In Vitro Characterization of Histone Chaperones using Analytical, Pull-Down and Chaperoning Assays
Summary
This protocol describes a battery of methods that includes analytical size-exclusion chromatography to study histone chaperone oligomerization and stability, pull-down assay to unravel histone chaperone-histone interactions, AUC to analyze the stoichiometry of the protein complexes, and histone chaperoning assay to functionally characterize a putative histone chaperone in vitro.
Abstract
Histone proteins associate with DNA to form the eukaryotic chromatin. The basic unit of chromatin is a nucleosome, made up of a histone octamer consisting of two copies of the core histones H2A, H2B, H3, and H4, wrapped around by the DNA. The octamer is composed of two copies of an H2A/H2B dimer and a single copy of an H3/H4 tetramer. The highly charged core histones are prone to non-specific interactions with several proteins in the cellular cytoplasm and the nucleus. Histone chaperones form a diverse class of proteins that shuttle histones from the cytoplasm into the nucleus and aid their deposition onto the DNA, thus assisting the nucleosome assembly process. Some histone chaperones are specific for either H2A/H2B or H3/H4, and some function as chaperones for both. This protocol describes how in vitro laboratory techniques such as pull-down assays, analytical size-exclusion chromatography, analytical ultra-centrifugation, and histone chaperoning assay could be used in tandem to confirm whether a given protein is functional as a histone chaperone.
Introduction
Nucleosomes composed of DNA and histone proteins form the structural unit of chromatin and regulate several critical cellular events. Nucleosomes are dynamically repositioned and remodeled to make DNA accessible to various processes such as replication, transcription, and translation1,2. Histones that are highly basic either tend to interact with acidic proteins in the cellular milieu or undergo aggregation, thus leading to various cellular defects3,4,5. A group of dedicated proteins called histone chaperones aid the transport of histones from the cytoplasm to the nucleus and prevent aberrant histone-DNA aggregation events6,7. Fundamentally, most histone chaperones store and transfer histones onto DNA at physiological ionic strength, thereby aiding the formation of nucleosomes8,9. Some histone chaperones have a definite preference for the histone oligomers H2A/H2B or H3/H410.
Histone chaperones are characterized based on their ability to assemble nucleosomes dependent or independent of DNA synthesis11. For example, chromatin assembly factor-1 (CAF-1) is dependent while histone regulator A (HIRA) is independent of DNA synthesis12,13. Similarly, the nucleoplasmin family of histone chaperones is involved in sperm chromatin decondensation and nucleosome assembly14. The nucleosome assembly protein (NAP) family members facilitate the formation of nucleosome-like structures in vitro and are involved in the shuttling of histones between cytoplasm and nucleus15. Nucleoplasmins and NAP family proteins are both functional histone chaperones but do not share any structural features. Essentially, no single structural feature allows the classification of a protein as a histone chaperone16. The usage of functional and biophysical assays along with structural studies work best in characterizing histone chaperones.
This work describes biochemical and biophysical methods to characterize a protein as a histone chaperone that aids nucleosome assembly. First, analytical size-exclusion chromatography was carried out to analyze the oligomeric status and stability of histone chaperones. Next, a pull-down assay was performed to determine the driving forces and the competitive nature of histone chaperone-histone interactions. However, the hydrodynamic parameters of these interactions could not be accurately calculated using analytical size-exclusion chromatography because of the protein's shape and its complexes that impact their migration through the column. Therefore, analytical ultracentrifugation was used, which provides in-solution macromolecular properties that include accurate molecular weight, the stoichiometry of interaction, and the shape of the biological molecules. Past studies have extensively used in vitro histone chaperoning assay to functionally characterize histone chaperones such as yScS11617, DmACF18, ScRTT106p19, HsNPM120. Histone chaperoning assay was also used to functionally characterize the proteins as histone chaperones.
Protocol
1. Analytical size-exclusion chromatography to elucidate the oligomeric status and stability of histone chaperones
- Analysis of the oligomeric status of histone chaperones
- Equilibrate a 24 mL analytical size-exclusion chromatography (SEC) column with 1.2 column volume (CV), i.e., 28.8 mL of degassed SEC buffer [20 mM of Tris-HCl (pH 7.5), 300 of mM NaCl, and 1 mM of β-mercaptoethanol (β-ME)] at 4 °C (see Table of Materials).
NOTE: Column type, buffer composition, and buffer pH may be selected based on the protein of interest. The sample injection volume should not exceed 500 µL for a 24 mL column. Also, the column pressure needs to be maintained below 5 MPa. - From a higher concentration protein stock solution, prepare 500 µL of 0.5 mg/mL protein sample in degassed SEC buffer and inject it into the pre-equilibrated column using a 500 µL injection loop. Allow the chromatography run to proceed at an isocratic flow rate of 0.2-0.3 mL/min with the SEC buffer at 4 °C.
- Monitor the elution profile of the protein by measuring absorbance at a wavelength of 280 nm. When dealing with proteins lacking aromatic residues, measure the absorbance at 214 nm.
- Use the elution volume of the protein to calculate its approximate molecular weight in kDa using the standard calibration curve21.
NOTE: Calibration curve is prepared by plotting the retention volume of known molecular weight proteins against the log of their respective molecular weights (log Mr), eluted using the same column.
- Equilibrate a 24 mL analytical size-exclusion chromatography (SEC) column with 1.2 column volume (CV), i.e., 28.8 mL of degassed SEC buffer [20 mM of Tris-HCl (pH 7.5), 300 of mM NaCl, and 1 mM of β-mercaptoethanol (β-ME)] at 4 °C (see Table of Materials).
- Analysis of the thermal stability of the histone chaperones
- Take 500 µL of 0.5 mg/mL of the protein sample prepared in degassed SEC buffer (same as used in 1.1.1) in individual microcentrifuge tubes and heat each tube to a particular temperature ranging between 20 °C and 90 °C (20 °C, 40 °C, 60 °C, and 90 °C) for 10 min in a water bath.
- Subsequently, centrifuge the heat-treated samples at 16,200 x g for 10 min at 4 °C, collect the supernatant with a micropipette, and inject each sample individually using a 500 µL injection loop into the analytical column, pre-equilibrated with the SEC buffer at 4 °C.
- Allow the chromatography run to proceed at an isocratic flow rate of 0.2-0.3 mL/min with the SEC buffer at 4 °C.
- Observe the position and height of the elution peaks and look for the appearance of additional peaks for the different samples.
- Analysis of the chemical stability of the histone chaperones
- To examine the salt stability of histone chaperones, incubate 500 µL of 0.5 mg/mL protein sample prepared in a Tris buffer [20 mM of Tris-HCl (pH 7.5), and 1 mM of β-ME] supplemented with increasing concentrations of NaCl (300 mM, 600 mM, 1 M, 1.5 M, and 2 M) in separate microcentrifuge tubes for 30 min at 4 °C. Centrifuge the samples at 16,200 x g for 10 min at 4 °C and retain the supernatant.
- Next, load the protein samples in different NaCl concentrations individually, using a 500 µL injection loop into the analytical column pre-equilibrated with 1.2 CV (28.8 mL) of the respective buffer containing increasing NaCl concentrations at 4 °C.
- Allow the chromatography run to proceed at an isocratic flow rate of 0.2-0.3 mL/min with 1 CV (24 mL) of the respective buffer at 4 °C.
- Observe the position and height of elution peaks and look for the appearance of additional peaks for the different samples.
- Similarly, for urea stability analysis, incubate 500 µL of 0.5 mg/mL protein sample prepared in a Tris buffer [20 mM of Tris-HCl (pH 7.5), and 1 mM of β-ME] supplemented with increasing urea concentrations (1 M, 2 M, 3 M, 4 M, and 5 M) in separate microcentrifuge tubes for 16 h at room temperature. Centrifuge the samples at 16,200 x g for 10 min at room temperature and retain the supernatant.
- Next, load the urea-treated protein samples individually using a 500 µL injection loop into the analytical column pre-equilibrated with 1.2 CV (28.8 mL) of the corresponding buffer containing different urea concentrations at room temperature.
- Allow the chromatography run to proceed at an isocratic flow rate of 0.2-0.3 mL/min with 1 CV (24 mL) of the respective buffer at room temperature.
CAUTION: Do not perform the experiments with buffer containing urea at a lower temperature as urea tends to crystallize and damage the column. - Observe the position and height of the elution peaks and look for the appearance of additional peaks for the different samples.
2. Salt gradient-based pull-down assays to understand the type of interactions contributing to the complex formation between histone oligomers and a histone chaperone
- For each reaction of pull-down assay, pipette 40 µL of Ni-NTA resin into a spin column and wash with sterile double-distilled water. Subsequently, equilibrate the resin with 100 CV (4 mL) of equilibration buffer [20 mM of Tris-HCl (pH 7.5), 300 mM of NaCl, 10 mM of imidazole, 10 µg/mL of BSA, and 1 mM of β-ME] (see Table of Materials).
NOTE: Pull-down can also be performed in a 1.5 mL microcentrifuge tube. - Prepare the sample by mixing 5 µM of the His-tagged histone chaperone with either 20 µM of histone H2A/H2B dimer or H3/H4 tetramer in the equilibration buffer. Incubate the sample on ice for 1 h.
NOTE: H2A/H2B dimer and H3/H4 tetramer are prepared from recombinant human histones21, and the integrity of the oligomers is confirmed based on the estimated molecular masses by analytical ultracentrifugation (AUC). The same histone oligomers have been used for all experiments mentioned below. - Load the samples into separate pre-equilibrated spin columns with Ni-NTA resin from step 2.1, each labeled for a particular salt concentration, and keep the columns for 30 min at 4 °C. Centrifuge the columns at 1000 x g for 1 min.
- Next, wash the columns with 100 CV (4 mL) of wash buffer [20 mM of Tris-HCl (pH 7.5), 50 mM of imidazole, 0.2% of Tween-20, and 1 mM of β-ME] containing different salt concentrations (i.e., 300 mM, 500 mM, 600 mM, 700 mM, 800 mM, 900 mM and 1 M NaCl). Wash each column with a buffer having a particular salt concentration.
- After the salt washing step, elute the protein from the different columns using 100 µL of elution buffer [20 mM of Tris-HCl (pH 7.5), 300 mM of NaCl, 300 mM of imidazole, and 1 mM of β-ME].
- Subsequently, subject the eluted samples to 18% SDS-PAGE22 and visualize the gel after staining with Coomassie Brilliant Blue R250 (see Table of Materials). Alternatively, you may directly load the resin onto the SDS-PAGE gel instead of eluting the bound protein from the Ni-NTA resin.
NOTE: Equilibration, wash, and elution buffer compositions and pH may be modified depending upon the protein of interest.
3. Competitive pull-down assay to identify the preference of a histone chaperone for H2A/H2B or H3/H4
- Prepare spin column as described in step 2.1
- Incubate 5 µM of the histone chaperone with 20 µM of H2A/H2B dimer in 300 µL of equilibration buffer (prepared in step 2.1) for 30 min on ice.
NOTE: The ratio of histone oligomer to histone chaperone in the reaction can be chosen based on known binding stoichiometry data; use five-fold excess histone if no information is available. - Centrifuge the histone chaperone-H2A/H2B complex at 16,200 x g for 5 min at 4 °C to remove any precipitate. Next, load the sample on the spin column pre-equilibrated with equilibration buffer (prepared in step 2.1) and incubate for 30 min at 4 °C.
- Wash the column with 100 CV (4 mL) of wash buffer [20 mM of Tris-HCl (pH 7.5), 300 mM of NaCl, 50 mM of imidazole, 0.2% of Tween-20, and 1 mM of β-ME] to remove excess H2A/H2B dimer. Next, mix the histone chaperone-H2A/H2B complex with 20-60 µM of H3/H4 tetramer and incubate for 30 min on ice.
- Rewash the column with 100 CV (4 mL) of wash buffer (prepared in step 3.4) to remove any unbound H3/H4 tetramer and elute the sample using elution buffer (prepared in step 2.5). Subject the eluted samples to 18% SDS-PAGE and visualize after staining with Coomassie Brilliant Blue R250.
NOTE: The assay could be reversed wherein, first, H3/H4 tetramer can be mixed with the chaperone, the complex allowed to bind to Ni-NTA beads, and the complex then be incubated with varying concentrations of H2A/H2B dimer.
4. Analytical ultracentrifugation – sedimentation velocity (AUC-SV) experiments to analyze the binding stoichiometry between histone chaperones and histones
- Sample preparation for AUC
- Dialyze the reconstituted histone H2A/H2B dimer, H3/H4 tetramer, and the histone chaperone separately through a 7 kDa cut-off dialysis tubing23, against a dialysis buffer [20 mM of Tris (pH 7.5), 300 mM of NaCl, and 1 mM of β-ME] (see Table of Materials). To minimize background error due to buffer mismatch, perform dialysis extensively against the dialysis buffer, preferably three times over a period of 24 h.
NOTE: The initial OD280 of the protein samples should have a two to a three-fold higher value to achieve a final OD280 of 0.3-0.5. This is essentially done to nullify the effects of dilution. - Purify H2A/H2B dimer, H3/H4 tetramer, and the histone chaperone individually with the dialysis buffer, using analytical size-exclusion chromatography (as mentioned in step 1). Save the buffer from the run to prepare further dilutions later on and to use as a reference in the AUC cell.
- Dialyze the reconstituted histone H2A/H2B dimer, H3/H4 tetramer, and the histone chaperone separately through a 7 kDa cut-off dialysis tubing23, against a dialysis buffer [20 mM of Tris (pH 7.5), 300 mM of NaCl, and 1 mM of β-ME] (see Table of Materials). To minimize background error due to buffer mismatch, perform dialysis extensively against the dialysis buffer, preferably three times over a period of 24 h.
- Sample loading for AUC
- Mix the purified proteins in a final volume of 450 µL using dialysis buffer from step 4.1.1 to reach an OD280 of 0.3-0.6. Mix the histone chaperone with H2A/H2B dimer or H3/H4 tetramer for complex formation in separate reaction tubes. Incubate the protein mixtures for 2-3 h.
NOTE: Alternatively, sedimentation data can be acquired with an interference optical scanning system in the analytical ultracentrifuge. Separately, for mixing purified proteins, fix the histone chaperone concentration and incubate it with increasing concentrations of the histone oligomers to obtain the exact stoichiometry. - Assemble the cell with a double sector centerpiece and quartz windows for the AUC-SV experiment using an absorbance detector of the analytical ultracentrifuge as described previously in detail24.
- Fill 400 µL of the sample solution and 420 µL of dialysis buffer into the two sectors (sample and reference sectors, respectively) of the cell.
NOTE: A larger volume of buffer is used in the reference sector to keep the reference meniscus above the meniscus of the sample. However, while using an optical interference system, fill the two sectors with equal volume. - Weigh and accurately balance the cells and load them into a four-place titanium rotor (see Table of Materials). Align the cells using the marks provided at the bottom of the cells and the rotor. Load the rotor in the centrifuge, close the lid and allow to develop a vacuum until the pressure drops to less than 15 microns of Hg and the rotor temperature stabilizes to 20 °C (usually takes 2-2.5 h).
NOTE: AUC operating parameters include experimental temperature, rotor speed, the interval between scans, and the number of scans to be collected. In the case of SV experiments, the scan interval is given according to the protein's molecular mass; smaller proteins require larger time intervals between the scans. The rotor speed is also set according to the protein's expected molecular mass, and the experiment is conducted at 20 °C. The absorbance data is monitored at 280 nm. - To obtain the exact stoichiometry, keep the histone chaperone concentration constant and incubate with increasing concentrations of histone oligomers to attain saturation.
- Mix the purified proteins in a final volume of 450 µL using dialysis buffer from step 4.1.1 to reach an OD280 of 0.3-0.6. Mix the histone chaperone with H2A/H2B dimer or H3/H4 tetramer for complex formation in separate reaction tubes. Incubate the protein mixtures for 2-3 h.
- AUC data analysis
- Perform the data analysis as previously described25. Briefly, calculate the density and viscosity for the buffer components using the program SEDNTERP26 (see Table of Materials). Similarly, calculate the partial specific volume of the protein based on its amino acid composition, also using SEDNTERP.
- Load the data from the AUC machine into the program SEDFIT27 and define the meniscus (red line), the cell bottom (blue line), and data analysis boundaries (green lines). Choose continuous C(s) distribution as a model.
- Next, set resolution maximum up to 100; set sedimentation coefficient (s), s min: 0 and s max: 10-15; set frictional ratio to 1.2 initially and opt to float to derive the ratio from the data; set confidence level (F-ratio; which determines the magnitude of regularization) to 0.68 for 1 sigma regularization; set partial specific volume, buffer density and buffer viscosity values as obtained from SEDNTERP.
- Press RUN to allow the software to solve the Lamm equation27. Adjust the parameters if there is a significant data mismatch. After adjusting the parameters, press FIT to refine all parameters. Assess the quality of fit based on the root-mean-square deviation (RMSD) value, which should be less than 0.01 signal units.
- Estimate the molecular masses of the peaks by choosing the option: show peak "Mw in c(s)" in the display function of the main toolbar, which will provide information about the 's' of the molecule/complex.
5. Plasmid supercoiling assay to confirm histone chaperoning function
- Nucleosome assembly reaction
- Mix 2 µM of H3/H4 tetramer and 4 µM of H2A/H2B dimer with increasing concentrations of the histone chaperone (1-6 µM) in an assembly buffer [20 mM of Tris HCl (pH 7.5), 1 mM of DTT, 1 mM of MgCl2, 0.1 mg/mL of BSA, and 100 mM of NaCl] to a final volume of 50 µL. Incubate the mixture at 4 °C for 30 min.
- Simultaneously, in a separate reaction, pretreat 500 ng of the negatively supercoiled pUC19 plasmid with 1 µg of topoisomerase I enzyme (see Table of Materials) in the assembly buffer in a final volume of 50 µL and incubate at 30 °C for 30 min.
NOTE: Topoisomerase I relaxes the supercoiled double-stranded plasmid DNA by generating a single-stranded nick. A topoisomerase I enzyme of eukaryotic origin, such as the commercially available wheat germ topoisomerase I or recombinantly expressed Drosophila melanogaster topoisomerase I, could be used. - Next, combine the H3/H4 tetramer, H2A/H2B dimer, histone chaperone mixture (from step 5.1.1), the relaxed plasmid DNA reaction mixture (from step 5.1.2), and incubate further at 30 °C for 90 min.
NOTE: Set up two control reactions for the assay; one having the histone chaperone and the relaxed plasmid DNA (but not the histones) and the other having the histone oligomers and the relaxed plasmid DNA (but not the histone chaperone). - Stop the assembly reaction by adding 100 µL of 2x stop buffer (40 mM of EDTA, 2% of SDS, and 0.4 mg/mL of proteinase K) and incubate at 37 °C for 30 min.
NOTE: Stop buffer deproteinizes the plasmid DNA by denaturation and proteolysis of bound histones.
- Phenol-chloroform extraction and ethanol precipitation
- Add an equal volume of Tris-saturated phenol in the tube containing the reaction mixture from step 5.1.4 and mix well, followed by centrifugation at 16,200 x g for 10 min at room temperature.
- Gently collect the upper aqueous phase having the plasmid DNA with a micropipette and mix with an equal volume of chloroform. Vortex the mixture and centrifuge at 16,200 x g for 10 min at room temperature.
NOTE: Isoamyl alcohol could be included at this step to avoid a fuzzy interface between the aqueous and organic phases. - Next, collect the upper aqueous phase, add 1/10th volume of 3 M sodium acetate (pH 5.5) and 2.5 volumes of ice-cold ethanol (see Table of Materials). Mix the solution well by inverting the tube 3-4 times and keep the mixture in a -20 °C freezer for 30 min for complete precipitation of the plasmid DNA.
- Centrifuge the sample from step 5.2.3 at 16200 x g for 10 min and gently discard the supernatant. Keep the tubes open at room temperature until even trace amounts of ethanol evaporate, leaving the precipitated plasmid DNA in the tube.
- Perform the agarose gel electrophoresis to observe the plasmid supercoiling effect.
- Dissolve the precipitated plasmid DNA from step 5.2.4 in sterile double-distilled water.
- Resolve the samples on a 1% agarose gel in 1x Tris-acetate-EDTA (TAE) buffer (40 mM of Tris, 20 mM of acetic acid, and 1 mM of EDTA) (see Table of Materials).
- Stain the gel with 0.2-0.5 µg/mL concentration of ethidium bromide and observe under UV to visualize the DNA bands on the gel.
Representative Results
The recombinant N-terminal nucleoplasmin domain of the protein FKBP53 from Arabidopsis thaliana was subjected to analytical SEC. The elution peak volume was plotted against the standard curve to identify its oligomeric state. The analytical SEC results revealed that the domain exists as a pentamer in solution, with an approximate molecular mass of 58 kDa (Figure 1A,B). Further, the nucleoplasmin domain was analyzed for thermal and chemical stability in conjunction with analytical SEC. The nucleoplasmin sample subjected to heat-treatment up to 90 °C displayed no apparent shift in the elution volume and the peak height compared to the samples maintained at 20 °C, suggesting that the domain is highly thermostable (Figure 1C). Likewise, the nucleoplasmin domain displayed salt stability up to 2 M of NaCl (Figure 1D) and urea stability up to 4 M (Figure 1E). However, the nucleoplasmin pentamer started dissociating in higher urea concentrations.
A pull-down assay was performed to determine the type of interactions contributing to the complex formation between the histone chaperone (nucleoplasmin domain of AtFKBP53) and the histone oligomers H2A/H2B dimer and H3/H4 tetramer using a gradient salt wash. The interaction of the nucleoplasmin domain with H2A/H2B dimer was stable up to a salt concentration of 0.4 M NaCl (Figure 2A). In comparison, the association with H3/H4 was reasonably stable up to 0.7 M NaCl (Figure 2B). The ability of the chaperone-histone complexes to withstand high salt concentration suggests the role of hydrophobic interactions in stabilizing the complexes. The chaperone complex with H3/H4 being stable even in high salt concentrations suggests a predominant role of hydrophobic interactions in the complex formation. The lower stability of the H2A/H2B-chaperone complex in high salt concentrations reveals a significant role for electrostatic interactions in the complex formation. In another experiment, the pull-down assay was used to examine whether the chaperone prefers either H2A/H2B dimer or H3/H4 tetramer. The results revealed that the chaperone binds to H2A/H2B dimer and H3/H4 tetramer simultaneously and irrespective of the order in which they are added to the chaperone (Figure 2C,D). This indicated that the chaperone possesses separate sites for its interaction with the histone oligomers.
AUC-SV experiments (Figure 3) were performed to study the stoichiometry of interaction between histone oligomers and chaperones. AUC-SV data analysis provided a sedimentation coefficient (s) value of 5.40 S for the AtFKBP53 nucleoplasmin domain in complex with H2A/H2B that corresponded to a molecular mass of 104 kDa. The complex of the nucleoplasmin domain with H3/H4 gave a sedimentation coefficient value of 7.35 S, corresponding to 129 kDa. The estimated molecular mass of the complexes reveals that the pentameric nucleoplasmin forms complex with both H2A/H2B dimer and H3/H4 tetramer in a 1:1 stoichiometry.
It is essential to show that the protein can deposit histone oligomers onto DNA to confirm that it is a histone chaperone. Towards this end, a plasmid supercoiling assay was adopted (Figure 4). The relaxed circular plasmid was incubated with the histone oligomers H2A/H2B and H3/H4 with the recombinant plant histone chaperones of the NAP family – AtNRP1 and AtNRP228. The presence of the chaperone increased the amount of supercoiled plasmid, suggesting it could deposit histones onto the DNA to form nucleosomes, causing DNA supercoiling.
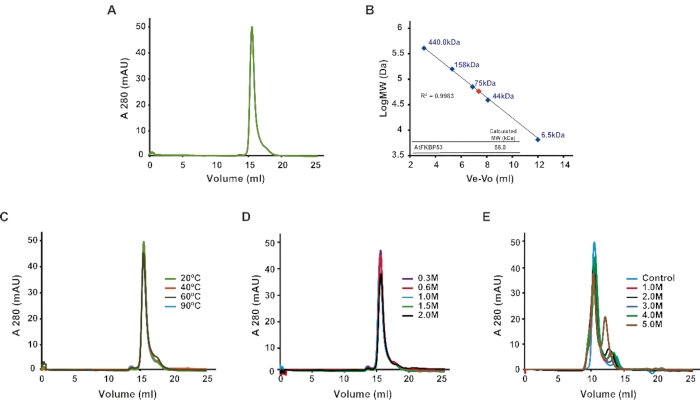
Figure 1: Oligomeric state and stability of the nucleoplasmin domain of AtFBP53. (A) Analytical size-exclusion chromatography profile of the AtFKBP53 nucleoplasmin domain. (B) Calibration curve obtained using globular proteins of known molecular mass. The blue dots represent the molecular mass of the known proteins, whereas the red dot represents the AtFKBP53 nucleoplasmin domain. (440 kDa – ferritin, 158 kDa-aldolase, 75 kDa-con albumin, 44 kDa-ovalbumin, 6.5 kDa-aprotinin). (C) Analytical size-exclusion chromatogram of 500 µL of 0.5 mg/mL AtFKBP53 nucleoplasmin domain subjected to heat treatment at different temperatures: 20 °C (green), 40 °C (orange), 60 °C (black), 90 °C (light blue). (D) Analytical size-exclusion chromatogram of 500 µL of 0.5 mg/mL AtFKBP53 nucleoplasmin domain in buffers containing different NaCl concentrations: 0.3 M (purple), 0.6 M (red), 1.0 M (light blue), 1.5 M (green), 2.0 M (black). (E) Analytical size-exclusion chromatogram of the AtFKBP53 nucleoplasmin domain in buffers with different urea concentrations: 0 M (control; light blue), 1.0 M (pink), 2.0 M (black), 3.0 M (dark blue), 4.0 M (green), 5.0 M (brown). The nucleoplasmin pentamer shows high stability to thermal and chemical stress conditions. The figure is adapted from Reference21. Please click here to view a larger version of this figure.
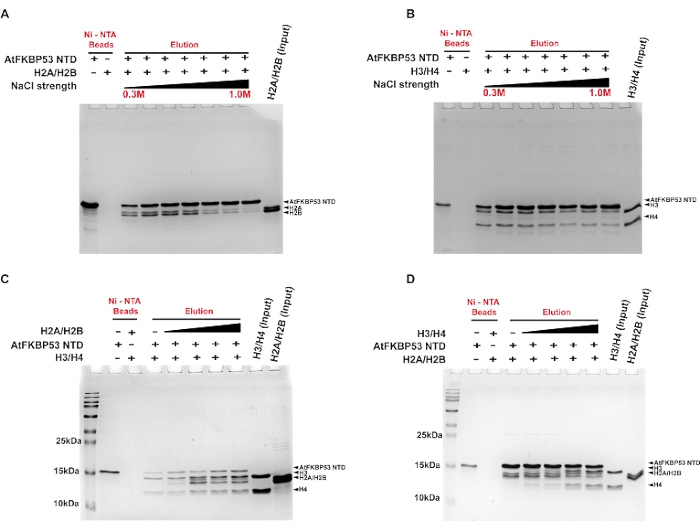
Figure 2: Pull-down assays for the interaction of the nucleoplasmin domain of AtFKBP53 with histone oligomers. 18% SDS-PAGE images of the elution fractions from the assays are presented here. Pull-down assay for (A) 20 µM H2A/H2B dimer and (B) 20 µM H3/H4 tetramer with 5 µM AtFKBP53 nucleoplasmin domain in increasing concentrations of NaCl in the range of 0.3 M, 0.5 M, 0.6 M, 0.7 M, 0.8 M, 0.9 M, and 1.0 M. 5 µM AtFKBP53 FKBD was used as a negative control here. For the competitive binding experiment, (C) a mixture of 5 µM AtFKBP53 nucleoplasmin domain and 20 µM H3/H4 tetramer incubated with a range of 20-60 µM H2A/H2B dimer and (D) a mixture of 5 µM AtFKBP53 nucleoplasmin domain and 20 µM H2A/H2B dimer incubated with a range of 20-60 µM H3/H4 tetramer has been used. The label AtFKBP53 corresponds to the nucleoplasmin domain of AtFKBP53. Elution fractions show simultaneous binding of both the histone oligomers to the nucleoplasmin. The figure is adapted from Reference21. Please click here to view a larger version of this figure.
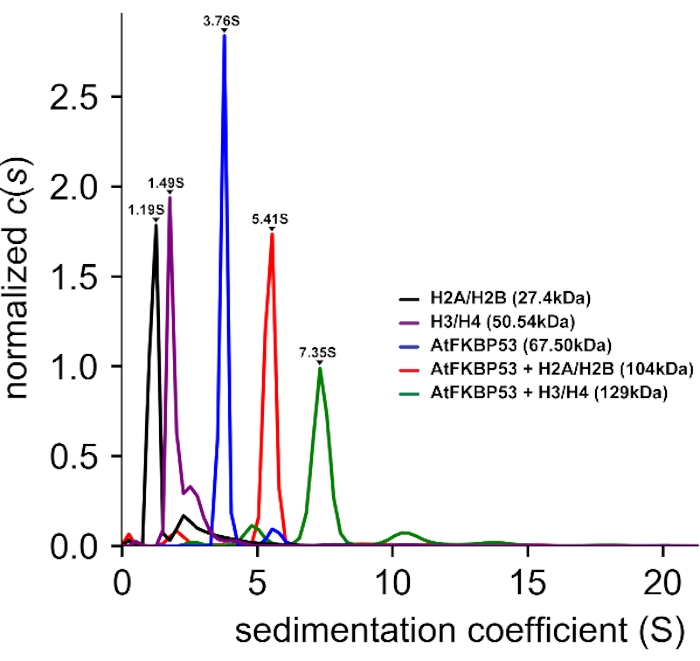
Figure 3: Analytical ultracentrifugation – sedimentation velocity (AUC-SV) experiment of histone oligomers, the nucleoplasmin domain of AtFKBP53, and their complexes. The AUC distance distribution vs. sedimentation coefficient (S) plot. The obtained sedimentation coefficient (s) values and molecular masses are also provided. The label AtFKBP53 corresponds to the nucleoplasmin domain of AtFKBP53. The estimated molecular masses reveal a 1:1 stoichiometry for the AtFKBP53 nucleoplasmin domain with the histone oligomers H2A/H2B dimer and H3/H4 tetramer. 450 µL of all the protein samples having an OD280 of 0.3-0.5 were used for the AUC-SV experiments. The figure is adapted from Reference21. Please click here to view a larger version of this figure.
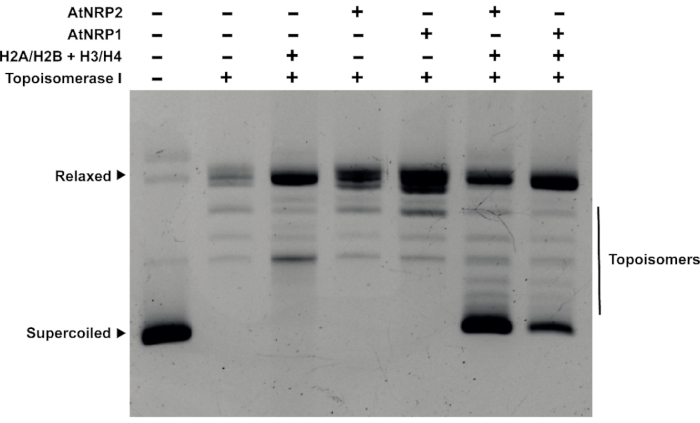
Figure 4: Plasmid supercoiling assay. Plasmid supercoiling assay for the histone chaperones AtNRP1 and AtNRP2. 500 ng of pUC19 plasmid DNA was pretreated with 1 µg of Topoisomerase I for the experiment. 4 µM AtNRP1, 4 µM AtNRP2, and a mixture of 4 µM H2A/H2B dimer and 2 µM H3/H4 tetramer were as control that shows no supercoiling activity when incubated with the pretreated pUC19 DNA. The lanes with a mixture of 4 µM H2A/H2B of dimer and 2 µM H3/H4 of tetramer and 4 µM each of AtNRP1 and AtNRP2 show the formation of supercoiled DNA upon incubation with the pretreated pUC19 DNA. Please click here to view a larger version of this figure.
Discussion
This work demonstrates and validates a comprehensive set of protocols for the biochemical and biophysical characterization of a putative histone chaperone. Herein, recombinantly expressed and purified NAP family proteins, AtNRP1 and AtNRP2, and the N-terminal nucleoplasmin domain of AtFKBP53 were used to demonstrate the protocols. The same set of experiments could very well be used to delineate the functional attributes of previously uncharacterized histone chaperones from any organism.
The first part of the protocol section involves investigating the oligomeric state and stability of a histone chaperone. Several reports indicate that histone chaperones exhibit considerable diversity in their oligomeric state. For example, human CAF-1 exists as a monomer29. NAP family members exist as dimer or tetramer29,30,31. Nucleoplasmins reveal pentameric and often decameric oligomeric states32,33. An analytical SEC experiment can determine the oligomeric state of a histone chaperone, and AUC-SV experiments can confirm the same. Several of the histone chaperones are known to be highly stable under various thermal and chemical stress conditions33,34. The thermal and chemical stability features of histone chaperones could also be explored in conjunction with analytical SEC. Further, circular dichroism spectroscopy could be effectively used for in-depth analysis of the changes in the secondary structure of the chaperone when subjected to increasing temperatures or higher concentrations of a chemical agent.
The second part of the protocol section covers pull-down assays that could examine the fundamental interactions that aid the association of histone oligomers with the chaperone by using a salt-gradient approach and a competitive pull-down assay to identify the histone oligomer preference of a chaperone. If the complex falls apart with a slight increase in salt concentration, that would suggest a major contribution of electrostatic interactions in stabilizing the complex. An intact complex in high salt would suggest a significant role for hydrophobicity in stabilizing the complex35. The competitive pull-down assay could be easily employed to determine the specificity or preference of a histone chaperone to a specific histone oligomer class. Based on their preference towards histone oligomers, histone chaperones can be classified into three categories such as H2A/H2B chaperones, H3/H4 chaperones, and H2A/H2B-H3/H4 chaperones10,36. In addition, if necessary, isothermal titration calorimetry (ITC) could be used to understand the histone oligomer specificity of a given chaperone and the thermodynamic characteristics of their interactions.
The third part of the protocol section covers the investigation of the interaction stoichiometry between a histone chaperone and the histone oligomers. In general, the different families of histone chaperones differ considerably for the stoichiometry of their association with H2A/H2B or/and H3/H421,28,37,38. AUC-SV experiment aids in obtaining sedimentation coefficient (s) and molecular mass of a protein or its complex, which becomes very useful in accurately estimating the stoichiometry in the complex formation. Alternatively, ITC can also be used to examine stoichiometry.
The fourth part of the protocol section focuses on investigating the nucleosome assembly function of histone chaperones. Histone chaperones play a crucial role in nucleosome assembly, which regulates vital cellular processes such as replication, transcription, and DNA repair39. Plasmid supercoiling assay that is typically employed for the in vitro assessment of histone chaperoning activity of histone chaperones is elaborated in this section.
It may be noted that not all histone chaperones are fully structured. Few are known to have intrinsically disordered regions40,41. Therefore, thermal and chemical stability assays may not be suitable for such proteins. Further, histone chaperones from different organisms have different oligomeric states and differential abilities to bind to histones. Therefore, this protocol may be a good starting point but would entail modifications as necessary.
Declarações
The authors have nothing to disclose.
Acknowledgements
The extramural grants to Dileep Vasudevan from the Science and Engineering Research Board, Government of India [CRG/2018/000695/PS] and the Department of Biotechnology, Ministry of Science and Technology, Government of India [BT/INF/22/SP33046/2019], as well as the intramural support from the Institute of Life Sciences, Bhubaneswar are greatly acknowledged. We thank Ms. Sudeshna Sen and Ms. Annapurna Sahoo for their help with histone preparation. The discussions with our colleagues Dr. Chinmayee Mohapatra, Mr. Manas Kumar Jagdev, and Dr. Shaikh Nausad Hossain are also acknowledged.
Materials
| Acetic acid (glacial) | Sigma | A6283 | |
| Acrylamide | MP Biomedicals | 814326 | |
| Agarose | MP Biomedicals | 193983 | |
| AKTA Pure 25M FPLC | Cytiva | 29018226 | Instrument for protein purification |
| Ammonium persulfate (APS) | Sigma | A3678 | |
| An-60Ti rotor | Beckman Coulter | 361964 | Rotor for analytical ultracentrifugation |
| Bovine serum albumin (BSA) | Sigma | A7030 | |
| Chloroform | Sigma | C2432 | |
| Coomassie brilliant blue R 250 | Sigma | 1.15444 | |
| Dialysis tubing (7 kDa cut-off) | Thermo Fisher | 68700 | For dialysing protein samples |
| Dithiothreitol (DTT) | MP Biomedicals | 100597 | |
| DNA Loading Dye | New England Biolabs | B7025S | |
| EDTA disodium salt | MP Biomedicals | 194822 | |
| Electronic balance | Shimadzu | ATX224R | |
| Ethanol | Sigma | E7023 | |
| Ethidium bromide (EtBr) | Sigma | E8751 | |
| Gel Doc System | Bio-Rad | 12009077 | For imaging gels after staining |
| Horizontal gel electrophoresis apparatus | Bio-Rad | 1704405 | Instrument for agarose gel electrophoresis |
| Hydrochloric acid (HCl) | Sigma | 320331 | |
| Imidazole | MP Biomedicals | 102033 | |
| Magnesium chloride (MgCl2) | Sigma | M8266 | |
| Micropipettes | Eppendorf | Z683779 | For pipetting of micro-volumes |
| Mini-PROTEAN electrophoresis system | Bio-Rad | 1658000 | Instrument for SDS-PAGE |
| N,N-methylene-bis-acrylamide | MP Biomedicals | 800172 | |
| Nano drop | Thermo Fisher | ND-2000 | For measurement of protein and DNA concentrations |
| Ni-NTA agarose | Invitrogen | R901-15 | Resin beads for pull-down assay |
| Optima AUC analytical ultracentrifuge | Beckman Coulter | B86437 | Instrument for analytical ultracentrifugation |
| pH Meter | Mettler Toledo | MT30130863 | |
| Phenol | Sigma | P4557 | |
| Plasmid isolation kit | Qiagen | 27104 | |
| Proteinase K | Sigma-Aldrich | 1.07393 | |
| pUC19 | Thermo Fisher | SD0061 | Plasmid for supercoiling assay |
| Refrigerated high-speed centrifuge | Thermo Fisher | 75002402 | |
| SDS-PAGE protein marker | Bio-Rad | 1610317 | |
| SEDFIT | Free software program for analytical ultracentrifugation data analysis | ||
| SEDNTERP | Free software program to estimate viscosity and density of buffer and partial specific volume of a protein | ||
| SigmaPrep Spin Columns | Sigma | SC1000 | For pull-down assay |
| Sodium acetate | Sigma | S2889 | |
| Sodium chloride (NaCl) | Merck | S9888 | |
| Sodium dodecyl sulfate (SDS) | MP Biomedicals | 102918 | |
| Superdex 200 Increase 10/300 GL | Cytiva | 28990944 | Column for analytical size-exclusion chromatography |
| Superdex 75 Increase 10/300 GL | Cytiva | 29148721 | Column for analytical size-exclusion chromatography |
| TEMED | Sigma | 1.10732 | |
| Topoisomerase I | Inspiralis | WGT102 | Enzyme used in plasmid supercoiling assay |
| Tris base | Merck | T1503 | |
| Tween-20 | Sigma | P1379 | |
| Urea | MP Biomedicals | 191450 | |
| Water bath | Nüve | NB 5 | For heat treatment of protein samples |
| β-mercaptoethanol (β-ME) | Sigma | M6250 |
Referências
- Hübner, M. R., Eckersley-Maslin, M. A., Spector, D. L. Chromatin organization and transcriptional regulation. Current Opinion in Genetics and Development. 23 (2), 89-95 (2013).
- Lai, W. K. M., Pugh, B. F. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nature Reviews Molecular Cell Biology. 18 (9), 548-562 (2017).
- Kim, U. J., Han, M., Kayne, P., Grunstein, M. Effects of histone H4 depletion on the cell cycle and transcription of Saccharomyces cerevisiae. EMBO Journal. 7 (7), 2211-2219 (1988).
- Prado, F., Aguilera, A. Partial depletion of histone H4 increases homologous recombination-mediated genetic instability. Molecular and Cellular Biology. 25 (4), 1526-1536 (2005).
- Meeks-Wagner, D., Hartwell, L. H. Normal stoichiometry of histone dimer sets is necessary for high fidelity of mitotic chromosome transmission. Cell. 44 (1), 43-52 (1986).
- Groth, A., et al. Human Asf1 regulates the flow of S phase histones during replicational stress. Molecular Cell. 17 (2), 301-311 (2005).
- Laskey, R. A., Honda, B. M., Mills, A. D., Finch, J. T. Nucleosomes are assembled by an acidic protein which binds histones and transfers them to DNA. Nature. 275 (5679), 416-420 (1978).
- Das, C., Tyler, J. K., Churchill, M. E. A. The histone shuffle: histone chaperones in an energetic dance. Trends in Biochemical Sciences. 35 (9), 476-489 (2010).
- Akey, C. W., Luger, K. Histone chaperones and nucleosome assembly. Current Opinion in Structural Biology. 13 (1), 6-14 (2003).
- De Koning, L., Corpet, A., Haber, J. E., Almouzni, G. Histone chaperones: An escort network regulating histone traffic. Nature Structural and Molecular Biology. 14 (11), 997-1007 (2007).
- Eitoku, M., Sato, L., Senda, T., Horikoshi, M. Histone chaperones: 30 years from isolation to elucidation of the mechanisms of nucleosome assembly and disassembly. Cellular and Molecular Life Sciences. 65 (3), 414-444 (2008).
- Quivy, J. P., Grandi, P., Almouzni, G. Dimerization of the largest subunit of chromatin assembly factor 1: importance in vitro and during Xenopus early development. EMBO Journal. 20 (8), 2015-2027 (2001).
- Ray-Gallet, D., et al. HIRA is critical for a nucleosome assembly pathway independent of DNA synthesis. Molecular Cell. 9 (5), 1091-1100 (2002).
- Frehlick, L. J., Eirín-López, J. M., Ausió, J. New insights into the nucleophosmin/nucleoplasmin family of nuclear chaperones. Bioessays. 29 (1), 49-59 (2007).
- Ito, T., Bulger, M., Kobayashi, R., Kadonaga, J. T. Drosophila NAP-1 is a core histone chaperone that functions in ATP-facilitated assembly of regularly spaced nucleosomal arrays. Molecular and Cellular Biology. 16 (6), 3112-3124 (1996).
- Elsässer, S. J., D’Arcy, S. Towards a mechanism for histone chaperones. Biochimica et Biophysica Acta. 1819 (3-4), 211-221 (2013).
- Rodríguez-Campos, A., Koop, R., Faraudo, S., Beato, M. Transcriptionally competent chromatin assembled with exogenous histones in a yeast whole cell extract. Nucleic Acids Research. 32 (13), 111 (2004).
- Levenstein, M. E., Kadonaga, J. T. Biochemical analysis of chromatin containing recombinant Drosophila core histones. Journal of Biological Chemistry. 277 (10), 8749-8754 (2002).
- Huang, S., et al. Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proceedings of the National Academy of Sciences of the United States of America. 102 (38), 13410-13415 (2005).
- Swaminathan, V., Kishore, A. H., Febitha, K. K., Kundu, T. K. Human histone chaperone nucleophosmin enhances acetylation-dependent chromatin transcription. Molecular and Cellular Biology. 25 (17), 7534-7545 (2005).
- Singh, A. K., Datta, A., Jobichen, C., Luan, S., Vasudevan, D. AtFKBP53: A chimeric histone chaperone with functional nucleoplasmin and PPIase domains. Nucleic Acids Research. 48 (3), 1531-1550 (2020).
- Scofield, B. T. K. H. . Protein Electrophoresis. , (2012).
- Andrew, S. M., Titus, J. A., Zumstein, L. Dialysis and concentration of protein solutions. Current Protocols in Toxicology, Appendix 3. , 1-5 (2002).
- Balbo, A., Zhao, H., Brown, P. H., Schuck, P. Assembly, loading, and alignment of an analytical ultracentrifuge sample cell. Journal of Visualized Experiments. (33), e1530 (2009).
- Padavannil, A., Brautigam, C. A., Chook, Y. M. Molecular size analysis of recombinant importin-histone complexes using analytical ultracentrifugation. Bio-protocol. 10 (10), 3625 (2019).
- Zhao, H., Brautigam, C. A., Ghirlando, R., Schuck, P. Overview of current methods in sedimentation velocity and sedimentation equilibrium analytical ultracentrifugation. Current Protocols in Protein Science. , (2013).
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modelling. Biophysical Journal. 78 (3), 1606-1619 (2000).
- Kumar, A., Kumar Singh, A., Chandrakant Bob de, R., Vasudevan, D. Structural characterization of Arabidopsis thaliana NAP1-related protein 2 (AtNRP2) and comparison with its homolog AtNRP1. Molecules. 24 (12), 2258 (2019).
- Liu, W. H., Roemer, S. C., Port, A. M., Churchill, M. E. A. CAF-1-induced oligomerization of histones H3/H4 and mutually exclusive interactions with Asf1 guide H3/H4 transitions among histone chaperones and DNA. Nucleic Acids Research. 45 (16), 9809 (2017).
- Bowman, A., et al. The histone chaperones Vps75 and Nap1 form ring-like, tetrameric structures in solution. Nucleic Acids Research. 42 (9), 6038-6051 (2014).
- Newman, E. R., et al. Large multimeric assemblies of nucleosome assembly protein and histones revealed by small-angle X-ray scattering and electron microscopy. Journal of Biological Chemistry. 287 (32), 26657-26665 (2012).
- Edlich-Muth, C., et al. The pentameric nucleoplasmin fold is present in Drosophila FKBP39 and a large number of chromatin-related proteins. Journal of Molecular Biology. 427 (10), 1949-1963 (2015).
- Franco, A., et al. Structural insights into the ability of nucleoplasmin to assemble and chaperone histone octamers for DNA deposition. Scientific Reports. 9 (1), 9487 (2019).
- Xiao, H., Jackson, V., Lei, M. The FK506-binding protein, Fpr4, is an acidic histone chaperone. FEBS Letters. 580 (18), 4357-4364 (2006).
- Graziano, G. Role of hydrophobic effect in the salt-induced dimerization of bovine beta-lactoglobulin at pH 3. Biopolymers. 91 (12), 1182-1188 (2009).
- Burgess, R. J., Zhang, Z. Histone chaperones in nucleosome assembly and human disease. Nature Structural and Molecular Biology. 20 (1), 14-22 (2013).
- Donham, D. C., Scorgie, J. K., Churchill, M. E. The activity of the histone chaperone yeast Asf1 in the assembly and disassembly of histone H3/H4-DNA complexes. Nucleic Acids Research. 39 (13), 5449-5458 (2011).
- Avvakumov, N., Nourani, A., Côté, J. Histone chaperones: Modulators of chromatin marks. Molecular Cell. 41 (5), 502-514 (2011).
- Ransom, M., Dennehey, B. K., Tyler, J. K. Chaperoning histones during DNA replication and repair. Cell. 140 (2), 183-195 (2010).
- Chu, X., et al. Importance of electrostatic interactions in the association of intrinsically disordered histone chaperone Chz1 and histone H2A.Z-H2B. PLoS Computational Biology. 8 (7), 1002608 (2012).
- Heidarsson, P. O., et al. Disordered proteins enable histone chaperoning on the nucleosome. bioRxiv. , (2020).

