National Science Foundation (NSF), almost all academic research is completed by a small percentage of higher education institutions in the United States. When examining over 4,400 post-secondary degree-granting institutions, the top 115 universities/institutes perform and publish 75% of all research1. In the United States, there are 131 research 1 (R1: The highest status level a university can achieve in terms of research ranking) universities which receive the bulk of federal funding.
This top-heavy funding disparity limits research options for many principal investigators as well as students; for example, only 1.9% of R1 universities are Hispanic-Serving institutes. Further, non-R1 institutes are limited in terms of research space, grants awarded, and time made available for research, and these schools often do not have medical school affiliations2. Given these obstacles, we provide the methods that have successfully allowed for the investigation of brain-behavior relationships in deception in a resource limited environment. While these methods are suitable for any institute, we believe that those at smaller/teaching intensive universities will receive the maximum benefit from these methods.
Our laboratory has focused primarily on the brain regions responsible for producing self-deception and self-enhancement. Establishing causation in terms of the underlying cortical regions is achievable by a number of techniques, and these data help confirm correlative neuroimaging methods and experimental patient trials3,4,5.
To investigate self-deception with causal neuroimaging techniques, a number of innovative methods have been employed, mainly with single pulse Transcranial Magnetic Stimulation (TMS) and repetitive TMS (rTMS6; Figure 1). While tDCS (transcranial Direct Cortical Stimulation) has been employed successfully7 and can be modified to replicate the methods, procedures, and results presented here, the flexibility of TMS still makes it the optimal choice for the neuromodulation of self-deception. At its most common implementation, researchers inhibit, excite, disrupt, or measure cortical excitability (not covered here, but see reference8).
The Medial Prefrontal Cortex (MPFC) appears to be involved in self-deceptive responding9. Given the role of the Cortical Midline Structures (CMS) in terms of self-awareness in general10, it is not surprising that self-deception is correlated with MPFC activity. To determine causation in terms of frontal regions, TMS was relied on to create 'virtual lesions' while measuring bouts of self-deception11. Measuring self-deception has been achieved via two main methods: Self-enhancement and overclaiming6.
We have found that disruption of the MPFC leads to the reduction of self-deception6,8,11,12,13. Furthermore, we have discovered that such a reduction (i.e., the lowering of self-deception) is related to a decrease in a person's affect (i.e., negative mood increases and positive mood decreases).
Because neuro-navigation/individual MRI's are not employed (due to expense, most laboratories do not have these resources), concern may be raised over positioning and accuracy in TMS targeting. We have compensated for this by occasionally doing fiducial procedures in which a contrast target (e.g., a vitamin E tablet) is placed on the cap and the participant(s) is/are subsequently scanned in a structural MRI11,12. These methods have confirmed the accuracy of the methods outlined here, and we are targeting the medial aspect of the MPFC at the border of BA 10/9 which lies above the Medial Frontal Gyrus (0, ~40, ~30).
Clearly, higher spatial resolution can be obtained using other methods such as neuro-navigation, however, these methods are not employed without drawbacks which include participant drop-out, participant exclusion, increased length of experimental duration, additional training and screening, added expense and often multiple site visits for participants. Therefore, the methods presented here offer an excellent alternative to neuro-navigation in many circumstances.
Protocol
The research presented here was approved by the Institutional Review Board (IRB) committee of Montclair State University. All participants have been treated within the ethical guidelines of the APA.
1. Participants
- First, obtain IRB Committee Review Approval for the protocol (see Discussion for non- Research 1 institutions). Consultation with experienced researchers is recommended. Obtain forms such as Screening (Supplementary File 1) and Side Effects (Supplementary File 2) forms from other researchers-they are readily shared across the TMS community. NOTE: For the purpose of this experiment, forms were obtained from Simone Rossi.
- Train all investigators on consenting and informing participants of all risks, side effects, and potential adverse events. If necessary, the Principal Investigator (PI) takes a TMS course if further knowledge is needed. Prior to running participants, ensure that the PI performs a pilot test of the protocol including consent and debriefing.
- Recruit participants through flyers around campus. Screen participants in person; initial contact need not be in person. Ensure that the flyers describe the compensation and risks in general terms only, including any special circumstances (e.g., COVID).
- Ensure participants read the consent form aloud including specific questions including: Are you a current student of ____PI______? Do you have a: history of epilepsy, family history of epilepsy? Do you have a history of seizures? Do you have any of the following-stroke, cranial metal implants, structural brain lesion, implanted device, pacemaker, medication pump, cochlear implant, implanted brain stimulator, metal worker? Do you have a history of head trauma with loss of consciousness? Do you have a high potential for pregnancy? Are you younger than 18? Are you older than 65?
- Excuse any participants affirming any questions from the study.
- Before being enrolled, ensure that the Screening Checklist is administered.
- Pay all participants $25 for their participation and treat in accordance with guidelines of the Institutional Review Board at Montclair State University and of the American Psychological Association.
- Deliver all TMS within the parameter appropriate for the institution (see Discussion).
- Participant safety and comfort is critical so at all points forward, ask and monitor the participants closely both verbally and visually. Nervousness can be the norm which in some cases leads to more difficult outcomes and this is monitored.
2. TMS equipment handling
- Use a single-pulse TMS device for all stimulation. Trigger the device by simultaneous depression of hand and foot switches manually by the PI. Use the maximum stimulation rate of the stimulator (i.e., 0.75 Hz).
- Use a 70 mm figure-of-eight coil throughout the experiment. Ensure that the coil never reaches dangerous/shut-off temperatures during the experiment. Backup coils are ready in case needed as replacements.
- Present all stimuli using a laptop computer. Open up the software (e.g., Testable) and login to the account. Click on the appropriate experiment.
- Scale the monitor using a credit card. Enter demographic information. Clean/sanitize the laptop before and after each participant is tested.
- Determine the motor threshold using visual inspection (5/10 evoked Abductor Pollicis Brevis) or via an EMG (Electromyograph).
- Use swim caps to preserve markings. Use a standard coil holder for training and as demonstration only, not for active stimulation.
- Use cloth tape measures to take coordinates for CZ and OZ from the 10/20 system and take MPFC from a previous study on overclaiming10. To determine MPFC, take 1/3 of the distance of nasion to inion, and MPFC is 1.5 cm anterior to this location. This will focus at the BA 10/9 (Medial Frontal Gyrus).
- Confirm measurements at the PI's discretion using the fiducial method in which a vitamin E tablet is adhered to the cap of the coil location which will contrast easily in a standard MRI. Due to the cost, this option is limited.
3. COVID – 19
- Due to COVID-19, include the following protocols14. In the Consent Form, add a disclaimer: "As a participant in this study, you will spend time in an indoor space within close proximity of the researcher. This poses a significant additional risk to getting COVID-19. We are taking the following precautions to protect you such as: Only PI will be within 6 ft of participant; Only one assistant is allowed in the vicinity but they must stay socially distanced; The participant must wear two masks; PI must wear two masks, gloves and a face shield; The assistant must wear a mask and a face shield; All contact equipment is sanitized."
- Perform all experiments in the lobby/hall outside of the normal lab as ventilation is significantly increased. All equipment is sanitizable and portable.
- Once COVID-19 protocols are relaxed, use normal procedures.
4. Motor threshold
- Mark swim caps along the nasion/inion line and the midpoint taken using a magic marker. Measure pre-auricular points and take those midpoints, as well. From here, plot 10/20 coordinates (see 2.6).
- Using the right hemisphere pre-auricular line, then go 33% down (in the ventral direction) and begin the search for the optimal location for the Abductor Pollicis Brevis (APB) using the TMS coil. Discharge the TMS machine using the coil trigger, the footswitch, and disengaging the safety.
- Orient the TMS coil at 45° for all searches and TMS deliveries.
- Start the stimulation output at 30% total machine output by using the dial on the front of the machine and raise in 2% increments using the dial until a movement is noted. Here, as the stimulation is increased in terms of intensity, move the location also. There is a careful interplay between coil movement and stimulation intensity.
- Once the optimal location is found (i.e., the site that provided the maximum APB response), determine the MT.
- Prior to starting MT determination, mark the coil tip site on the cap to allow accurate placement. Trace the entire anterior portion of the coil on to the swim cap using a magic marker.
- For the visual inspection method, use approximately 20 pulses (varying machine intensity) to find what stimulation level results in 5/10 (50%) APB responses. The dial should be raised and lowered in response to increased or decreased finger movements. Start at 20% of machine intensity and work up. Once 5/10 responses have been obtained, record the individual's MT by noting what the machine is displaying as the intensity.
- For the (preferred) MEP method, place disposable electrodes on the APB and the tendon of the thumb and a ground (usually around the back of the wrist), and instead of using visual inspection, a positive MEP must be observed on the recording unit.
- Define a positive MEP response as an MEP with ≥50 µV peak-to-peak amplitude.
- Similar to visual inspection, stimulate until 5/10 positive MEPs are observed. The MEPs should be greater than 50 µV. If 50% of the MEPs are above (and 50% below), MT has been identified.
- Once established, set the TMS machine to the appropriate stimulation level. 90% of the motor threshold is an ideal balance between effective active TMS and safety. Do not exceed 45% of the machine's total output. There are occasions that a person's MT is 60% of the machine's total output, but this is rare.
5. Active single-pulse TMS
- Randomly select the order of all sites (e.g., SMA, PZ, MPFC, or Sham over CZ; Figure 5).
- Place the coil over the active site and start a presentation software, (e.g., Testable (see below)). Stimulation should proceed automatically and in sync with stimuli.
- Always have a spare coil in case of overheating.
6. Presentation
- Collect all behavioral data using a presentation software (e.g., Testable) This software is easily configured, and scripts are simple.
NOTE: Three separate blocks are created-one for each of the brain conditions. Demographics to be collected are first chosen using Testable's automatic selection routine. Then real words and fake words are placed into the scripting software. The size and duration of the words are chosen, as is the location on the screen of the stimulus words. - Once the script is made, collect demographics first and perform screen calibration. This is done matching the slider to a credit card. Perform all experiments on a computer. All responses are made on the built-in keyboard and sensor pad.
- Give two practice trials and introduce the analog scale. All participants easily adjust to the equipment. The instructions are given orally, and the participants are told to rate how well they know the word.
- If the word is familiar to them (such as 'desk') it should be given a high rating.
- If they 'sort of' know the word, they are to give a medium rating (such as 'chlorophyll').
- If it is not that familiar to them, they will assign a low rating (such as '5HTTlpr'). A total of 144 words should be used (36 per brain site).
- Participants have unlimited time to respond. Following the response on the analog scale, the next word is presented.
Representative Results
Figure 2, from Taylor-Lilquist et al.14, involved four brain sites: MPFC, SMA, PZ, and a Sham site. These sites were utilized to determine the correlates of overclaiming. Overclaiming is a participant indicating that they know a word when it is actually not a word. 12 participants were tested in both social and non-social settings. The social settings represented pressure to either know a word (high-social pressure; n=6) or not know a word (low-social pressure; n=6). The social pressure was a series of verbal prompts that indicated that every person knows these words and they were easy (high-social pressure) or that they words were difficult and most people did not know them (low-social pressure).
When TMS was delivered to the MPFC, the participants were less likely to overclaim in the social condition (p < .05). That is, overclaiming when under social pressure conditions was much more disrupted than any other condition (following MPFC TMS). This can be seen in Figure 2 where the participants are being much less deceptive (i.e., more honest) in the MPFC/social condition. When the MPFC is inhibited, overclaiming goes down as does the influence of social pressure. Because social cognition and overclaiming are thought to be MPFC properties, this is not surprising.
These data are in line with previous studies that demonstrated MPFC disruption leads to more honest responding12,13.
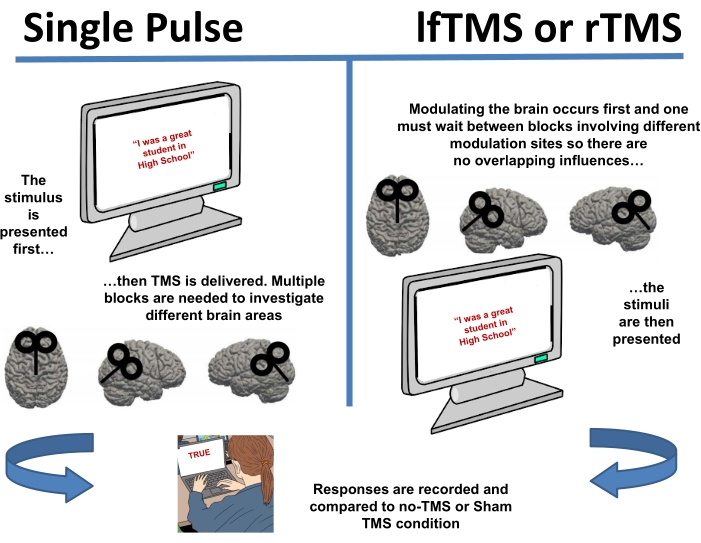
Figure 1: Two different TMS methods have been employed to disrupt self-deception. Single-pulse techniques are timed so that the pulse is delivered with the stimuli to disrupt self-deception while the task is being done. Both lfTMS and rTMS modulate the brain before the task, potentially altering self-deception. All techniques vary brain sites and provide a number of controls including Sham TMS. Please click here to view a larger version of this figure.
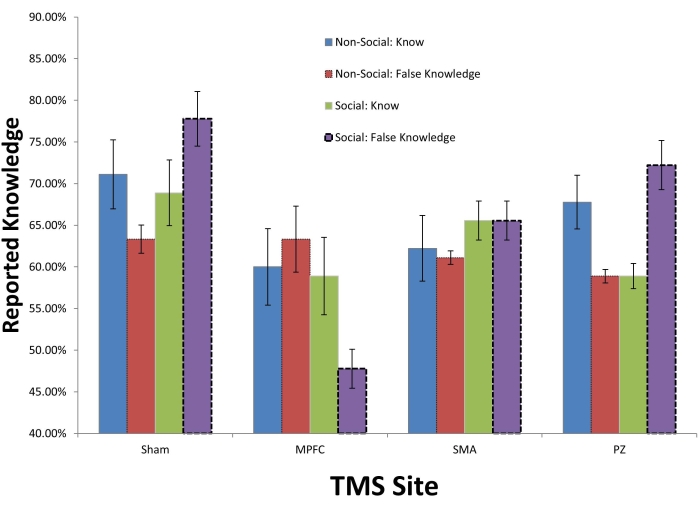
Figure 2: Self-deception can be modulated using TMS. Participants (n=12) were given social pressure to know words in one condition and no social pressure in another condition. The social pressure was a series of verbal prompts that indicated that every person knows these words and they were easy (high-social pressure; n=6) or that they words were difficult and most people did not know them (low-social pressure; n=6). Means and SEs presented. Because social cognition is an MPFC function, we thought that TMS delivered in an inhibitory manner would decrease overclaiming (also a MPFC function). This was found to be the case. Please click here to view a larger version of this figure.
Supplementary File 1: Even though our Informed Consent addresses these questions, we advise a separate screening form. Both the Informed Consent and the Screening Checklist are administered orally. This allows us and the participant to ask follow up questions and clarify any confusion. The form presented here is IRB approved. Please click here to download this File.
Supplementary File 2: Tracking side effects can be achieved with this or similar forms. Headaches, though rare, are not unseen. This form is a standard use TMS form and has not been modified for the experimental use. Pre- and post-TMS (the entire session, not per block) responses are recorded. Please click here to download this File.
Discussion
The protocol (and variations of) outlined here has been used in over 50 studies at Montclair State University. The entire set-up can be created for under $15,000 (US). Further, we have found our coordinates match well with underlying brain structures using fiducial procedures.
Variations of this method are often used. For example, control conditions can include stimulating different brain areas, applying TMS different timings (i.e, apply TMS at a timing that should have no effect), using a Sham coil, using different levels of total machine output etc. Safety concerns may be different at a smaller teaching/non-teaching institution as medical personnel are not readily available. Any study or research in which neuromodulation is conducted must meet safety protocols. Performing single-pulse TMS is safer than rTMS but still poses significant risks. We have performed TMS and lfTMS at Montclair State University for 20 years without major incident by adhering to published safety guidelines16,17,18.
Establishing TMS and lfTMS at non-R1 institutions may require educating one's Institutional Review Board (IRB) and being open to concerns that may not exist at larger institutions. Such an arrangement allowed for back and forth questioning and answering between neutral experts, the Principal Investigator (PI), and the IRB Chair. A number of important precedents were established that may be unique to non-R1 institutions. First, Transcranial Magnetic Stimulation (TMS and lfTMS) will be administered only by the PI. Post-docs, graduate students, undergraduate students are not to administer TMS during experiments. Second, payment ($25 per session) was set by the IRB chair in a manner that balanced enticement and fair compensation. Further, Motor Threshold (MT) was determined to be the manner in which all stimulation was to be set in terms of intensity and this could be done either via visual inspection or measured Motor Evoked Potentials (MEP). Also, we agreed that active TMS will be delivered at 90% MT unless noted. Exceptions to this number (higher) have been granted in particular when collecting MEPs for hypothesis testing9. Finally, we agreed that consent forms will be read in part or in full to participants so that they fully understand the protocol and do not 'just sign a form' without completely understanding TMS. Many participants have challenges with English and they often appreciate having the consent explained and read to them while they also read it.
Our procedures are extremely conservative in terms of safety. One principle that we have followed is treating TMS as if it were rTMS. In our consent, we use the following language:
Risks associated with TMS include seizure, headache, neck ache, hearing loss or disruption, and possible short term memory loss, as well as possible long term, unknown effects. The most serious known risk of TMS is the production of a convulsion (seizure). TMS can produce a convulsion when a series of pulses are given at high power and when the series are given extremely close together. This study follows published safety guidelines for the use of TMS that are designed to avoid known risk factors for convulsions with TMS. Although accidental seizures occur at a frequency of <0.1%, there are factors that may increase the risk of TMS triggering a seizure such as family history of seizures or previous neurological condition. Persons with epilepsy cannot participate in this study. Also, if you have a history of head trauma, or implanted metallic objects, you cannot participate in this study. If you are pregnant, you may not participate in this study. The most commonly reported side effect of TMS is headache. Neck pain may also occur. If a headache or neck pain occurs, it is usually easily managed with pain medications. One may also experience some discomfort on their head where the coil is held. This is due to contraction of scalp muscles. The clicking noise produced during the stimulation may temporarily affect hearing. Earplugs have been shown to reduce this risk, therefore, you will be asked to wear earplugs during TMS.
Because TMS is now a common technique, finding both a consultant and sample IRB/safety forms should involve minimal effort. A search in PubMed as of March 21, 2021 of "TMS or rTMS" resulted in 24,435 citations.
Acknowledging that seizure is the primary risk, we query about seizures multiple times as these questions are posed in both the Consent Form and the Screening Checklist (Supplementary File 1). The Screening Checklist is also administered orally. We have not had a seizure incident since TMS was established at Montclair State University and have rejected about 5% of our initially recruited participants because of seizures. To put this into context, we estimate that 20% of recruited participants are rejected for other reasons (e.g., previous head trauma with loss of consciousness). Beyond medical considerations, practical considerations are extremely pertinent in the United States. In the state of New Jersey, physicians are required by law to report reoccurred seizures to the MVC (Motor Vehicles Commission), individuals who suffer from seizures are required to go through medical review, once revoked licenses are suspended 6-months-post 'last seizure' and "a person is disqualified from driving a commercial motor vehicle if he/she has an established medical history or diagnosis of epilepsy" (https://www.state.nj.us/mvc).
More power and more robust results are likely if stronger TMS intensities are employed. This may be ideal and in fact called for in a number of settings including clinical ones. For example, most laboratories will stimulate at 100%120% above MT. Furthermore, many laboratories employ neuro-navigation for improved accuracy17,18. If available and safety can be assured, these are considered best practices.
Excellent neuroscience research can be achieved at any institution. By implementing these procedures, we believe research will be furthered as more institutions can contribute to the academic knowledgebase. In addition, students normally underrepresented will gain access to the sciences.
Declarações
The authors have nothing to disclose.
Acknowledgements
LSAMP (Louis Stokes Alliance for Minority Participation), Wehner, and The Crawford Foundation, the Kessler Foundation are all thanked for their support.
Materials
| Android Samsung Tablet (for MEPs) | Samsung | SM-T500NZSAXAR | |
| Cloth Measuring Tape | GDMINLO | B08TWNCDNS(AMZ) | |
| Figure of 8 Copper TMS Coil | Magstim | 4150-00 | This is the current model |
| Lenovo T490 Laptop | Lenovo | 20RY0002US | |
| Magstim 200 Single Pulse | MagStim | Magstim200/2 | This is the current model |
| Magstim Standard Coil Holder | MagStim | AFC/SS | This is the current model |
| Speedo Swim Caps | Speedo | 751104-100 | |
| Testable.Org Account and Software | Testable | NA | |
| Trigno 2 Lead Sensor (for MEPs) | DelSys | SP-W06-018B | |
| Trigno Base and Plot Software (for MEPs) | DelSys | DS-203-D00 |
Referências
- Academic Research and Development. Science and Engineering Indicators 2020. National Science Board, National Science Foundation Available from: https://incses.nsf.gov/pubs/nsb20202 (2020)
- . Rutgers School of Graduate Education. Overview of R1 Serving Hispanic Institutions Available from: https://cmsi.gse.rutgers.edu/sites/default/files/HSI_Report_R2_0.pdf (2022)
- Maeda, F., Keenan, J. P., Pascual-Leone, A. Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. The British Journal of Psychiatry. 177 (2), 169-173 (2000).
- Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., Pascual-Leone, A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical Neurophysiology. 111 (5), 800-805 (2000).
- Pascual-Leone, A., Bartres-Faz, D., Keenan, J. P. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 354 (1387), 1229-1238 (1999).
- Amati, F., Oh, H., Kwan, V. S., Jordan, K., Keenan, J. P. Overclaiming and the medial prefrontal cortex: A transcranial magnetic stimulation study. Cognitive Neuroscience. 1 (4), 268-276 (2010).
- Tang, H., et al. Stimulating the right temporoparietal junction with tDCS decreases deception in moral hypocrisy and unfairness. Frontiers in Psychology. 8, 2033 (2017).
- Kelly, K. J., et al. The effect of deception on motor cortex excitability. Social Neuroscience. 4 (6), 570-574 (2009).
- Farrow, T. F., Burgess, J., Wilkinson, I. D., Hunter, M. D. Neural correlates of self-deception and impression-management. Neuropsychologia. 67, 159-174 (2015).
- Uddin, L. Q., Iacoboni, M., Lange, C., Keenan, J. P. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 11 (4), 153-157 (2007).
- Luber, B., Lou, H. C., Keenan, J. P., Lisanby, S. H. Self-enhancement processing in the default network: a single-pulse TMS study. Experimental Brain Research. 223 (2), 177-187 (2012).
- Barrios, V., et al. Elucidating the neural correlates of egoistic and moralistic self-enhancement. Consciousness and Cognition. 17 (2), 451-456 (2008).
- Kwan, V. S., et al. Assessing the neural correlates of self-enhancement bias: a transcranial magnetic stimulation study. Experimental Brain Research. 182 (3), 379-385 (2007).
- Taylor-Lillquist, B., et al. Preliminary evidence of the role of medial prefrontal cortex in self-enhancement: a transcranial magnetic stimulation study. Brain Sciences. 10 (8), 535 (2020).
- Bikson, M., et al. Guidelines for TMS/tES clinical services and research through the COVID-19 pandemic. Brain Stimulation. 13 (4), 1124-1149 (2020).
- Lerner, A. J., Wassermann, E. M., Tamir, D. I. Seizures from transcranial magnetic stimulation 2012-2016: Results of a survey of active laboratories and clinics. Clinical Neurophysiology. 130 (8), 1409-1416 (2019).
- Pascual-Leone, A., et al. Safety of rapid-rate transcranial magnetic stimulation in normal volunteers. Electroencephalography and Clinical Neurophysiology. 89 (2), 120-130 (1993).
- Rossi, S., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical Neurophysiology. 132 (1), 269-306 (2021).
- Wassermann, E. M. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology. 108 (1), 1-16 (1998).

