A Highly Scalable Approach to Perform Ecological Surveys of Selfing Caenorhabditis Nematodes
Summary
This protocol can be used to perform large-scale ecological surveys of selfing Caenorhabditis nematodes. The primary advantage of this method is the efficient organization and analysis of ecological and molecular data associated with the nematodes collected from nature.
Abstract
Caenorhabditis elegans is one of the major model organisms in biology, but only recently have researchers focused on its natural ecology. The relative sparsity of information about C. elegans in its natural context comes from the challenges involved in the identification of the small nematode in nature. Despite these challenges, an increasing focus on the ecology of C. elegans has caused a wealth of new information regarding its life outside of the laboratory. The intensified search for C. elegans in nature has contributed to the discovery of many new Caenorhabditis species and revealed that congeneric nematodes frequently cohabitate in the wild, where they feed on microbial blooms associated with rotting plant material. The identification of new species has also revealed that the androdioecious mating system of males and self-fertilizing hermaphrodites has evolved three times independently within Caenorhabditis. The other two selfing species, C. briggsae and C. tropicalis, share the experimental advantages of C. elegans and have enabled comparative studies into the mechanistic basis of important traits, including self-fertilization. Despite these advances, much remains to be learned about the ecology and natural diversity of these important species. For example, we still lack functional information for many of their genes, which might only be attained through an understanding of their natural ecology. To facilitate ecological research of selfing Caenorhabditis nematodes, we developed a highly scalable method to collect nematodes from the wild. Our method makes use of mobile data collection platforms, cloud-based databases, and the R software environment to enhance researchers' ability to collect nematodes from the wild, record associated ecological data, and identify wild nematodes using molecular barcodes.
Introduction
The last two decades have brought an increased interest in the ecology of Caenorhabditis nematodes. From these studies, we know that the free-living Caenorhabditis species can be isolated from ephemeral micro-habitats in both temperate and tropical regions, where they feed on microbial blooms associated with decomposing plant material, sometimes in sympatry1,2,3,4,5,6,7,8. We have also learned that convergent evolution of self-fertilization has occurred in the genus three times, and selfing is the dominant mode of reproduction for C. briggsae, C. elegans, and C. tropicalis9,10. Among these selfers, C. elegans is one of the most widely studied animals on Earth and has been used by researchers to make critical advances in biology. Importantly, the other selfing Caenorhabditis species share many of C. elegans experimental advantages and are rapidly advancing comparative studies in the genus. However, the cryptic nature of these nematodes in the wild makes it difficult to study their ecology and natural diversity, which is critical for understanding the biological functions of their genes and the ways in which evolution has shaped genetic diversity among the species10,11.
The greatest challenge to studying the ecology of selfing Caenorhabditis nematodes in the wild is their small size; adult nematodes are often 1 mm in length or less. This challenge requires that researchers sample substrates from the wild and attempt to separate nematodes of interest from the substrates in the laboratory without the ability to observe animals in the wild. Because even trained experts find it difficult to discriminate selfing Caenorhabditis nematodes from other free-living nematodes under the microscope, nematodes are typically removed from the substrate, isolated, and left to proliferate before they are identified by sequence identity using established molecular barcodes3,12,13,14. The time and effort required to process each nematode in this way present an organizational challenge, as researchers must be able to trace the identity of each nematode isolated in the laboratory back to the exact substrate and associated ecological data sampled in the field. Here, we describe a step-by-step process to efficiently collect and identify selfing Caenorhabditis nematodes from the field and faithfully link these isolates with their associated spatial and ecological data at a high scale.
This collection method increases the scale and accuracy of ecological surveys by using mobile data collection platforms, cloud-based databases, and the R software environment. Fulcrum is a customizable data-collection platform that works with most mobile devices and allows users to build custom applications to gather and organize location-based data (https://www.fulcrumapp.com). This protocol provides detailed instructions on how to use customized data-collection applications to organize spatially explicit ecological data from the field and accurately link those data with the identity of nematodes isolated in the laboratory. The protocol also explains how to efficiently identify selfing Caenorhabditis nematodes using established molecular barcodes. The data from these methods can be processed simply and reproducibly with the accompanying R software package easyFulcrum15 to explore the ecology and genetic diversity of natural Caenorhabditis populations.
Protocol
1. Collection preparation
- Identify a location to survey Caenorhabditis nematodes.
NOTE: In most temperate regions, C. elegans and C. briggsae can be isolated easily from human-associated habitats like agricultural fields or rural and urban gardens1. In subtropical and tropical regions, C. briggsae, C. elegans, and C. tropicalis can all be found in the human-associated habitats listed above, sometimes in close proximity to one another. However, C. elegans seems to prefer cooler, drier habitats than the other species in tropical habitats7,8. Each of the species can also be isolated from wild habitats that are not associated with humans, but these habitats are sampled less often. - Create a Fulcrum project to organize the collection and isolation data with the mobile data-collection applications.
- Create an account with Fulcrum online using a no-cost educational agreement16. Add the Nematode Field Sampling application to a Fulcrum account by clicking on ADD APP button17.
- Add the Nematode Isolation application to an account by clicking on ADD APP button18.
NOTE: It is recommended that each trip to a location is organized as its collection project using the naming convention 'YearMonthLocation', e.g., 2020FebruaryAustralia.
- Add users to the Fulcrum account to grant them access to the collection project. Ensure that each user downloads the Fulcrum mobile application to participate in the project.
- Print a set of QR-code labels to track the collections (C-labels) and the nematode isolations (S-labels) with the mobile application. Attach the C-labels to zip-lock plastic bags, roll the labeled bags into groups of 25, and wrap them with a rubber band for packing. Keep the set of S-labels for use in the laboratory.
NOTE: Throughout this protocol, the collections (substrates from the field) are contained in bags or on plates and are labeled with C-labels. The isolated nematodes are labeled with S-labels. The C-labels are used to identify unique collections, and the S-labels are used to identify unique nematode isolates. These two types of labels are used to make the connection between a particular collection (C-label) and the nematodes isolated from that collection (S-labels) in the Fulcrum database. Print twice the number of S-labels as C-labels for a collection project because, on average, two nematodes are isolated per collection. More S-labels can be printed later if needed. 2,500 unique C-labels (Supplemental File 1) and 5,000 unique S-labels (Supplemental File 2) are provided in the supplement. - Prepare 10 cm NGMA plates for collections and 3.5 cm NGMA plates for isolating nematodes. Make one 10 cm plate and at least two 3.5 cm plates per collection21. These plates are seeded with Escherichia coli strain OP50 following established protocols. Store the plates prior to use at 4 °C for no more than 1 month.
2. Field collection
NOTE: Caenorhabditis nematodes are most often isolated from rotting vegetable material, including fruits, nuts, seeds, pods, flowers, stems, vegetal litter, and compost1,5,6,8. The best substrates are rotten and almost unrecognizable as fruits or flowers; avoid substrates that are too dry or wet (Figure 1). Substrates are most efficiently collected from the field by working in pairs. The individual with the non-contact infrared thermometer will select a substrate for collection and collect the sample while their partner uses the Nematode Field Sampling application in Fulcrum to record the collection data. The pair of collectors will repeat this process until the desired number of samples is collected. The list of materials required for fieldwork is found in (Supplemental Table 1).
- Open the Fulcrum mobile app, select Nematode Field Sampling from the drop-down menu. Press + to start a new record in the project (Figure 2A). Take a photo of the substrate.
- Click on the box in the top center to select the correct collection project made in step 1.2 (Figure 2B). Tap on the C-label field at the bottom of the collection record and choose Scan when the prompt appears. Scan the barcode on the collection bag using the mobile device camera, then tap on Done in the upper right of the screen.
- Tap on the Substrate field and select a substrate type from the drop-down menu. Add notes about the substrate by tapping the Substrate Notes field and manually entering notes.
- Choose a landscape from the drop-down menu. Pick the landscape that best represents the sampling site.
- Choose a sky view. When choosing sky view, describe the sky visibility at the sampling site (e.g., A full-sky view without obstructed views from trees or other structures = full).
- Measure the surface temperature of the substrate using the non-contact thermometer and record the value in the substrate temperature field.
NOTE: Hold the non-contact thermometer no more than 14 inches from the substrate while recording the temperature. - Measure the ambient temperature and humidity with the handheld device and record these data in the proper fields.
NOTE: Check that the ambient temperature and humidity device are not on Hold. The measurement unit will change when the button is released. Keep the device in an outside pocket to avoid irregular readings. - Save the record in Fulcrum by tapping on Save in the upper left of the screen.
- Collect about a tablespoon of the substrate without sticks or other hard pieces by inverting the collection bag to use it as a "glove" to pick up the substrate, then seal the bag. Put a paper towel in the bag if the sample is particularly moist.
NOTE: In hot climates, place the bags in soft coolers with cooler packs to keep the collections cool. - After all the samples have been collected for the day, clean the collection equipment, take batteries out of probes, recharge batteries, re-freeze freezer packs. Sync the Fulcrum collection data by tapping the Sync button on the top left of the Nematode Field Sampling application.
NOTE: The uploads can take several minutes without a strong cellular connection so it might be best to wait for WiFi access. The data will remain on mobile devices and will be synchronized to the cloud. - Ship the samples to a home institution by placing them in an overnight shipping box. Minimize the time the samples are exposed to temperatures less than 11 °C or greater than 25 °C by shipping packages on days when cargo is transported.
NOTE: Most shipping facilities do not ship cargo overnight on weekends in remote locations.
3. Plating out field collections in the laboratory
NOTE: This section details how to organize the transfer of samples from labeled collection bags to labeled plates. The samples may arrive from an overnight shipment or directly from the field.
- Receive the shipment of collections and inspect for broken bags or other evidence of damage. If bags are broken, discard the material and clean the unbroken collection bags with 70% ethanol; avoid the C-label on the bag with the ethanol as it will discolor the label and make it difficult to read.
- For each zip-lock bag, note the C-label on the bag and attach a matching C-label to the lid of a 10 cm plate spotted with OP50 bacteria.
NOTE: The labeled 10 cm plates are referred to as 'C-plates' for the rest of the protocol. The easiest way to organize the samples is to place the collection bags on a lab bench with the matching C-plate on top (Figure 3). - For each collection, transfer about one tablespoon of sample from the collection bag to the C-plate using a clean plastic spoon. Add the sample around the bacterial lawn in a crescent or ring shape, do not completely cover the bacterial lawn (Figure 4).
NOTE: Keep the tablespoon clean by placing it in a beaker of 95% ethanol when it is not in use. Use a paper towel to dry the tablespoon before transferring additional samples. - Record the time the collections were transferred from collection bags to C-plates and keep the C-plates at room temperature (RT) for at least 24 h before attempting to isolate nematodes in section 4.
4. Isolating nematodes from collections
- Open the Fulcrum application on the mobile device and choose Nematode Isolation from the application menu (Figure 5A). Make a new isolation record by tapping the + icon in the lower right (Figure 5B).
- In the new isolation record screen, confirm the correct collection project by checking the project name displayed in the box at the top center. If the wrong project is displayed, tap the project name to switch to the correct project (Figure 5C).
- Tap on the Select button under the C-label field to find the C-label associated with the sample from which nematodes are being isolated (Figure 5D). Tap on the Buscar icon, then tap on the Scan icon to scan the C-label QR code on the C-plate with the device camera. Once the QR code is scanned, a C-label record will appear in the C-label field.
- Tap on the Camera icon in the Photos field to open the device camera and use it to take a photo of the sample on the C-plate with the QR code visible (Figure 5E). Tap on Done to return to the Isolation screen.
NOTE: These isolation record photos can be used to explore specific attributes of the substrate at a later time. - Use a dissecting microscope to look for nematodes on the C-plate. Tap on the Worms on Sample field to record the presence of nematodes on the sample (Figure 5F). Tap on Yes if nematodes are present on the C-plate and tap on No if no nematodes are present.
- Tap on Tracks if only nematode tracks are present. If no nematodes are present, parafilm the C-plate and dispose of it in a biohazard bin.
NOTE: Invert the C-plate over the biohazard waste bin and gently tap the back of the plate to dislodge all of the sampled substrates. This step makes it easier to find and isolate nematodes that can be under the substrate on the C-plate. - If nematodes are present, isolate up to five nematodes from the C-plate. To isolate a nematode, transfer one nematode from the C-plate to an S-plate using a platinum wire pick. Isolate healthy, gravid adults if possible. However, isolate other stages if adults are not found.
NOTE: After isolation, up to five S-plates will each have a single nematode on them. Keep these S-plate(s) with isolated nematodes from the same C-plate organized together in a neat stack away from other S-plates until they are entered into Fulcrum. - Tap on the S-labeled Plates field to enter the S-plate(s) used for this isolation. Tap on the + in the lower right. Tap on S-label and then click on Scan to open the device camera. Use the device camera to scan the S-label QR code on the S-plate.
NOTE: Ensure that the S-label code matches the code on the plate. If it matches, tap on Done. If it does not, tap on Cancelar and rescan until it matches, then click on Done. Sometimes QR codes of nearby plates are accidentally scanned. - After entering each S-plate, save the entry with the Save button on top right. The entry will be lost if it is not saved. Tap on the + in the lower right to add more S-labeled plates if necessary until all nematodes isolated from the C-plate are entered. After adding all the S-labeled plates for the isolation record, tap on the < button on the upper left to go back to the isolation record screen.
NOTE: To cancel an isolation record because mistakes cannot be resolved, click on Cancelar in the upper left. This step will open a dialog asking whether the record can be discarded without saving. If desired, click on Yes, Discard. - Tap on the Save button on the upper right once the isolation record has all information added correctly. Then parafilm the S-plates with isolated nematodes and set them aside in an area designated to hold S-plates with nematodes.
- Parafilm the C-plate and discard it in the biohazard bin. Tap on the Sync icon to upload all the data to Fulcrum.
- Sort all the S-plates into alphanumeric order, then place the S-plates into cardboard boxes. Make sure the S-plates are lid-side down and parafilmed. Stack up to four S-plates into one position in the box and label the cardboard box with the project name, date, time, and a unique box number.
- Store the labeled boxes at RT. These isolates will be checked for proliferation at 48 h and again at 168 h if necessary.
5. Exporting S-plates from Fulcrum
NOTE: This section details how to export S-labels used in the isolation process from the Fulcrum project database. These S-labels will be used to track proliferating isofemale lines while they are being identified by sequence identity in sections 6-9.
- Sign in to the Fulcrum website and select the Nematode Isolation application. Click on Exporter from the left-hand side of the screen. Click to select the desired project, and check the Nematode Isolation box. Click Next to download a .zip file that contains the 'nematode_isolation_s_labeled_plates.csv' file.
- Open the 'nematode_isolation_s_labeled_plates.csv' file and sort it by the 'S-label' column in ascending order (the smallest S-label will be on top). Select all the S-labels and copy them from the spreadsheet.
- Navigate to the wild isolate genotyping template google sheet (wild_isolate_genotyping_template) using a web browser19.
- Make a copy of this google sheet by right-clicking on the Genotyping Template tab then selecting the Copy to New Spreadsheet option. Select Open Spreadsheet to view the new google sheet.
- Name this new sheet with the fulcrum project name followed by 'wild_isolate_genotyping', e.g., '2020FebruaryAustralia_wild_isolate_genotyping.'
NOTE: This sheet is referred to as the 'genotyping sheet' throughout the rest of the protocol.
- Paste the S-labels copied from the 'nematode_isolation_s_labeled_plates.csv' 's_label' column into the genotyping sheet column titled 's_label'. Check the 's_label_repeat_error' column for '1's. A value of '1' in this column means the S-label is duplicated somewhere on the genotyping sheet. If duplications are discovered, investigate and correct them before moving forward.
- Fill in the genotyping sheet 'isolation_box_number' column for all S-labels.
6. Check for proliferation on S-plates
- Check for proliferating animals on S-plates 48 h after isolation (use the date and time of last isolation on the box from step 4.11 to guide timing).
NOTE: Proliferating nematodes are characterized by offspring on the S-plate. - If an S-plate is proliferating, enter '1' in the proliferation_48 column on the genotyping sheet, then move the S-plate to a box labeled '48 h proliferation, box 1'. Place a maximum of 88 S-plates in a proliferation box, then start filling a new box labeled '48 h proliferation, box 2'. Ensure the S-labels are organized in alphanumeric order in the 48 h proliferation boxes.
NOTE: Do not dispose of the non-proliferating S-plates; these plates will be checked again at 168 h post-isolation. If desired, consolidate these S-plates in numeric order in boxes labeled '48 h non-proliferating, box X', but remember to record when the 168 h check must occur on the new box. - After identifying all the proliferating S-labels at 48 h, move on to section 7 for S-plates with proliferation at 48 h.
- Check the S-plates that were not proliferating at 48 h post-isolation again at 168 h post-isolation.
- If an S-plate is now proliferating, enter '1' in the proliferation_168 column on the genotyping sheet and then move the S-plate to a box labeled '168 h proliferation, box 1'.
- Place a maximum of 88 S-plates in a proliferation box, then start filling a new box labeled '168 h proliferation, box 2'. Be sure to organize S-labels in alphanumeric order in the 168 h proliferation boxes.
- Discard the S-plates that have no proliferation after 168 h. Move on to section 7 for S-plates with proliferation at 168 h.
7. Lysis of isofemale lines
NOTE: This step will use the data filter tool in google sheets to help print lysis worksheets for the S-plates in the proliferation boxes. The purpose of the lysis worksheets is to provide personnel with the correct positions for S-labels in lysis strip tubes at the bench.
- Open the genotyping sheet for the desired project and select all cells by typing Cmd+A. Click on Data > Create a Filter to add a filter button to each column header. Use the Filter buttons to display only the S-plates that will be genotyped. For example, if all the S-plates with proliferation at 48 h are to be lysed: Click on the Filter button in the 'proliferation_48' column and select '1'.
- Once the genotyping google sheet has been filtered, review the list of S-labels displayed to ensure they are the S-labels to be printed on the worksheet.
- In the 'strip_tube_number' column of the genotyping google sheet, enter a unique number every 11 rows.
- Enter the strip tube numbers for a project in successive order starting at 1 and never duplicated. In the 'strip_tube_position', enter 2 through 12 for each strip tube number.
NOTE: Use 12-tube strip tubes for lysis. The first position (strip_tube_position 1) will be control, but the controls are not added to the lysis worksheets (only the strip_tube_positions are added, 2-12). At the time of lysis, the positive control strain 'N2' will be added to position 1 of every even-numbered strip tube as a positive control. No worms are added to position 1 of every odd-numbered strip tube as a negative control.
- Filter the genotyping google sheet further to include just the S-labels in one proliferation box that are to be lysed, then select the columns 's_label' through 'lysis_notes'. Print a lysis worksheet for each proliferation box to be lysed.
- Click on the drop-down menu in the Print field and select Selected Cells. Click on Next in the upper right, then use the dialogue to print the lysis worksheet for the proliferation box.
- Repeat steps 7.3-7.5 to print a lysis worksheet for each proliferation box.
NOTE: Each proliferation box holds up to 88 S-plates, which corresponds with eight 12-well strip tubes. - Prepare 12-well strip tubes for all the samples that will be lysed. Label a strip tube with a unique 'strip_tube_number' assigned in the lysis worksheet. This label must be written on the cap strip and the strip tube to avoid confusion if they are separated. The EVEN strip tubes have a positive control (N2 worms) in position 1. The ODD strip tubes have a negative control (no worms) in position 1.
- Make up enough lysis buffer (100 mM KCl, 20 mM Tris pH 8.2, 5 mM MgCl2, 0.9% IGEPAL, 0.9% Tween 20, 0.02% gelatin with proteinase K added to a final concentration of 0.4 mg/mL) for all of the samples and add 5% extra for pipette error. Scale as necessary.
NOTE: The lysis buffer is best prepared by combining all ingredients except for proteinase K and freezing in 10-50 mL aliquots at -20 °C. Thaw aliquots and keep at 4 °C prior to use; immediately before use, add proteinase K and mix thoroughly. Keep the lysis buffer on ice while working. - Arrange the S-plates for a particular strip tube in order using the printed lysis worksheet as a guide.
- Uncap one strip tube and add 8 µL of lysis buffer to each cap with a repeat pipettor. Add the lysis buffer to one strip of caps at a time because the lysis buffer will evaporate if left at RT and uncovered. Pick 3-5 animals from the source plates (S-plate or N2 stock plate for positive controls) into the appropriate cap positions indicated on the lysis worksheet. Record notes for any S-plate with fewer than 5 worms picked to the lysis in the lysis_notes section of the lysis worksheet.
- After loading nematodes into each position of the strip tube, place the cap strip back on the strip tube. Match the marked cap (position 1) with the marked tube (position 1). Once capped, centrifuge the strip tube briefly until the nematodes are at the bottom of the tube.
- Place the strip in the -80 °C freezer until completely frozen (at least 10 min). Repeat steps 7.9 to 7.11 until all strips have nematodes added for lysis. Organize the tube strips in numeric order.
- Remove the sets of strip tubes and run the lysis program in a thermocycler: 1 h at 60 °C, 15 min at 95 °C, hold at 12 °C. When the lysis program is done, spin down the samples at 300 x g for 15 s at RT and store the lysates at -80 °C for up to 1 week.
- Organize the tube strips in numeric order using 96-well plate holders and include a label with a proliferation box number, strip tube number range, date, and the initials of the researcher. Update the genotyping sheet columns 'lysis_date' and 'lysis_notes' with information from the lysis worksheet.
8. PCR of SSU and ITS2 sequences
NOTE: This section will provide instructions on how to perform two separate PCRs for each lysed S-plate. The first primer set amplifies a 500-bp fragment of the 18S rDNA small subunit gene (SSU); oECA1271 = forward primer TACAATGGAAGGCAGCAGGC, oECA1272 = reverse primer CCTCTGACTTTCGTTCTTGATTAA 12. This PCR is used to check the quality of the template DNA. The PCR amplifies the SSU region for nearly all nematode species. If the SSU PCR fails to amplify, this result suggests that the lysis quality is poor and the lysis must be repeated for this S-plate. The second primer set amplifies a 2,000-bp fragment of the internal transcribed spacer region between the 5.8S and 28S rDNA genes (ITS2); oECA1687 = forward primer CTGCGTTACTTACCACGAATTGCARAC, oECA202 = reverse primer GCGGTATTTGCTACTACCAYYAMGATCTGC3. The ITS2 PCR product is Sanger sequenced and the sequence is used to identify nematodes in the Caenorhabditis genus to the species level by sequence similarity.
- Use the filtering tool in the genotyping sheet to view only the S-labels to be used for PCR.
- Update the pcr_plate_number, and pcr_well columns in the genotyping sheet. To prevent degradation of lysis material, the SSU and ITS2 PCRs are run at the same time.
- Use the same pcr_plate_number for the ITS2 and SSU PCRs even though these are separate reactions in separate plates. They will be distinguished with 'SSU' or 'ITS2' labels.
- Assign a pcr_plate_number to eight or fewer strip tubes (one strip tube per row of the 96-well PCR plate, arranged in ascending order, e.g., lowest strip tube number on top). Then assign a pcr_plate_well to each S-label in the strip tubes.
NOTE: The strip tubes are arranged in ascending order, with the lowest strip tube number assigned to row A and the highest number in row H. Position 1 of all strip tubes is assigned to column 1. Therefore, strip tube number 1, position 1 will be assigned to PCR plate number 1, well A01. - Label 96-well PCR plate(s) to accommodate the samples that will be used for PCR. Label each PCR plate with the following information: project name, PCR type, PCR plate number, and date of PCR (e.g., 2020FebruaryAustralia_SSU_1_20200304). Also, label the plate with the strip tube numbers that will be loaded into each row.
- Remove the lysis material from the -80 °C freezer and thaw the strip tubes containing the lysis material on ice. While the lysis material is thawing, prepare ITS2 and SSU master mixes in separate tubes on ice. The SSU and ITS2 PCR recipes are found in Supplemental Table 2.
NOTE: Prepare 100 reactions of PCR master mix for each 96-well plate to allow for pipetting error. Use a 15 mL or 50 mL conical to hold the master mix if large volumes are to be used. - Vortex the master mix gently until Taq is distributed throughout the mix. Once mixed, aliquot 38 µL of the master mix to the appropriate wells of the PCR plates on ice. Use sterile single-use v-bottom troughs and a 12-well multi-channel pipette to transfer the master mix to the PCR plates.
- Spin down the thawed lysis strip tubes to remove lysis material from the caps. Carefully remove the lids of all the strip tubes that will be loaded into the first PCR plate. Use a low-volume multi-channel pipette (either 12-well or 8-well) to add 2 µL of lysate to the appropriate well in the PCR plate. Gently pipette the lysate up and down once before removing the 2 µL.
NOTE: Check the tips to ensure they contain the lysis before the transfer. Remember to change tips between rows or columns. - Cover the PCR plate with PCR adhesive foil and use a roller to create a tight seal. After the foil is applied, briefly spin down the PCR plates in a centrifuge. Keep the plate on ice until it is ready to run in the thermocycler.
- Run the PCRs with the appropriate thermocycler program. Refer to Supplemental Table 2 for the details of the SSU and ITS2 PCR programs.
- Repeat steps 8.4- 8.8 until all the PCRs are run.
- While the PCR reactions are running, pour a 100 mL 1.5% agarose gel. Each gel will hold samples or a single PCR plate.
- Add 1.5 g of agarose to a 500 mL flask, then add 100 mL of 1x TAE buffer (Supplemental Table 3) and swirl to mix. Microwave to dissolve and cool the gel.
- Once the solution is cooled, add 5 µL of 10 mg/mL ethidium bromide solution and mix to combine. Pour the solution into a casting tray with four 25-well combs so that the gel can accommodate 96 samples plus a ladder for each row in the gel.
NOTE: Ethidium bromide is a potent mutagen. When handling ethidium bromide, use a lab coat, chemical resistant gloves, and chemical safety goggles.
- Just before the PCR is finished, add 6x loading dye to a disposable trough and use a multi-channel pipette to add 2 µL of 6x loading dye to each well of a new 96-well PCR plate. This plate will be used to load the samples into the gel. Make enough of these plates to accommodate all the samples.
- When the PCRs are done, remove the PCR plates and briefly centrifuge them at 300 x g for 15 s at RT. Store the PCR plates on ice until the PCR products can be run out on a gel.
- To run the products out on a gel, use a 12-well multi-channel pipette to add 5 µL of each sample to the appropriate well of a 96-well plate containing 2 µL of 6x loading dye.
- Then load 6 µL of this mixture into each well of a recently cast gel. Load 6 µL of 1 KB plus ladder into the first well of each row of the gel.
NOTE: To fill the wells of the gel, it may be necessary to intersperse Row A and Row B from the PCR plate in the first row of the gel. To avoid confusion, record the gel_number and gel_position in the genotyping sheet for each PCR sample.
- Place a new foil lid on the remaining PCR in the plate(s) and store them at 4 °C. These reaction products will be used for sequencing in step 9.
- Run the PCR products out on the gel at 120 V for 20 min. Image the gel and record which S-labels yield ITS2 and/or SSU PCR products in the 'pcr_product_its2' and 'prc_product_ssu' columns of the genotyping sheet. Mark the presence of a band with a '1'; mark a '0' for no band.
9. Identifying nematodes with Sanger sequencing and sequence BLAST
NOTE: This section provides instructions for sequencing the ITS2 amplicons from the S-labels, aligning those sequences to the National Center for Biotechnology Information (NCBI) database using the BLAST algorithm, and parsing the BLAST results to identify the nematodes on the S-plates.
- For each sample that is ITS2-positive, use the remaining ITS2 PCR product for Sanger sequencing using the forward primer oECA306 (CACTTTCAAGCAACCCGAC). Arrange for the sequencing output files to be easily linked to an S-label by recording the `sequencing_plate' and 'sequencing_well' columns of each S-label in the genotyping sheet.
- Obtain the .seq output files for each S-label from the sequencing platform. Arrange the .seq files for a project in a single directory with .seq files for each batch of sequencing located in subdirectories.
- Open the command-line interface tool and navigate to the top directory containing the .seq files by entering the command: cd <path_to_directory>. If it does not already exist, create a merged FASTA for all the .seq files by entering the following command: for dir in */; do cd $dir; for file in *.seq; do echo ">"$file; cat $file; done >>../all_seqs.fa; cd ..; done.
NOTE: This code will create a merged FASTA file named 'all_seqs.fa' from all the .seq files in the project directory. This file can be used in the NCBI online nucleotide BLAST tool to rapidly align the ITS2 sequence of each S-label to NCBI's sequence database. - In a web browser, navigate to the NBCI BLAST website20 and click on the Choose File button. Select the all_seqs.fa file that was just created, then click on the button Somewhat Similar Sequences (BLASTn). Click on the BLAST button at the bottom of the page to begin the BLAST search.
- Update the genotyping sheet with the BLAST results for each S-label. Use the filter tool to make updating the genotyping google sheet easier. Click on Data > Create a Filter to add a filter button to each column header. Filter the sequencing_plate column to select the sequencing plates that are to be updated with BLAST results.
- Use the drop-down menu on the NCBI BLAST results page to check the results for each S-plate ITS2 sequence (Figure 6).
- Check for no BLAST hits. A sequence ID in the drop-down prefixed with * has no blast hits. For these S-labels, enter 'no hit <current date>' in the manual_blast_notes column of the genotyping sheet.
- Check for a possible new Caenorhabditis species. Click the link on the top hit to visualize the alignment (Figure 6). If the top hit is (1) a Caenorhabditis species, (2) the alignment contains more than five mismatches in the center of the sequence, and (3) the query coverage is greater than 50%, this result suggests the isolate may be a new Caenorhabditis species (Figure 7). For these S-plates enter, the species of the top BLAST hit in the 'species_id' column, enter a 1 in the 'possible_new_caeno_sp column', and 'possible new Caeno sp.' into the 'manual_blast_notes' column along with percent identity, (e.g., 'possible new Caeno sp. 89% identity').
- For S-plate sequences that BLAST to a Caenorhabditis species, enter the full genus and species name of the top BLAST hit in the 'species_id' column. For example, 'Caenorhabditis elegans'.
- For S-plate sequences that BLAST to a non-Caenorhabditis species, enter only the genus of the top BLAST hit followed by 'sp.' in the 'species_id' column. This notation means the isolate is an unknown species within the named genus. For example, 'Oscheius sp.'.
NOTE: The ITS2 sequence can not be used to reliably identify isolates to the species -level outside of the Caenorhabditis genus3,13. - Enter 1 in the 'make_strain_name column' of the genotyping sheet if 'species_id' = 'Caenorhabditis elegans', 'Caenorhabditis briggsae', or 'Caenorhabditis tropicalis', OR 'possible_new_caeno_sp' = 1.
- Name the strains with unique names following Caenorhabditis nomenclature conventions, i.e., a unique laboratory designation consisting of 2-3 uppercase letters followed by a number for each unique strain23. Enter the strain names in the 'strain_name' column.
- After strains are named, they can be cryopreserved using established protocols 24.
10. Processing the collection data with the easyFulcrum package in R
NOTE: This step describes how to link the collection data (C-labels) and the nematode isolation data (S-labels) together using the easyFulcrum R package. The software contains functions that will further join the Fulcrum data with the genotyping data from the genotyping sheet so that S-label species identities and strain names are organized in a single data frame.
- Create a new directory named for the collection project. Arrange the folder structure within the directory to match the requirements described in the R package easyFulcrum15.
- Navigate to the Fulcrum website and sign in. Export the raw project data from the Fulcrum database using the Fulcrum website's data export tool on the left and selecting the following checkboxes: project, include photos, include GPS data, field sampling, and isolation.
NOTE: The Fulcrum data for the project will be exported as five comma-separated value (.csv) files. The complete project data will be joined together into a single data frame using the easyFulcrum package in R. - Move the five .csv files exported from Fulcrum into the project directory created in step 10.1 as instructed in the easyFulcrum vignette21.
- Open an Rstudio session and install easyfulcrum package in R by entering the following commands in the R console 'install.packages("devtools")' and 'devtools::install_github("AndersenLab/easyfulcrum")'.
- Open a new R script and follow the directions in the easyfulcrum vignette to process the collection data21.
Representative Results
This protocol has been used to collect Caenorhabditis nematodes from multiple locations, including Hawaii and California. The isolation success rate for Caenorhabditis nematodes varies with collection location, climate, sampling experience, and substrate types sampled. The protocol has been used to extensively sample the Hawaiian Islands, where nine collection projects have been conducted over multiple years and seasons. The isolation success rates for selfing Caenorhabditis species are nearly identical for C. briggsae (162 of 4,506 samples, 3.6%) and C. elegans (163 of 4,506 samples, 3.6%), and much lower for C. tropicalis (26 of 4,506 samples, 0.58%)8. Each of the selfing species is enriched on rotting fruit and flower substrates relative to the other substrate categories. Sample rotting fruit and flower substrates if the researcher is attempting to maximize the success rate rather than characterize substrate preferences. However, the success rate varies with the quality of the substrate selected. For example, among fruit and flower substrates, those substrates that are too dry, wet, or fresh will likely not yield Caenorhabditis nematodes.
The scalability of this collection protocol is evident from the number of collections a single pair of researchers can collect from the wild. For example, in October of 2018, a pair of researchers using this collection protocol was able to collect a total of over 1,000 samples in 7 days from multiple locations on two Hawaiian Islands. This field team shipped the samples overnight to the laboratory, where a team of eight researchers isolated over 2,000 nematodes from the samples as they arrived. A key advantage of this protocol is that it minimizes the cost associated with sampling in remote locations by reducing the equipment and personnel required in the field. Using this protocol, a small field team can focus on sampling while the isolation team can process the samples at their home institution using fragile and heavy equipment like dissecting microscopes and agar plates for isolating nematodes. Moreover, the implementation of the mobile data-collection application allows all the field data associated with the samples to be linked directly to the C-label, which enables the isolation team to work independently from the field team while processing samples.
Researchers that use this collection protocol must consider the effort that is required to isolate nematodes prior to a collection project. The isolation and identification steps are rate-limiting, and a small collection team can quickly overwhelm isolators with samples. Moreover, the laboratory space required to process many collections can interfere with ongoing research (Figure 3). Additionally, some isolated nematodes require additional effort to genotype. For example, approximately 2% of isolates fail to amplify with the SSU PCR primer set after the first lysis attempt and must be re-lysed to ensure that the lysis material is suitable for amplification with the ITS2 primer set (Figure 8). Furthermore, approximately 3% of isolates fail to produce quality sequences after an initial round of Sanger sequencing. For these isolates, another round of lysis, ITS2 PCR, and Sanger sequencing is often required, which can increase handing time for the isolation team. Importantly, sequence identity alone is not sufficient evidence to justify a new Caenorhabditis species (Figure 7). To properly justify raising an isolate as a new Caenorhabditis species, additional effort must be made to perform mating experiments and establish a typed specimen13. A formal morphological description of the typed specimen is also preferred but not required3. Together, these considerations suggest that researchers adopting this collection protocol will benefit from trial tests of the isolation and identification steps to ensure resources are properly allocated before a collection project begins. Importantly, even small collection projects can benefit from this protocol because the process is highly reproducible, and the data can easily be audited for quality control purposes across laboratory groups.

Figure 1: Substrate examples. (A) An ideal rotting fruit is shown in the center of the image (1), the fruit is almost unrecognizable. Less rotted fruit is shown nearby; avoid sampling freshly fallen fruits (2). (B) An ideally decomposed flower is shown at the top (3). Avoid sampling freshly fallen flowers (4). (C) The dark leaf litter under the top layer of dry leaves is ideal when sampling for selfing Caenorhabditis nematodes (5). Avoid sampling dry leaf litter (6). Please click here to view a larger version of this figure.
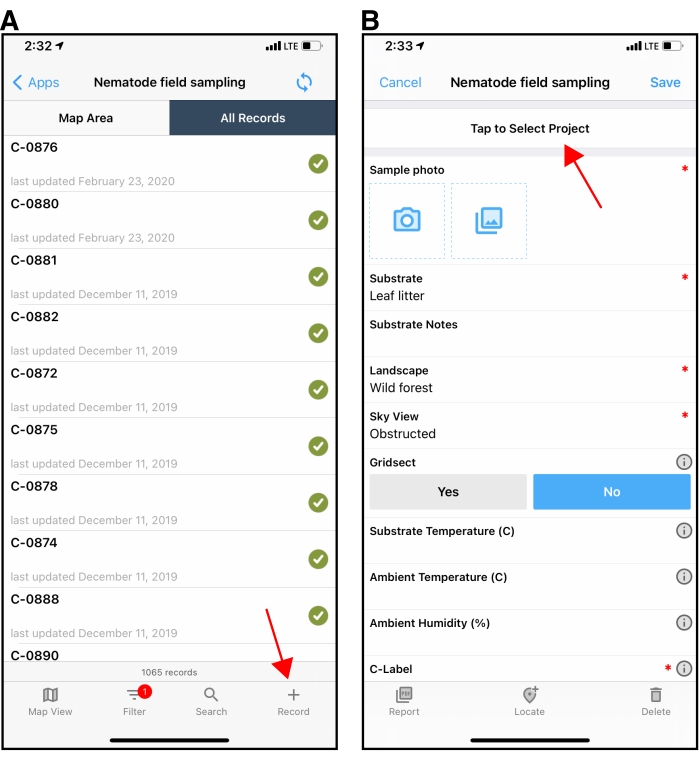
Figure 2: The Nematode Field Sampling mobile application. (A) The initial screen after opening the Nematode Field Sampling application on an Apple device in Fulcrum. The red arrow in the lower right points to the + button used to create a new collection record. (B) An example of a new collection record shown on an Apple device. The red arrow points to the 'Project' field at the top of the collection record screen. Be sure to select the correct project when sampling in the field. The project field will default to the last project used when creating subsequent collection records. Please click here to view a larger version of this figure.

Figure 3: Collection bags and collection plates organized prior to plating out samples. This figure shows the samples in C-labeled collection bags on the left. Each collection bag has a matching C-labeled 10 cm plate on top of it. On the right are 10 cm collection plates that contain sample material after it was transferred from the collection bags. Please click here to view a larger version of this figure.

Figure 4: A collection plate (C-plate) with properly a transferred sample. A 10 cm C-plate with decomposing fruit placed on the edge of the bacterial lawn. The C-label is attached to the plate lid. Please click here to view a larger version of this figure.
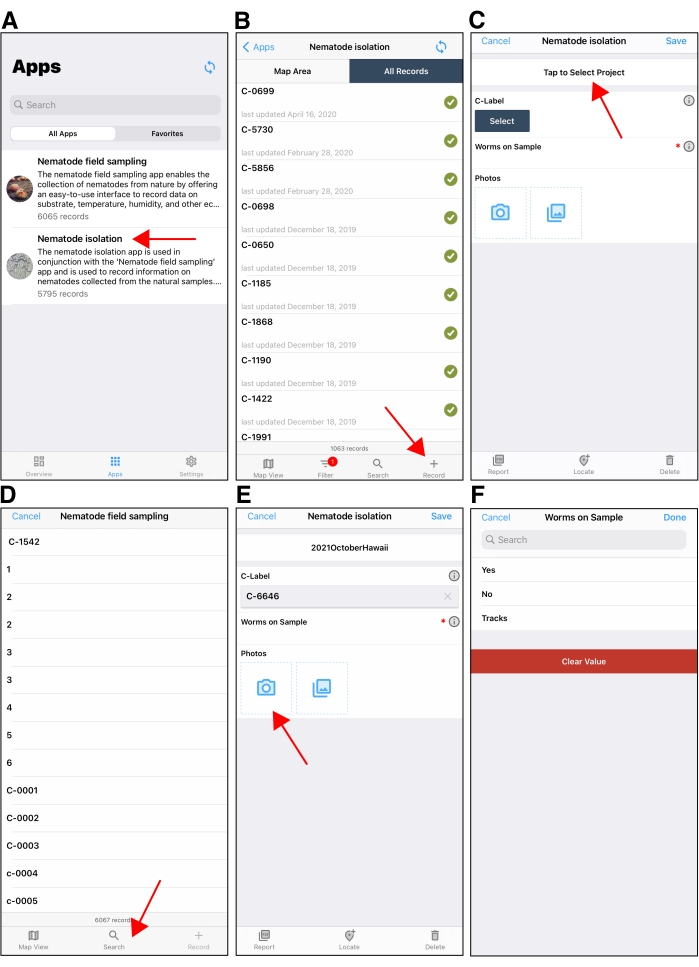
Figure 5: The Nematode isolation mobile application. (A) The application selection screen in the Fulcrum mobile application. The red arrow points to the Nematode Isolation application. (B) The initial screen after opening the Nematode Isolation application on an Apple device in Fulcrum. The red arrow in the lower right points to the + button used to create a new isolation record. (C) An example of a new isolation record shown on an Apple device. The red arrow points to the 'Project' field at the top of the isolation record screen. Be sure to select the correct project when isolating. The project field will default to the last project used when creating subsequent isolation records. (D) After tapping the Select field under C-label, users will tap the search button (red arrow) to find the C-label from which they are isolating nematodes. (E) After the C-label is selected, users will photograph the C-plate using the device camera. (F) Users then input whether there are nematodes on the C-plate or not. S-labels are added to the isolation record if there are nematodes to be isolated. Please click here to view a larger version of this figure.
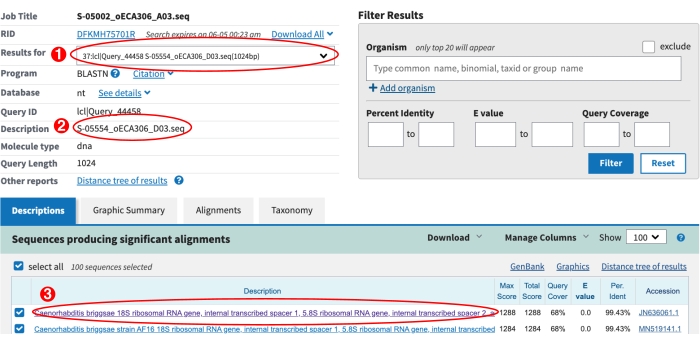
Figure 6: NCBI BLAST results page. (1) The drop-down menu used to view the BLAST results for all sequences. (2) The description of the current sequence selected from the drop-down. In this case the results for S-label S-05554 are shown. (3) The top BLAST hit for S-05554 is shown. The purple text indicates the link to visualize this alignment has been clicked. Please be sure to inspect the alignments by eye to identify possible new Caenorhabditis species, see step 9.8 above. Please click here to view a larger version of this figure.
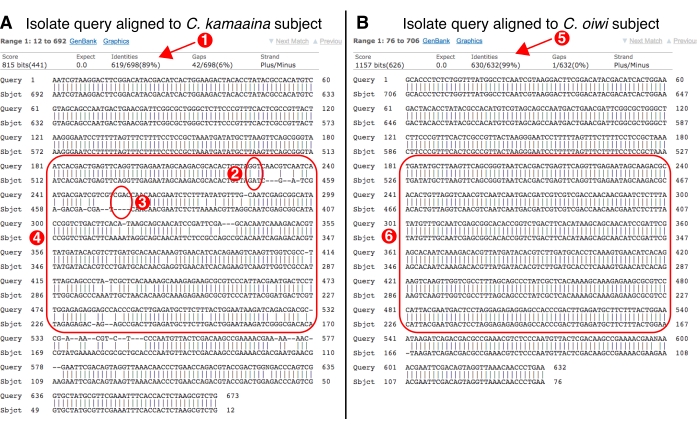
Figure 7: NCBI BLAST alignment visualization examples. (A) An example of an isolate's ITS2 query sequence aligned to a C. kamaaina subject sequence. (1) The percent identity of the alignment (89%), which is low for a top BLAST hit. (2) A mismatch between the query and subject sequence (G to A). (3) A four base pair gap in the subject sequence made by the alignment algorithm; gaps in the query or subject indicate poor alignment. (4) A generalized region in the center of the alignment with many mismatches and gaps. A region like this one suggests that the query sequence might come from a new Caenorhabditis species. Shown is an actual alignment example of a new species, C. oiwi, that was discovered in 2017. (B) An example of a good alignment between an isolate's ITS2 query sequence and a subject sequence. (5) The percent identity of the alignment (99%), which usually means the query sequence comes from an isolate of the same species as the subject. (6) A central region of the alignment with perfect identity. A region like this one suggests that the query isolate is likely the same species as the subject. Please click here to view a larger version of this figure.
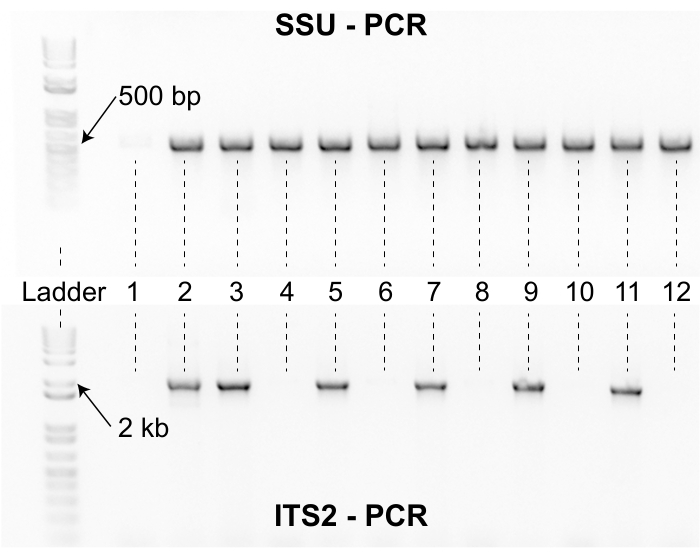
Figure 8: SSU and ITS2 PCR products. The top gel shows PCR products generated with the SSU primer set for 12 representative samples. A DNA ladder is included on the left as a reference. The SSU PCR products for Caenorhabditis nematodes are approximately 500 bp in length. Samples 2-12 amplified with the SSU primer set but sample one did not. The absence of a 500 bp SSU amplicon for sample one suggests that the lysis material was of poor quality and the sample must be re-lysed. The bottom gel shows PCR products generated with the ITS2 primer set for the same 12 Samples shown in the top gel. The ladder and samples are in the same orientation for both gels. Six of the 12 samples did not amplify with the ITS2 primer set. The samples with SSU and ITS2 bands are Sanger sequenced and identified by sequence similarity using the NCBI BLAST algorithm. Please click here to view a larger version of this figure.
Supplemental File 1: C-labels. A PDF file containing 2500 unique C-labels. Please click here to download this File.
Supplemental File 2: S-labels. A PDF file containing 5000 unique S-labels. Please click here to download this File.
Supplemental Table 1: Field Materials. A packing list of materials used in the field to sample nematodes. Please click here to download this Table.
Supplemental Table 2: PCR recipes and thermocycler conditions. A table of PCR recipes and thermocycler conditions for the ITS2 and SSU PCRs. Please click here to download this Table.
Supplemental Table 3: Electrophoresis buffer recipes. A recipe for 0.5 M pH 8.0 Ethylenediaminetetraacetic acid solution (EDTA) and the TRIS-acetate-EDTA (TAE) buffer solution. Please click here to download this Table.
Discussion
This protocol contains critical steps that must be executed with caution. For example, it is important that the field and isolation teams are careful to select the correct collection project in the application prior to collecting samples from the field or isolating nematodes from the samples in the laboratory. In the event that the wrong collection project is selected, the errant data records are best corrected in the Fulcrum database using the record editing tools online. This process can be tedious for many misplaced records. However, the database retains any changes to records so that a complete auditing of collection and isolation records is possible. The other critical steps in this protocol involve the handling of samples from the field and the nematodes isolated from those samples. To ensure Caenorhabditis nematodes survive the sampling and shipment steps, the temperature of samples should be maintained between 4 °C and 25 °C. Temperatures above 25 °C can cause sterility in C. elegans14. Ensure samples are transferred from collection bags to collection plates within five days whenever possible to minimize the loss to nematodes. After nematodes are isolated, it is critical that they are genotyped and cryopreserved before they perish. It is difficult to find living nematodes on S-plates that are more than two to three weeks old because fungal and bacterial contamination can make the S-plates inhospitable.
This protocol can be modified easily to accommodate different types of data that researchers may want to collect while in the field. For example, it is easy to customize the 'Nematode field sampling' application with new data entry fields using Fulcrum's online GUI for application editing. Moreover, the data analysis package, easyFulcrum, can accommodate these edits when processing the new data15. Another modification users may find appealing is to use a different sampling method in the field. Rather than sampling discrete substrates, researchers may desire to sample larger areas containing multiple substrate types. These larger samples are best processed in the laboratory using the Baermann funnel or tray extraction methods13. Importantly, the use of C-labels and S-labels still apply for these techniques and are therefore compatible with mobile applications.
The primary limitations of this protocol relate to the handling time of nematodes prior to isolation in the laboratory. First, the lag time between sample collection and nematode isolation makes it impossible to record the developmental stages of nematodes on a given sample at the time of collection. Second, the frequency of males and outcrossing in nature are key evolutionary questions for selfing Caenorhabditis nematodes10. This method is not well suited to addressing these questions because nematodes are likely to have gone through multiple generations prior to isolation. Delayed isolation means that direct evidence of male frequency in nature is impossible. Furthermore, the multi-generational delay during the genotyping steps means that genomic evidence of outcrossing (heterozygosity) will be eroded before a nematode strain can be sequenced. To identify heterozygosity in nature, the offspring produced by a nematode directly isolated from nature are used for sequencing2. Another potential limitation of this protocol is that it is biased toward the identification of selfing Caenorhabditis. This is because isolated nematodes of selfing species have a greater chance of proliferating than obligate outcrossers, which will only proliferate if a fertilized female is isolated.
This collection method is based on existing collection protocols13,14. The major advancement of this technique is the use of mobile technology and customized software to facilitate the organization of vast quantities of ecological and molecular data associated with large-scale collection projects. The ecological data generated using this collection protocol can be used to address outstanding questions for natural populations of Caenorhabditis species. For example, data generated with this method have been used to discover niche preferences for the species across the Hawaiian Islands. Moreover, by sequencing the genomes of cryopreserved nematodes, researchers can investigate how patterns of genetic variation are correlated with ecological data. Research of this kind can uncover signatures of local adaptation in Caenorhabditis populations and provide important insights into the relevance of genetic variation in natural contexts8. To gain a functional understanding of many genes in Caenorhabditis nematodes, ecological studies are likely required11. Even for C. elegans a large fraction of genes lacks functional annotations, despite it being the first multicellular animal to be sequenced and one of the most thoroughly studied animals on Earth. This collection protocol was developed to help address this knowledge gap by facilitating the collection of wild Caenorhabditis nematodes and the study of their ecology and natural genetic diversity.
Declarações
The authors have nothing to disclose.
Acknowledgements
This research was supported by start-up funds from Northwestern University and a National Science Foundation CAREER Award (IOS-1751035), both granted to E.C.A.
Materials
| Adhesive labels | Avery | 61533 | Printing the C-labels and S-labels |
| Agarose | Thomas Scientific | C748D75 | agarose gel preparation |
| Backpack | A backpack for each team member to hold equipment, samples, food, and water for the day. | ||
| Cardboard boxes | Fisher Scientific | AS261 | Storage of S-plates |
| Centrifuge | NA | NA | Neamtode lysis, SSU and ITS2 PCR |
| Collection bags | Zip-lock | NA | Collection of substrates |
| Digital temperature/humidity meter | Preciva | HT-86 | Measurement of ambient temperature and humidity |
| Disecting scope | NA | NA | Nematode isolation, lysis |
| Disposible reservoirs | USA Scientific | 1306-1010 | Aliquoting PCR master mixes and loading dye |
| DNA ladder, 1 KB plus | Thermo Scientific | SM0243 | Visualizing SSU and ITS2 PCR products |
| dNTPs | Thomas Scientific | CB4430-2 | PCR master mix component |
| easyFulcrum R package | NA | NA | An R package for processing collection data http://andersenlab.org/easyfulcrum/articles/easyFulcrum.html |
| EDTA solution (0.5 M pH 8.0) | NA | NA | See supplemental table 3 for recipe |
| Ethanol (95%) | NA | NA | Plating out samples, spoon sterilization |
| Ethidium bromide solution (10 mg/mL) | VWR | 97064-602 | Visualizing SSU and ITS2 PCR products |
| External battery charger for mobile device | NA | NA | External battery charger and charging cable for iPhone or Android |
| Flask (500 mL) | Fisher Scientific | FB501500 | agarose gel preparation |
| Food | NA | NA | Pack accordingly |
| Gel electrophoresis apparatus | Thermo Scientific | B2 | Visualizing SSU and ITS2 PCR products |
| Gel electrophoresis power supply | BioRad | 1645050 | Visualizing SSU and ITS2 PCR products |
| Gel loading dye, 6x | NEB | B7022S | Visualizing SSU and ITS2 PCR products |
| ITS2 primer set | NA | NA | oECA1687 = forward primer CTGCGTTACTTACCACGAATTG CARAC, oECA202 = reverse primer GCGGTATTTGCTACTACCAYYAM GATCTGC |
| ITS2 sequencing primer | NA | NA | oECA306 = forward primer CACTTTCAAGCAACCCGAC |
| Lysis buffer | NA | NA | Nematode lysis, see protocol for recipe |
| Microwave | NA | NA | agarose gel preparation |
| Mobile device | NA | NA | Charged phone in airplane mode. The Fulcrum app GPS positions are inaccurate compared to the GPS positions extracted from pictures. This setting ensures that you use less power and get more precise GPS measurements. |
| NGMA plates, 10 cm | Fisher Scientific | FB0875713 | C-plates, see Andersen et al. 2014 for NGMA plate protocol. |
| NGMA plates, 3.5 cm | Greiner Bio-One | 5662-7102Q | S-plates, see Andersen et al. 2014 for NGMA plate protocol. |
| Non-contact infrared thermometer | Etekcity | B00837ZGRY | Measurement of substrate temperature |
| Paper towels | NA | NA | Paper towels for absorbing excess moisture in a bagged sample. |
| Parafilm | Fisher Scientific | 13-374-12 | Plate sealing |
| PCR adhesive foil | VWR | 60941-126 | PCR adhesive foil |
| PCR buffer (10x) | Thomas Scientific | CB3702-7 | PCR master mix component |
| PCR plates (96-well) | Thomas Scientific | 1149K04 | SSU and ITS2 PCRs |
| Pencil | NA | NA | |
| Plastic spoons | NA | NA | Plating out samples |
| Platinum pick | NA | NA | Nematode isolation, lysis |
| Pre-labeled ziplock collection bags | NA | NA | Bundles of zip-lock plastic bags pre-labelled with C-label barcodes. Each bundle should contain 25 barcoded bags wrapped with a rubber band. |
| Proteinase K | Sigma | 3115879001 | Nematode lysis, added to lysis buffer just prior to use |
| R software | NA | NA | R software: A language and environment for statistical computing https://www.R-project.org/ |
| Soft cooler bag and ice pack | NA | NA | Collection coolers and cooler packs to keep samples cool when ambient temperature is above 25 °C. |
| Spare batteries | NA | NA | Extra batteries for sampling equipment |
| SSU primer set | NA | NA | oECA1271 = forward primer TACAATGGAAGGCAGCAGGC, oECA1272 = reverse primer CCTCTGACTTTCGTTCTTGATTAA |
| Strip tube caps (12-well) | Thomas Scientific | 1159V29 | Nematode lysis |
| Strip tubes (12-well) | Thomas Scientific | 1159V31 | Nematode lysis |
| TAE buffer (1x) | NA | NA | Visualizing SSU and ITS2 PCR products. See supplemental table 3 for recipe |
| Taq polymerase | Thomas Scientific | C775Y45 | PCR master mix component |
| Thermocycler | NA | NA | Neamtode lysis, SSU and ITS2 PCR |
| Water | NA | NA | Pack accordingly |
Referências
- Frézal, L., Félix, M. -. A. C. elegans outside the Petri dish. eLife. 4, 05849 (2015).
- Sivasundar, A., Hey, J. Sampling from natural populations with RNAI reveals high outcrossing and population structure in Caenorhabditis elegans. Current Biology: CB. 15 (17), 1598-1602 (2005).
- Kiontke, K. C., et al. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evolutionary Biology. 11, 339 (2011).
- Petersen, C., et al. Ten years of life in compost: temporal and spatial variation of North German Caenorhabditis elegans populations. Ecology and Evolution. 5 (16), 3250-3263 (2015).
- Richaud, A., Zhang, G., Lee, D., Lee, J., Félix, M. -. A. The local coexistence pattern of selfing genotypes in Caenorhabditis elegans natural metapopulations. Genética. 208 (2), 807-821 (2018).
- Ferrari, C., Salle, R., Callemeyn-Torre, N., Jovelin, R., Cutter, A. D., Braendle, C. Ephemeral-habitat colonization and neotropical species richness of Caenorhabditis nematodes. BMC Ecology. 17 (1), 43 (2017).
- Crombie, T. A., et al. Deep sampling of Hawaiian Caenorhabditis elegans reveals high genetic diversity and admixture with global populations. eLife. 8, 50465 (2019).
- Crombie, T. A., et al. Local adaptation and spatiotemporal patterns of genetic diversity revealed by repeated sampling of Caenorhabditis elegans across the Hawaiian Islands. bioRxiv. , (2021).
- Noble, L. M., et al. Selfing is the safest sex for Caenorhabditis tropicalis. eLife. 10, 62587 (2021).
- Cutter, A. D., Morran, L. T., Phillips, P. C. Males, Outcrossing, and Sexual Selection in Caenorhabditis Nematodes. Genética. 213 (1), 27-57 (2019).
- Petersen, C., Dirksen, P., Schulenburg, H. Why we need more ecology for genetic models such as C. elegans. Trends in Genetics: TIG. 31 (3), 120-127 (2015).
- Haber, M., Schüngel, M., Putz, A., Müller, S., Hasert, B., Schulenburg, H. Evolutionary history of Caenorhabditis elegans inferred from microsatellites: evidence for spatial and temporal genetic differentiation and the occurrence of outbreeding. Molecular Biology and Evolution. 22 (1), 160-173 (2005).
- Barrière, A., Félix, M. -. A. Isolation of C. elegans and Related Nematodes. WormBook. , (2018).
- Poullet, N., Braendle, C. Sampling and isolation of C. elegans from the natural habitat. Methods in Molecular Biology. 1327, 221-229 (2015).
- Di Bernardo, M., Crombie, T. A., Cook, D. E., Andersen, E. C. easyFulcrum: An R package to process and analyze ecological sampling data generated using the Fulcrum mobile application. PloS one. 16 (10), 0254293 (2021).
- . Mobile data collection & workflow automation in NCAP Available from: https://www.fulcrumapp.com/ (2021)
- . Nematode Isolation Application Available from: https://www.fulcrumapp.com/apps/nematode-isolation (2021)
- . Nematode Field Sampling Application Available from: https://www.fulcrumapp.com/apps/nematode-field-sampling (2021)
- . JOVE wild_isolate-genotyping-template Available from: https://docs.google.com/spreadsheets/d/1oEP40jy6_K6Wkwn7Qm4iF58rTUe4JWk2zhJ20-keoOk/edit (2021)
- . Nucleotide BLAST: Search nucleotide databases using a nucleotide query Available from: https://blast.ncbi.nim.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch (2021)
- Andersen, E. C., Bloom, J. S., Gerke, J. P., Kruglyak, L. A variant in the neuropeptide receptor npr-1 is a major determinant of Caenorhabditis elegans growth and physiology. PLoS Genetics. 10 (2), 1004156 (2014).
- Tuli, M. A., Daul, A., Schedl, T. WormBook: Caenorhabditis Nomenclature. WormBook. , (2018).
- Stiernagle, T. WormBook: Maintenance of C. Elegant. WormBook. , (2006).

