Transplantation of Human Induced Pluripotent Stem Cell-Derived Microglia in Immunocompetent Mice Brain via Non-Invasive Transnasal Route
Summary
The protocol presented here allows the transplantation of induced pluripotent stem cell-derived human microglia (iPSMG) into the brain via a transnasal route in immunocompetent mice. The method for the preparation and transnasal transplantation of cells and the administration of cytokine mixture for the maintenance of iPSMG is shown.
Abstract
Microglia are the specialized population of macrophage-like cells of the brain. They play essential roles in both physiological and pathological brain functions. Most of our current understanding of microglia is based on experiments performed in the mouse. Human microglia differ from mouse microglia, and thus response and characteristics of mouse microglia may not always represent that of human microglia. Further, due to ethical and technical difficulties, research on human microglia is restricted to in vitro culture system, which does not capitulate in vivo characteristics of microglia. To overcome these issues, a simplified method to non-invasively transplant induced pluripotent stem cell-derived human microglia (iPSMG) into the immunocompetent mice brain via a transnasal route in combination with pharmacological depletion of endogenous microglia using a colony-stimulating factor 1 receptor (CSF1R) antagonist is developed. This protocol provides a way to non-invasively transplant cells into the mouse brain and may therefore be valuable for evaluating the in vivo role of human microglia in physiological and pathological brain functions.
Introduction
Microglia are a specialized population of macrophage-like cells in the central nervous system (CNS) and play essential roles in controlling various brain functions like neural circuit development, modulating neurotransmission, and maintaining brain homeostasis1,2,3. Although murine microglia share many functions with ones from humans, they show species-specific differences. Thus, the response of mouse microglia to various stimuli may not always represent that of human microglia4,5,6. Although many studies have analyzed human microglia, those experiments are limited to in vitro studies. In vitro cultured human microglia show morphological features and gene expression that are very different from those in vivo. Thus, in vitro experiments may not always capitulate the in vivo characteristics of human microglia. Therefore, an experimental system to study human microglia in vivo is needed.
Recently, to study the in vivo characteristics of human microglia, in vitro generated induced pluripotent stem cells (iPSCs)- or embryonic stem cells-derived human microglia are surgically transplanted into mice brain7,8,9,10,11,12,13,14. Using this approach, various in vivo features of human microglia have been characterized. However, the widespread use of this method is limited for two reasons. First is the requirement of immune-deficient mice. Thus, to study the role of human microglia in various neurodegenerative diseases, disease mutation-carrying mice have to be crossed into immune-deficient mice, which requires significant time and effort. Furthermore, in various neurological disorders, peripheral immune cells, like T cells, can modulate microglial functions15,16,17. Therefore, experiments performed in immune-deficient mice may not represent bona fide characteristics of human microglia in vivo. Second, invasive surgeries to transplant microglia require additional equipment and training. Further, brain injury during invasive transplantation may change microglial phenotypes.
In this protocol, non-invasive transnasal transplantation (Tsn) of iPSMG into immunocompetent wild-type mice is described18. Combining pharmacological ON/OFF of a CSF1R antagonist PLX5622 which depletes endogenous mouse microglia19 and Tsn, iPSMG can be non-invasively transplanted into the mouse brain. Further, with the application of exogenous human cytokine, the transplanted iPSMG remains viable for 60 days in a region-specific manner without any immunosuppressants.
Protocol
All animals used in this study were obtained, housed, cared for, and used in accordance with the "Guiding Principles in the Care and Use of Animals in the Field of Physiologic Sciences" published by the Physiologic Society of Japan20, and with the previous approval of the Animal Care Committee of the University of Yamanashi (Yamanashi, Japan).
1. Preparation of cell medium, transplantation medium, and anesthesia mixture
- Prepare the cell medium by adding 10% fetal bovine serum and 0.1% penicillin/streptomycin in DMEM.
- Prepare the transplantation medium by adding hCSF1 (250 ng/mL) and hTGF-β1 (100 ng/mL) to the cell medium.
- Prepare the anesthesia mixture for intraperitoneal injection by mixing 0.45 mL of medetomidine hydrochloride, 1.2 mL of midazolam, and 1.5 mL of butorphanol tartrate in 11.8 mL of normal saline.
2. Preparation of iPSMG
NOTE: Frozen iPSMG (Table of Materials) were kept at -80 °C until use.
- Thaw the frozen cells quickly in a 37 °C water bath. Swirl the samples until all visible ice has melted.
- Add the thawed iPSMG to the culture media warmed to 37 °C. Add 1 mL of thawed cells containing medium (1 × 106 cells) to 10 mL of the culture media.
- Centrifuge the cells at 300 x g for 5 min to obtain a cell pellet.
- After centrifugation, remove all supernatant without disturbing the cell pellet. Complete removal of supernatant is desirable to reduce dilution of the cytokine concentration in the transplantation medium.
- Add the transplantation medium to obtain a cell concentration of 1 x 105 cells/µL.
- Place the iPSMG on ice and immediately proceed to transplantation.
NOTE: Preparation of iPSMG for transplantation is performed on a clean bench to avoid contamination.
3. Preparation of mouse for transnasal transplantation (Tsn)
- Feed the wild-type male mice (C57BL/6J, 8 weeks old) with PLX5622 containing diet for 7 days.
- At the end of the 7th day, cease the PLX diet and feed the mice with a normal diet till the end of study.
NOTE: PLX5622 containing diet is prepared by adding 1.2 g of PLX5622 in 1 kg of AIN-93G (Table of Materials).
4. Transnasal transplantation of cells
- 24 h after the cessation of PLX feeding, weigh the mice and anesthetize them using an intraperitoneal injection of the anesthesia mixture (0.2 mL/20 g).
- After the mice are completely anesthetized as assessed by unresponsiveness to pedal withdrawal reflex (firm toe pinch), administer 2.5 µL of hyaluronidase in PBS (100 U/mL) at 1 h before Tsn of iPSMG to each nostril twice using a 10 µL pipette tip to increase the permeability of the nasal mucosa.
- After the application of hyaluronidase, place the mice in the supine position.
- Repeat step 4.2 10 min before the transnasal transplantation of iPSMG.
- Apply 2.5 µL of cell suspension into one nostril of the mouse using a 10 µL pipette tip.
- Place the mouse in the supine position for 5 min before administration of cell suspension to the other nostril.
- Repeat steps 4.5 and 4.6 four times, applying a total volume of 20 µL per animal.
- Place the mouse in the supine position on a 37 °C heat pad until recovery from anesthesia.
- At 48 h after the cessation of PLX feeding, repeat steps 4.1-4.7 on the same mice once again.
NOTE: It should be noted that iPSMG are placed on ice during the transplantation.
5. Application of cytokines
- Anesthetize the mice by an intraperitoneal injection of anesthesia mixture (0.2 mL/20 g).
- Apply 2.5 µL of the transplantation medium into one nostril of the mouse using a 10 µL pipette tip.
- Place the mouse in the supine position for 5 min before administering the transplantation medium to the other nostril.
- Repeat steps 5.2 and 5.3 four times, applying a total volume of 20 µL per animal.
NOTE: It should be noted that transnasal administration of transplantation medium (human cytokines) is required every 12 h for the viability of transplanted iPSMG till the end of the study.
Representative Results
This technique allows the investigator to non-invasively transplant iPSMG into the hippocampus and cerebellum but not into the cortex of the mouse brain. After completing the study, anesthetized mice were subjected to transcardial perfusion of ice-cold PBS(−), followed by ice-cold 4% (w/v) paraformaldehyde in PBS. The brains were isolated, postfixed overnight in 4% (w/v) paraformaldehyde, and cryoprotected in a PBS containing 30% (w/v) sucrose. Further, the brains were frozen in an embedding compound and sectioned (20 µm thick coronal sections) on a cryostat. The sections were washed thrice in PBS(−) (10 min each) and permeabilized and blocked with 0.5% (v/v) Triton X-100 in 10% normal goat serum for 1 h. The sections were then incubated with anti-human-specific cytoplasm marker, STEM121 (1:100), and anti Iba1 (1:1000) for 5 days. Next, the sections were washed three times with PBS(−) for 10 min each and were incubated with secondary antibodies: Alexa Fluor 488-, or 546-conjugated mouse, or rabbit IgGs (1:1000) for 2 h at room temperature. After washing with PBS(-) three times, sections were mounted on slides using an antifade mounting medium. A confocal microscope equipped with a 40x objective lens was used for acquiring fluorescence images. The viability of iPSMG at 2 months post-transplantation in the hippocampus and the cortex are shown in Figure 1. The number of transplanted cells can be determined by counting cells that are positive for both human-specific antibodies and pan-microglial/monocyte markers, whereas endogenous mouse microglia are positive for pan-microglial/monocyte marker only as previously described18. The transplanted iPSMGs replace mouse microglia, show ramified morphologies in the hippocampus, and are not detected in the cortex18.
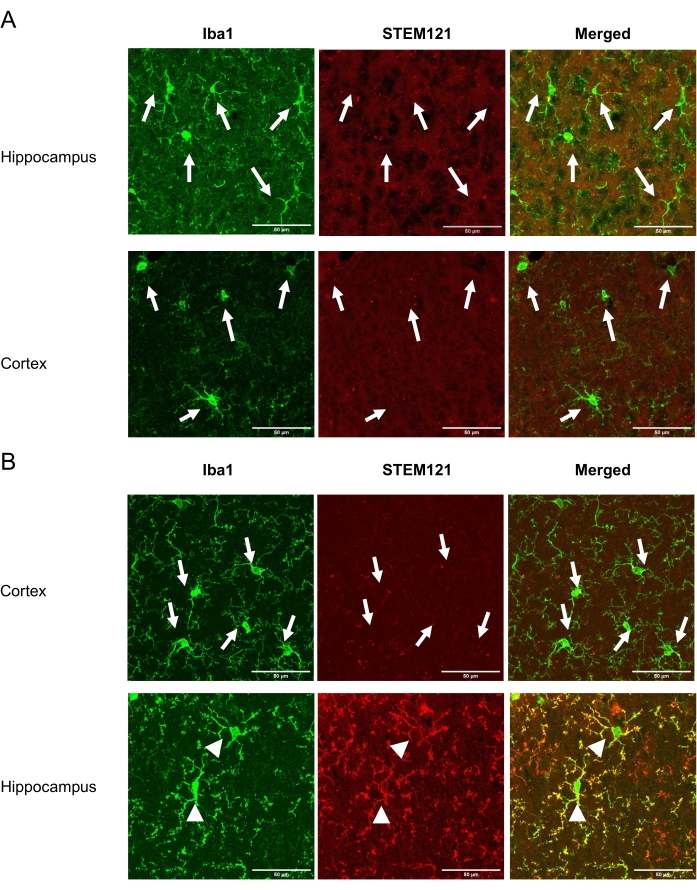
Figure 1: Viability of iPSMG in the cortex and the hippocampus at 2 months after Tsn. The left panel shows immunostaining with a pan-microglial/monocyte marker, Iba1 (green). The middle panel shows immunostaining with human-specific cytoplasm marker STEM121 (red). The right panel shows a merged image of Iba1 and STEM121 immunostaining. (A) In control mice, only mouse microglia (Iba1+/STEM121-) were detected in both cortex and hippocampus. (B) In iPSMG transplanted mice, in the cortex, only mouse microglia (Iba1+/STEM121–) were detected, whereas in the hippocampus iPSMG (Iba1+/STEM121+) were detected. The arrows in the image show mouse microglia, whereas arrowheads show iPSMG. A confocal microscope equipped with a 40x objective lens was used for fluorescence image acquisition. Maximum image size: 1024 x 1024 pixels. Zoom factor: 2. Scale bars = 50 µm Please click here to view a larger version of this figure.
Discussion
The protocol here describes the non-invasive transplantation of iPSMG into the mouse brain. The uniqueness of the current protocol is that by combining pharmacological PLX ON/OFF methods and intranasal transplantation, iPSMG can be non-invasively transplanted into the immunocompetent mouse brain. Transplanted iPSMG formed the majority of microglia in the hippocampus and cerebellum by occupying the vacant niche for up to 60 days but not in the cortex.
The critical points for the efficient Tsn of iPSMG are (i) depletion efficiency of endogenous mouse microglia (ii) the administration of human cytokines every 12 h. Microglia maintain their own territory in the brain. Efficient depletion of mouse microglia is required to provide a niche for the engraftment of transplanted iPSMG. When depletion of endogenous mouse microglia is insufficient, colonization of the mouse hippocampus and cerebellum by iPSMG are not observed. The viability of microglia depends on CSF1R and TGFBR signaling19,21,22. hCSF1 is reported to selectively increase the viability of human microglia, and hTGF-β1 is required for the viability of microglia as well as dampens inflammation when administered every 12 h21,23,24. In the absence of exogenous human cytokines, iPSMG are not observed in mouse brain. Further, care must be taken not to mechanically activate iPSMG by excessive pipetting or by any other means prior to Tsn, as it irrevocably alters iPSMG characteristics as well as transplantation efficiency. If the satisfactory Tsn of iPSMG is not seen, the viability of iPSMG before transplantation as well as the depletion of endogenous microglia, must be determined. If the depletion of endogenous mouse microglia is not more than 90%, PLX5622 feeding time may be modified to increase depletion.
Compared to a conventional surgical transplantation method that is invasive and requires additional equipment and training, Tsn allows transplantation in a non-invasive, simple, stable, and easy way. In addition, this method allows the transplantation of iPSMG into immunocompetent mice brains; thus, immunocompetent disease model mice can be used to study the response of iPSMG.
The greatest disadvantage of the current method is the regional heterogeneity in the engraftment of iPSMG. If the brain region-specific iPSMG transplantation is required, the current protocol is not suitable as the transplanted iPSMG remains engrafted for 60 days only in the hippocampus and the cerebellum but not in the cortex. Further, the need to administer exogenous human cytokines intranasally every 12 h is also a limitation of the current protocol as it requires extensive labor and is expensive.
In conclusion, a detailed protocol for Tsn of iPSMG into the brains of immunocompetent mice is provided. When combined with pharmacological ON/OFF of mouse microglia by PLX5622, this protocol allows successful engraftment of iPSMG. As transplanted cells can be observed in the hippocampus and cerebellum for a sustained period of time when exogenous cytokines are applied, the current method may be valuable for evaluating the role of human microglia in both physiological and pathological states in those regions.
Declarações
The authors have nothing to disclose.
Acknowledgements
Grant sponsors: This study was supported by JSPS KAKENHI 17K14961 (PB), 20K15899 (PB), JP18K06481 (YS), JP20KK0366 (YS), 20H05902 (SK), 20H05060 (SK), 19H04746 (SK), 21H04786 (SK), 21K19309 (SK), AMED-CREST (SK), CREST (SK), the Mitsubishi Science Foundation (SK), the Takeda Science Foundation (SK), and a Frontier Brain Science Grant from the University of Yamanashi (SK).
Materials
| Dulbecco's Modified Eagle Medium (DMEM) | Thermo Fisher Scientific | 10566 | |
| AIN 93G | Oriental Yeast Co | ||
| Anti-Iba1 antibody | FUJIFILM | 019–19741 | |
| Anti-STEM121 antibody | Takara Bioscience | Y40410 | |
| Butorphanol tartrate | Kyoritsu Seiyaku | 8019 | |
| Confocal microscope | Olympus | FV1200 | |
| Fetal bovine serum | GE Healthcare Life Sciences | SH30070.03 | |
| Frozen iPSMG | Shionogi & Co., Ltd | Laboratory for Drug Discovery and Disease Research | |
| Human colony stimulating factor 1 (hCSF1) | PeproTech | 300-25 | |
| Hyaluronidase | Sigma-Aldrich | H-3506 | |
| Medetomidine hydrochloride | Meiji Seika | VETLI5 | |
| Midazolam | Astellas | 18005A2 | |
| Paraformaldehyde | Wako Pure Chemical Industries | 162-16065 | |
| Penicillin/streptomycin | Thermo Fisher Scientific | 15140-122 | |
| Pipette | Eppendorf | 3120000011 | |
| Pipette tip | Eppendorf | 30076028 | |
| PLX5622 | Amadis Chemical | A930097 | |
| Transforming growth factor-β1 (Tgf-b1) | PeproTech | 100-21 | |
| Triton X-100 | Sigma-Aldrich | X-100 | |
| VECTA SHIELD Hard Set Mounting Medium | Vector Laboratories | H-1400-10 | antifade mounting medium |
Referências
- Hanisch, U. K., Kettenmann, H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience. 10 (11), 1387-1394 (2007).
- Nimmerjahn, A., Kirchhoff, F., Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308 (5726), 1314-1318 (2005).
- Paolicelli, R. C., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 333 (6048), 1456-1458 (2011).
- Smith, A. M., Dragunow, M. The human side of microglia. Trends in Neurosciences. 37 (3), 125-135 (2014).
- Galatro, T. F., et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nature Neuroscience. 20 (8), 1162-1171 (2017).
- Gosselin, D., et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 356 (6344), (2017).
- Abud, E. M., et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 94 (2), 278-293 (2017).
- Brownjohn, P. W., et al. Functional studies of missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Reports. 10 (4), 1294-1307 (2018).
- Douvaras, P., et al. Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Reports. 8 (6), 1516-1524 (2017).
- Haenseler, W., et al. A highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Reports. 8 (6), 1727-1742 (2017).
- Hasselmann, J., et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron. 103 (6), 1016-1033 (2019).
- Mancuso, R., et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nature Neuroscience. 22 (12), 2111-2116 (2019).
- Muffat, J., et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nature Medicine. 22 (11), 1358-1367 (2016).
- Pandya, H., et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nature Neuroscience. 20 (5), 753-759 (2017).
- Schetters, S. T. T., Gomez-Nicola, D., Garcia-Vallejo, J. J., Van Kooyk, Y. Neuroinflammation: Microglia and T cells get ready to tango. Frontiers in Immunology. 8, 1905 (2017).
- Sulzer, D., et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature. 546 (7660), 656-661 (2017).
- Togo, T., et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. Journal of Neuroimmunology. 124 (1), 83-92 (2002).
- Parajuli, B., et al. Transnasal transplantation of human induced pluripotent stem cell-derived microglia to the brain of immunocompetent mice. Glia. 69 (10), 2332-2348 (2021).
- Elmore, M. R., et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 82 (2), 380-397 (2014).
- Zasshi, N. S. Guiding principles for the care and use of animals in the field of physiological sciences. Journal of the Physiologic Society of Japan. 64 (7-8), 143-146 (2002).
- Bohlen, C. J., et al. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron. 94 (4), 759-773 (2017).
- Butovsky, O., et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature Neuroscience. 17 (1), 131-143 (2014).
- Regateiro, F. S., Howie, D., Cobbold, S. P., Waldmann, H. TGF-beta in transplantation tolerance. Current Opinion in Immunology. 23 (5), 660-669 (2011).
- Yoshimura, A., Muto, G. TGF-beta function in immune suppression. Current Topics in Microbiology and Immunology. 350, 127-147 (2011).

