A Rodent Model of The Ross Operation: Syngeneic Pulmonary Artery Graft Implantation in A Systemic Position
Summary
We demonstrate how to establish a murine model of pulmonary root implantation into the descending aorta to simulate the Ross procedure. This model enables the medium/long-term evaluation of pulmonary autograft remodeling in a systemic position, representing the basis of developing therapeutic strategies to promote its adaptation.
Abstract
The Ross operation for aortic valve disease has regained new interest due to its outstanding long-term results. Nonetheless, when employed as freestanding root replacement, the possible dilation of the pulmonary autograft and subsequent aortic regurgitation is described. Several animal models have been proposed. However, these are usually limited to ex-vivo models or in-vivo experiments with relatively expensive large animal models. In this study, we sought to establish a rodent model of pulmonary artery graft (PAG) implantation in a systemic position. A total of 39 adult Lewis rats were included. Immediately after euthanasia, the pulmonary root was harvested from a donor animal (n=17). Syngeneic recipient (n=17) and sham-operated (n=5) rats were sedated and ventilated. In the recipient group, the PAG was implanted with an end-to-end anastomosis in infra-renal abdominal aortic position. Sham-operated rats underwent only transection and re-anastomosis of the aorta. Animals were followed with serial ultrasound studies for two months and post-mortem histological analysis. The median PAG diameter in the native position was 3.20 mm (IQR=3.18-3.23). At follow-up, the median diameter of the PAG was 4.03 mm (IQR=3.74-4.13) at 1 week, 4.07 mm (IQR=3.80-4.28) at 1 month, and 4.27 mm (IQR=3.90-4.35) at 2 months (p<0.01). Peak systolic velocity was 220.07 mm/s (IQR=210.43-246.41) at 1 week, 430.88 mm/s (IQR=375.28-495.56) at 1 month, and 373.68 mm/s (IQR=305.78-429.81) at 2 months (p=0.02) and did not differ from the sham-operated group at the end of the experiment (p=0.5). Histological analysis did not show any sign of endothelial thrombosis. This study showed that rodent models may allow for the evaluation of the long-term adaptation of the pulmonary root to a high-pressure system. A systemically placed syngeneic PAG implantation represents a simple and feasible platform for the development and evaluation of novel surgical techniques and drug therapies to further improve the outcomes of the Ross operation.
Introduction
Congenital aortic valve stenosis is a subgroup of congenital heart disease characterized by an obstruction of the left ventricular tract in which the lesion is located at the valvular level. The malformation affects approximately 0.04-0.38 per 1000 live births1.
The available options for the correction are many, each with its own advantages and disadvantages. For patients suitable for a biventricular correction2, the approach may be aimed at valve repair (percutaneous or surgical valvulotomy) or its replacement3. The latter is preferred when the aortic valve is considered unsalvageable; however, the available options are limited for pediatric patients. Indeed, bioprosthetic valves are not indicated for aortic replacement in the young population due to their early calcification4. On the other hand, degeneration in mechanical valves is considerably slower, but these require lifelong anticoagulant therapy5. In addition, the major limitation of these prostheses is represented by the lack of growth potential, which predisposes the patients to additional reinterventions.
An interesting therapeutic option in the pediatric population is the transfer of the pulmonary autograft to the aortic position named "Ross operation". In this case, the pulmonary valve is then replaced with a homograft (Figure 1)6. This procedure can possibly represent the best surgical choice for children because the pulmonary autograft preserves its growth potential and does not carry the risks of lifelong anticoagulant therapy. Furthermore, the Ross procedure can be of great value also in young adults to avoid a mechanical or biological valve, having the potential to become the best surgical solution.
Results after aortic valve replacement with pulmonary autograft are excellent, with survival greater than 98% and good long-term outcomes7. Literature studies report 93% and 90% freedom from replacing the pulmonary homograft at 4 and 12 years, respectively8.
The major limitation of this procedure is the tendency of the autograft to dilate in the long term, especially when employed as a freestanding root replacement. This can cause valvular incompetence which may require a reintervention. Indeed, the longest follow-up study performed so far reports freedom from reoperation for autograft replacement of 88% at 10 years and 75% at 20 years9.
The possibility of recreating a Ross operation in an experimental setting represents a fundamental prerequisite to investigate the underlying mechanism of the pulmonary autograft adaption to systemic pressures. Several models have been proposed in the past. However, these are usually limited to ex-vivo experiments or in-vivo animal models with a relatively expensive large animals. In this study, we sought to establish a rodent model of pulmonary artery graft (PAG) implantation in a systemic position, as freestanding root.
Protocol
All procedures have been approved by the University of Padova Animal Care Committee (OPBA, protocol number n° 55/2017) and authorized by the Italian Ministry of Health (Authorization n° 700/2018-PR), in compliance with the European Union Directive 2010/63/UE and the Italian Law 26/2014 for the Care and Use of Laboratory Animals.
1. Animal care and experimental model
- Ensure all Lewis rats are obtained from a single company (Table of materials). Maintain the rats in conventional facilities with free access to food and water.
- Ensure that the weight of the rats ranges from 320-400 g for the recipient group and 200-250 g for the donor group.
2. Preoperative protocol
NOTE: All operations must be performed under clean conditions. Use male and female adult Lewis rats as recipients and donors as well in order to perform a syngeneic transplant.
- Perform an intraperitoneal injection of tramadol (5 mg/kg) 15 min before surgery.
- Administer a single dose of intramuscular Gentamicin (5 mg/kg) immediately before surgery.
- For anesthesia induction, supply 4% sevoflurane in 1 L/min of oxygen to a poly(methyl methacrylate) chamber where the animal is placed. For anesthesia maintenance, use 2.0-2.5% sevoflurane in 1 L/min of oxygen throughout the procedure.
- Shave the animal along the midline for 2 cm width from the sternum to 1 cm above the genital area with a razor. Then, sterilize the skin with iodine solution.
- In order to prevent the animal from getting wet and to prevent heat dispersion during the surgery, cover the animal with a transparent plastic film.
- Evaluate the level of anesthesia before performing the procedure by assessing the absence of response to a noxious stimulus.
3. Donor operation
- Animal and heart preparation:
- Place the anesthetized animal on a cork tray with the caudal side facing the surgeon. Perform a xipho-pubic incision of about 5-6 cm, and retract the two musculocutaneous flaps laterally.
- Administer a volume of 1 mL of saline solution at 4 °C containing 500 IU of heparin through the abdominal vena cava.
- After 1 min, cut the diaphragm from left to right and perform an anterior thoracotomy to expose the heart.
- Cool the beating heart by dripping saline solution at 4 °C.
- Perform a pericardiectomy and a thymectomy in order to obtain a complete view of the aortic arch. Remove the remaining fatty tissues surrounding the aorta.
- Cut at the arch, just above the origin of the innominate artery; sever the latter one, too.
- Cut the thoracic inferior vena cava (IVC) and insert a 22 G cannula to infuse the heart with 20-25 mL of saline solution at 4 °C, exerting light pressure. Discontinue the perfusion when the heart stops beating and flow from the aorta became clear.
- PAG explant:
NOTE: An accurate harvesting and delicate handling of the PAG is mandatory for achieving optimal implantation in the recipient. Do not touch it directly with instruments, instead use cotton swabs.- Perform an ultrasound study to assess the PA diameter in its native position.
- Insert a micro-plier under the posterior wall of the vessel and cut the latter using a micro-scissor as close as possible to its bifurcation to maximize the length of the PAG.
- Gently hold the PA with the ring-tipped micro-forceps and separate it from the right ventricle with the micro-spring scissors. Harvest the PAG, including some right ventricular muscle.
- PAG preparation:
- Place the PAG on a gauze moistened with cold saline on the operating table and inspect the vessel under the operating microscope.
- Cut any abundant surrounding tissue, leaving only 1 mm of ventricular muscle. Set the length of the vessel at 5 mm.
4. Pulmonary artery graft (PAG) implantation
- Preparation of the recipient animal:
- Place the anesthetized animal on a cork tray with the caudal side facing the surgeon.
- Perform a median longitudinal incision and use two mini retractors to keep the abdomen open.
- Extract the intestines with two cotton swabs and cover them with a gauze soaked with 39 °C saline allowing the visualization of the retroperitoneal area with exposure of the infra-renal abdominal aorta (AA).
NOTE: During the surgery, it is important to occasionally moisten the intestines using a syringe containing 39 °C saline to prevent hypothermia, a critical condition common in rodents. - Strip the posterior parietal peritoneum between the two renal arteries and the iliac bifurcation using two cotton swabs and remove the fatty tissue around the infrarenal AA. Leave only a small portion of fat above the AA, to facilitate handling on the vessel.
- Separate the AA from the IVC. To perform this procedure, first, pass a curved forceps behind the posterior aortic wall and use it to open a passage between the AA and IVC. Then, use a 2-0 silk suture to create a loop around the AA, in order to lift the vessel and separate the AA from IVC. Ligate any lumbar artery arising from the infrarenal AA with 6/0 silk suture and divide it.
- Rotate the animal 90° counterclockwise, placing the head on the operator's left side. The AA now lay horizontally in the microscopic field.
- Use two Yasargil clips to clamp the infrarenal AA and place them at a distance of 1.5 cm from each other. Transect the AA at the midpoint between the two clips.
- Irrigate the two ends of the vessels with heparin (1 UI/mL) in saline solution to remove any clots. Remove any adventitial debris from the vessels.
- PAG implantation:
- Place the PAG between the two ends, with the ventricular end towards the cranial portion of the animal.
- Use a 10-0 polypropylene suture to perform two landmark single stitches connecting the PG to the AA. Perform the procedure on both ends of the PAG by placing the suture on opposite sides of the vessel circumference.
- Perform an end-to-end anastomosis between PAG and AA, beginning with the distal end. Use one of the two ends of the distal landmark suture for the posterior anastomosis using a recipient-to-graft out-in/in-out sequence to perform a running suture of about six stitches.
- Once the suture reaches the proximal landmark, perform a double half hitch completed by a square knot using the suture and one of the two ends of the proximal landmark suture. Apply rubber-shod mosquito forceps to the sutures to provide traction.
- Perform the same anastomosis on the anterior wall. Carry on the entire procedure on the proximal end of the PAG. Take particular attention when performing the proximal anastomosis to avoid including any leaflet in the suture line.
- Release the distal clip first to let the PAG be filled with retrograde blood (low-pressure flow) in order to check the anastomosis. Repair any blood leakage with a single suture. Once the distal anastomosis is evaluated, perform the same procedure on the proximal end.
- Final stages of the operation on the recipient:
- Assess the patency of the PAG and apply two strips of gelatin sponge over the suture lines on both sides of the PAG (if necessary). Exert gentle pressure for a few seconds with two cotton swabs to help hemostasis.
- Relocate the intestines into the abdominal cavity and close the walls with a 4/0 polypropylene running suture.
5. Sham-operated procedure
- Perform an identical preparation of the animal as previously illustrated for recipient rats.
- Cut the infra-renal AA, midway between the renal and the iliac arteries' origin.
- Reapproximate the two ends of the AA using an end-to-end anastomosis, as previously described. Remove the two clips and perform an accurate hemostasis procedure.
- Reposition the intestines and close the abdominal wall in layers, as for the recipient animals.
6. Postoperative care and follow-up
- Administer warm saline solution (5 mL) into the subcutaneous tissue of the animal's back for hydration. Place the rat under a heating lamp and visually monitor it until awakening, which usually takes up to 5 min after anesthesia is stopped. Place the animal in a cage at a room temperature of 22-24 °C, with immediate and unrestricted access to food and water.
- Administer intramuscular tramadol (5 mg/kg) for postoperative analgesia twice daily for the first 48 h after surgery. Thereafter, monitor the recipient's health status and body weight daily, on a regular basis.
- Follow-up: During the follow-up, perform seriate ultrasound studies at one week, one month, and two months to evaluate PAG function. During these studies, measure the vessel diameter, the peak systolic velocity (PSV), and the end-diastolic velocity. Measure these parameters inside the PAG and at the level of proximal and distal AA.
- Euthanize the animals after two months of follow-up by application of CO2 for a few minutes, and then explant the PAG, which will undergo histopathological analysis.
Representative Results
A total of 39 adult Lewis rats were included in this study: 17 animals were used as PAG donors, 17 animals as recipients and 5 as sham-operated (control group) (Table 1). Male rats were 22 (56%) and female 17 (44%); the latter were used only in the donor group.
No fatal event occurred during the operation with 100% survival. During the follow-up, two animals of the transplant group had a fatal outcome, at 12 and 51 days, respectively; the survival rate at the end of the study was 91% (Table 1).
The median weight of the rats was 387 g (interquartile range, IQR, 358-394 g) for the recipient group and 328 g (IQR=304-337 g) for the donor group. At one week after the surgery, the median weight was 363 g (IQR=350-376 g) with a 6% decrease compared to the preoperative weight. The animals regained their weight within the first month of follow-up (median 387 g, IQR 369-392 g), with a final weight at two months of 397 g (IQR=391-402 g) (Figure 2).
The median follow-up time was 62.5 days (IQR=60-68 days) in the transplant group and 62 days (IQR=61-67 days) in the sham-operated group (p=0.68).
The preoperative PA median diameter in its native position was 3.20 mm (IQR=3.18-3.23 mm). The median diameter of the PAG was 4.03 mm (IQR=3.74-4.13 mm) at one week, 4.07 mm (IQR=3.80-4.28 mm) at one month, and 4.27 mm (IQR=3.90-4.35 mm) at two months (Figure 3A). This was a 25.9%, 27.2% and 33.5% increase compared to the diameter in the native position, respectively. The increase in diameter was significantly different when comparing the value in the native position and the value at one week (p=0.003), while no significant increase was found during the following studies. Aorta diameter in the sham-operated group was 1.41 mm (IQR=1.35-1.62 mm) at one week and 1.41 mm (IQR=1.29-1.70 mm) at two months. Median PSV at the level of the PAG was 220.07 mm/s (IQR=210.43-246.41 mm/s) at one week, 430.88 mm/s (IQR=375.28-495.56 mm/s) at one month, and 373.68 mm/s (IQR=305.78-429.81 mm/s) at two months. When compared to the sham-operated group, a significative difference in PSV was found at one week (median 419.12 mm/s, IQR=408.42-561.32 mm/s; p<0.001), while no difference was found at the end of the study (392.92 mm/s, IQR=305.89-514.27 mm/s; p=0.5) (Figure 3B).
At the end of the study, histological analysis showed no signs of endothelial thrombosis and wall calcification was not significant in most cases (Figure 4).
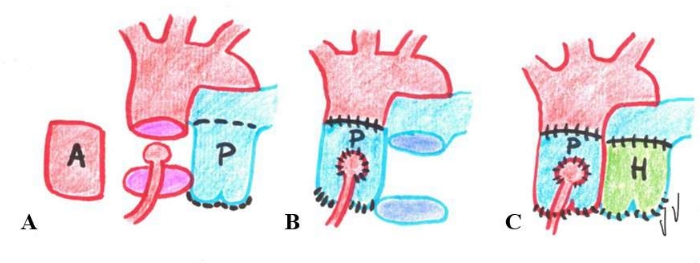
Figure 1: Representative image of the Ross operation. The photo shows the phases of the Ross operation. (A) Aortic valve and root explant; (B) Pulmonary artery autograft transposition in the aortic position; (C) Pulmonary artery autograft replacement with a homograft. A: aortic valve and root; H: homograft; P: pulmonary valve and root. Please click here to view a larger version of this figure.
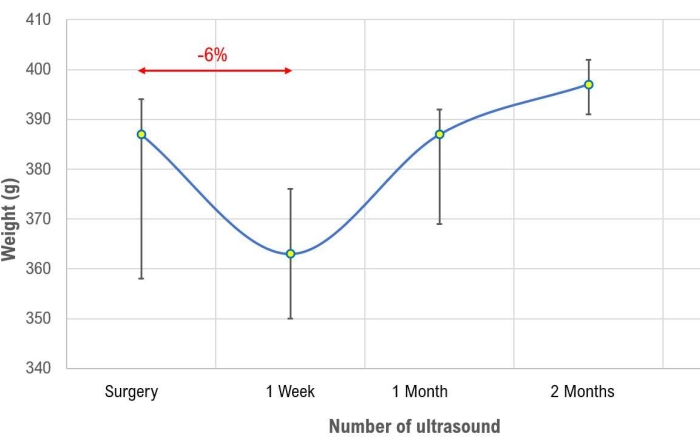
Figure 2: Time course of body weight in the transplant group. The graph shows the course over the time of the rat's weight in the transplant group. Values are expressed as median and interquartile range. Please click here to view a larger version of this figure.
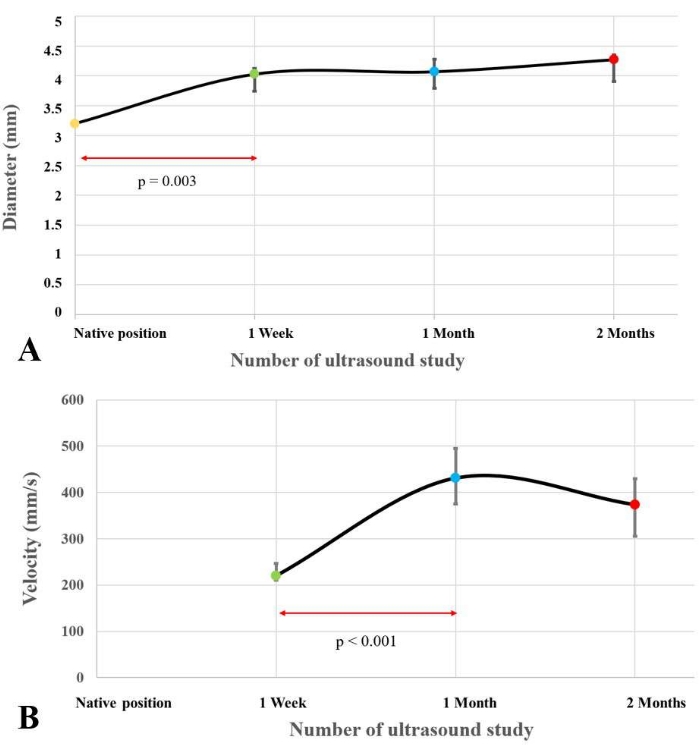
Figure 3: Variation of the diameter and the peak systolic velocity into the pulmonary artery graft. The graphs show the variation of the diameter (A) and the peak systolic velocity (B) inside the pulmonary artery graft during the seriate ultrasonographic evaluations. Values are expressed as median and interquartile range. Please click here to view a larger version of this figure.
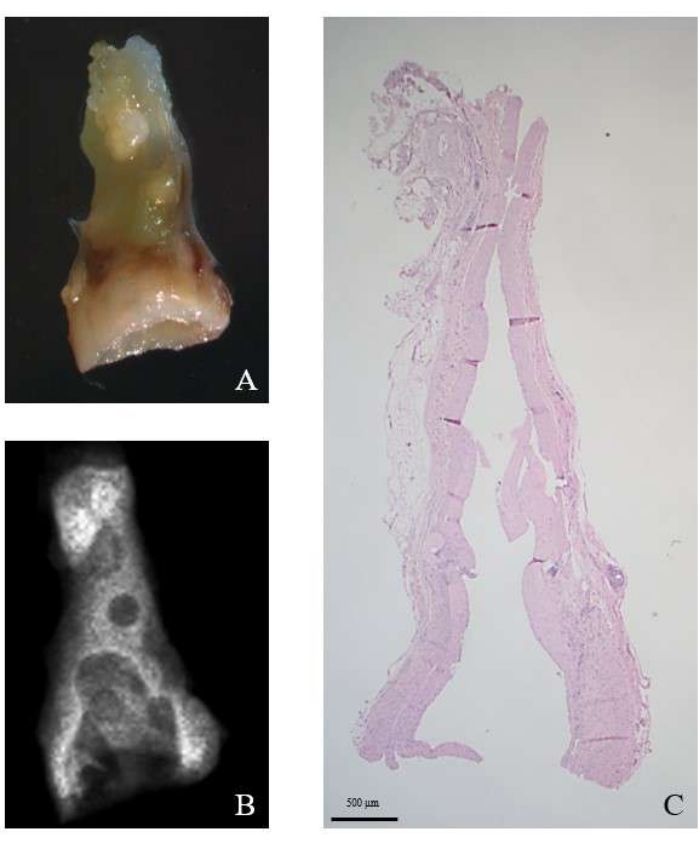
Figure 4: Microscopic evaluation of the PAG. The image shows the PAG after explant (A). (B) Radiographic evaluation; (C) Hematoxylin and Eosin stain, original magnification 12.5x. Please click here to view a larger version of this figure.
| VARIABLE | TRANSPLANT | DONORS | SHAM-OPERATED | TOTAL |
| Number of events | 17 | 17 | 5 | 39 |
| Fatal events at surgery | 0 | // | 0 | 0 |
| Fatal events during follow-up (%) | 2 | // | 0 | 2 (91) |
| Weight at surgery* | 387 (358-394) | 327,5 (303-337) | 389 (321-404) |
Table 1: Characteristics and outcomes of the study. *Values are expressed as median and interquartile range.
Discussion
Aortic valve replacement with the autologous pulmonary root (Ross operation) represents an attractive option for congenital aortic valve stenosis repair due to the favorable profile and potential growth of the autograft10. The major limitation to this procedure is the potential dilatation of the aortic neo-valve, which predisposes to the development of long-term regurgitation. The possibility of characterizing the modifications on the pulmonary artery after exposure to systemic pressures could represent the basis for understanding the causes of pulmonary autograft failure. For this reason, we developed an experimental model of syngeneic PAG implantation in a systemic position in a rodent model
The reported surgical technique is safe, effective, and reproducible. The small size of the animals that were used simplifies the surgical and postoperative management. This allowed us to obtain a useful model with limited materials and animal expenses. Lewis rats were chosen because, as an inbred strain, these rats are isogenic, with over 99% of their alleles fixed. Thus, they are an appropriate model for the study of transplantation of pulmonary valves between animals. We decided to set a two-month endpoint for the study because literature data indicate a 1:11 ratio between human and rat days11. Therefore, we can suppose that our follow-up time would correspond to about five years, which allow us to evaluate PAG adaptation in the medium-long term period.
Our initial results showed a rapid increase in the PAG diameter and a decrease in the PVS measured at its level within the first week after implantation. Subsequently, a partial plateau of the diameter increase was observed. We can speculate that the decrease in PSV seen in the short-term period can be related to the increased PAG diameter, causing a deceleration of the blood flow into the PAG itself.
Further studies aimed at indagating the PAG modification in a systemic position after shorter follow-up endpoints will help clarify the evolution of this adaptation over time. Possible future development of this model using different strategies to modulate PAG maladaptation could possibly prevent its dilatation and, thus, improve outcomes after Ross intervention. These strategies can be a pharmacological treatment, such as pressure control (i.e., using ACE inhibitors or angiotensin II receptor blockers) antioxidant therapies, or a mechanical containment to PAG dilatation with an external reinforcement (as recently proposed by some authors12).
Some critical steps in the procedure should be performed with particular attention. First, it is fundamental to include the right amount of right ventricle muscle when harvesting the pulmonary artery. As a matter of fact, when too much muscular tissue is preserved, the risk of leaking of the anastomosis increases, while an insufficient amount of muscle could predispose to damage to the leaflets of the valve. When performing the proximal end-to-end anastomosis between PA and AA, particular attention should be taken not to include the leaflets of the valve in order to avoid affecting their range of motion. Finally, adequate hemostasis is fundamental to avoid excessive blood loss which could compromise the postoperative course.
A weight reduction of up to 6% is considered acceptable during follow-up. However, animals should regain their initial weight within the first month of follow-up and keep increasing their weight afterwards. If a failure to reach the initial weight is associated with evidence of an upward trend can also be considered an index of animal well-being. On the other hand, any weight reduction of more than 6%, and any failure to reach the initial weight at one month with a downward trend should raise concerns about potential poor conditions of the animals.
The principal technical suggestion for investigators approaching this model is the use of continuous suturing to perform the end-to-end anastomosis. While microsurgery textbooks suggest using separate stitches for this kind of anastomosis, we prefer continuous suturing because it tightens better the pulmonary root. In addition to this, we observed that in this way it's easier to reduce the potential mismatch with the recipient aorta, which is still present despite the use of a smaller animal for pulmonary root harvesting.
Other animal models for the study of pulmonary root pressure overload have already been described in the current literature. These usually involve PA banding13. Despite the effective increase in upstream pressure, these models do not completely reproduce a Ross procedure. In fact, the first limitation is a high variability in pressure overload which depends on how tight the bandage is as compared to the PA diameter. For these reasons, pulmonary overload may not always reflect the actual systemic pressures. Preservation of the pulmonary root in its native position represents the second limitation of PA banding models. In a Ross procedure the PA loses all vascular and nervous connections which can affect its further adaptation to systemic pressures.
The scientific community has also already described some animal models of heterotopic transposition of the PA in a systemic position. However, all these models involve the use of large size animals such as lambs or sheep14,15. These animals could undoubtedly simplify under some aspects the surgical procedure by providing the possibility to perform an actual Ross procedure. However, the need for a cardiopulmonary bypass as well as the need for more people involved in surgical and postoperative management increases enormously the costs, thus limiting the use of this model on a large scale. Furthermore, small animal models, such as rats, would allow to perform a numerous casuistic, thus reducing variability and enabling different time endpoint as well as the possibility to compare multiple groups.
Although it provides the possibility to evaluate the modification of the PA root to systemic pressures as in Ross operation, this model has some limitations. The main limitation is the impossibility to perform an actual Ross operation with coronary arteries detachment and reimplantation. However, for our purposes this was only a minor limitation as the study was focused on the pulmonary wall. The pressure in the infrarenal abdominal aorta differ from those in the ascending aorta, thus limiting the comparison with Ross operation as regard to valve leaflets motion; however, again our main focus was the PA root as the primum movens of PAG failure. In addition, rodent use may have some limitations related to a different systemic pressure scale compared to large animals. However, this difference is proportional to the pressures to which the native root is subjected.
In conclusion, the current study showed that a systemically placed syngeneic PAG implantation in a rodent model represents a simple and feasible platform for the development and evaluation of novel surgical techniques and drug therapies to further improve the outcomes of the Ross operation.
Declarações
The authors have nothing to disclose.
Acknowledgements
The study was funded by the integrated budget for interdepartmental research (BIRD) 2019.
Materials
| 0.9% Sodium Chloride | Monico SpA | AIC 030805105 | Two bottles of 100 mL. The cold one (4°C) for flushing the harvesting organ; the warm one (39°C) for moistening, and rehydration of the recipient |
| 7.5% Povidone-Iodine | B Braun | AIC 032151211 | |
| Barraquer | Aesculap | FD 232R | Straight micro needle holder for the vascular anastomoses |
| Castroviejo needle holder | Not available | J 4065 | To close the animal |
| Clip applying forceps | Rudolf Medical | RU 3994-05 | For clip application |
| Cotton swabs | Johnson & Johnson Medical SpA | N/A | Supermarket product. Sterilized |
| Curved micro jeweller forceps | Rudolf Medical | RU 4240-06 | Used to pass sutures underneath the vases. |
| Depilatory cream | RB healthcare | N/A | Supermarket product |
| Electrocautery machine | LED SpA | Surton 200 | |
| Fine scissors | Rudolf Medical | RU 2422-11 | For opening the abdomen (recipient) |
| Fine-tip curved Vannas micro scissors | Aesculap | OC 497R | Only for preparing the pulmonary root, cut the lumbar vases and the 10/0 Prolene |
| Fluovac Isoflurane/Halotane Scavanger unit | Harvard Apparatus Ltd | K 017041 | Complete of anesthesia machine, anesthesia tubing, induction chamber and scavenger unit with absorbable filter |
| Gentamycin | MSD Italia Srl | AIC 020891014 | Antibiotic. Single dose, 5 mg/kg intramuscular, administered during surgery |
| Heparin | Pharmatex Italia Srl | AIC 034692044 | 500 IU into the recipient abdominal vena cava |
| I.V. Catheter | Smiths Medical Ltd | 4036 | 20G |
| Insulin Syringe, 1 mL | Fisher Scientific | 14-841-33 | To inject heparin in the harvesting animal and to flush the sectioned aorta in the recipient |
| Jeweler bipolar forceps | GIMA SpA | 30665 | 0.25 mm tip. For electrocautery of very small vases |
| Lewis rats (LEW/HanHsd) | Envigo RMS SRL, San Pietro al Natisone, Udine, Italy | 86104M | Male or female, weighing 200-250 g (pulmonary root harvesting animals) and 320-400 g (recipients) |
| Micro-Mosquito | Rudolf Medical | RU 3121-10 | In number of four, with tips covered with silicon tubing. To keep in traction the Prolene suture during anastomosis |
| Operating microscope | Leica Microsystems | M 400-E | Used with 6x, 10x and 16x in-procedure interchangeable magnifications |
| Perma-Hand silk 2-0 | Johnson & Johnson Medical SpA | C026D | To lift the aorta |
| Petrolatum ophthalmic ointment | Dechra | NDC 17033-211-38 | |
| Prolene 10-0 | Johnson & Johnson Medical SpA | W2790 | Very fine non-absorbable suture, with a BV75-3 round bodied needle, for the vascular anastomoses |
| Retractors | Not any | N/A | Two home-made retractors |
| Ring tip micro forceps | Rudolf Medical | RU 4079-14 | For delicate manipulation |
| Sevoflurane | AbbVie Srl | AIC 031841036 | Mixed with oxygen, for inhalatory anesthesia |
| Spring type micro scissors | Rudolf Medical | RU 2380-14 | Straight; 14 cm long |
| Standard aneurysm clips | Rudolf Medical | RU 3980-12 | Two clips (7.5 mm; 180 g; 1.77 N) to close the aorta |
| Sterile gauze of non-woven fabric material | Luigi Salvadori SpA | 26161V | 7.5×7.5 cm, four layers |
| Straight Doyen scissors | Rudolf Medical | RU/1428-16 | For use to the donor |
| Straight micro jeweller forceps | Rudolf Medical | RU 4240-04 | 10.5 cm long. Used throughout the anastomosis |
| Syringes | Artsana SpA | N/A | 20 mL (for the harvesting animal) and 5 mL (for the recipient). For saline flushing and dipping |
| TiCron 4-0 | Covidien | CV-331 | For closing muscles and skin |
| Tissue forceps V. Mueller | McKesson | CH 6950-009 | Used for skin and muscles |
| Tramadol | SALF SpA | AIC 044718029 | Analgesic. Single dose, 5 mg/kg intramuscular |
| Virgin silk 8-0 | Johnson & Johnson Medical SpA | W818 | For arterial branch ligation |
Referências
- Botto, L. D., Correa, A., Erickson, J. D. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 107 (3), 32 (2001).
- Vergnat, M., et al. Aortic stenosis of the neonate: A single-center experience. The Journal of Thoracic and Cardiovascular Surgery. 157 (1), 318-326 (2019).
- Hraška, V., et al. The long-term outcome of open valvotomy for critical aortic stenosis in neonates. The Annals of Thoracic Surgery. 94 (5), 1519-1526 (2012).
- Kaza, A. K., Pigula, F. A. Are bioprosthetic valves appropriate for aortic valve replacement in young patients. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 19 (1), 63-67 (2016).
- Myers, P. O., et al. Outcomes after mechanical aortic valve replacement in children and young adults with congenital heart disease. The Journal of Thoracic and Cardiovascular Surgery. 157 (1), 329-340 (2019).
- Donald, J. S., et al. Ross operation in children: 23-year experience from a single institution. The Annals of thoracic surgery. 109 (4), 1251-1259 (2020).
- Khwaja, S., Nigro, J. J., Starnes, V. A. The Ross procedure is an ideal aortic valve replacement operation for the teen patient. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. , 173-175 (2005).
- Elkins, R. C., Lane, M. M., McCue, C. Ross operation in children: late results. The Journal of Heart Valve Disease. 10 (6), 736-741 (2001).
- Chambers, J. C., Somerville, J., Stone, S., Ross, D. N. Pulmonary autograft procedure for aortic valve disease: long-term results of the pioneer series. Circulation. 96 (7), 2206-2214 (1997).
- Mazine, A., et al. Ross procedure in adults for cardiologists and cardiac surgeons: JACC state-of-the-art review. Journal of the American College of Cardiology. 72 (22), 2761-2777 (2018).
- Sengupta, P. The laboratory rat: Relating its age with humans. International Journal of Preventive Medicine. 4 (6), 624-630 (2013).
- Ashfaq, A., Leeds, H., Shen, I., Muralidaran, A. Reinforced ross operation and intermediate to long term follow up. Journal of Thoracic Disease. 12 (3), 1219-1223 (2020).
- Vida, V. L., et al. Age is a risk factor for maladaptive changes of the pulmonary root in rats exposed to increased pressure loading. Cardiovascular Pathology: The Official Journal of the Society for Cardiovascular Pathology. 21 (3), 199-205 (2012).
- Nappi, F., et al. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. The Journal of Thoracic and Cardiovascular Surgery. 149 (4), 1134-1142 (2015).
- Vanderveken, E., et al. Mechano-biological adaptation of the pulmonary artery exposed to systemic conditions. Scientific Reports. 10 (1), 2724 (2020).

