Analysis of Granulocyte-Macrophage Colony-Stimulating Factor-Producing T Helper Cells in a Mouse Model of Contact Hypersensitivity
Summary
Here, we present a simple and standardized method of analyzing the granulocyte-macrophage colony-stimulating factor-producing T helper subset in vivo.
Abstract
Parallel to traditional Th1/Th2/Th17/Treg lineages, granulocyte-macrophage colony-stimulating factor-producing T helper (Th-GM) cells have been identified as a distinct subset of T helper cells (GM-CSF+ IFN-γ– IL-17A– IL-22– effector CD4+ T cells) in human and mice. Contact hypersensitivity (CHS) is considered an excellent animal model for allergic contact dermatitis (ACD) in human, manifesting an intact T cell-mediated immune response. To provide a standardized and comprehensive assay to analyze the Th-GM cell subset in the T cell-dependent immune response in vivo, a murine CHS model was induced by sensitization/challenge with a reactive, low-molecular-weight, organic hapten, 2,4-dinitrofluorobenzene (DNFB). The Th-GM subset in effector CD4+ T cells generated upon immunization with the hapten was analyzed by flow cytometry. We found that Th-GM was mainly expanded in lesions and draining lymph nodes in the DNFB-induced CHS mouse model. This method can be applied to further study the biology of Th-GM cells and pharmacological research of therapeutic strategies centered on GM-CSF in various conditions, such as ACD.
Introduction
The granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing T helper cells-the Th-GM subset-has been emerging as a distinct subset of T helper cells in human and mice and is considered to comprise "GM-CSF-expressing only" (GM-CSF+ IFN-γ– IL-17A– IL-22–) CD4 T cells identified by single-cell RNA analysis, mass cytometry, and GM-CSF fate mapper mice1,2,3. In 2014, Sheng et al. reported signal transducer and activator of transcription 5 (STAT5) programming of the Th-GM subset and conceptualized the "Th-GM" subset for the first time4,5. Th-GM cells are characterized by cytokine expression of GM-CSF, IL-2, TNF-α, IL-3, CCL20, and chemokine receptors C-X-C chemokine receptor type (CXCR) 4 or CXCR61,2. STAT and/or the NF-κB pathway are essential for Th-GM lineage differentiation. An in vitro method was established to differentiate naïve CD4 T cells into Th-GM cells using IL-7 in the presence of TCR stimuli6. Meanwhile, the cytokines IL-23 and IL-1β were shown to maintain the expression and pathogenicity of Th-GM cells ex vivo3,7.
Elevation of Th-GM cells has been associated with several autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis2,8,9, suggesting a potential role in the pathogenesis of autoimmunity10. Accumulating evidence suggests that GM-CSF can function as an inflammatory mediator. Mice genetically overexpressing Csf2 (gene encoding GM-CSF) in CD4+ T cells spontaneously developed neurological deficits accompanied by the infiltration of phagocytes into the central nervous system. In a T cell-transfer colitis model, the adoptive transfer of Csf2−/− T cells into Rag1−/− mice significantly reduced the clinical and histopathological features of the disease. However, there are few reports of the roles of the Th-GM subset in allergic diseases, such as ACD.
ACD is among the most common inflammatory dermatological conditions with high prevalence in work and life environments11,12. It is a type IV delayed-type hypersensitivity response mediated by an intact immune circuit that develops in two temporally segmented phases: sensitization and elicitation. Human ACD is triggered by exposure to some chemicals (haptens or metals) that lead to sensitization. During this phase, a T cell-mediated response is primed by hapten-protein complexes presented by antigen-presenting cells. Upon subsequent exposure to the same hapten, hapten-specific effector and memory T cells are reactivated and localize to the skin, a process involving the infiltration of a variety of immune cell populations. This acute inflammatory response is known as elicitation, resulting in the full development of lesions13. Human ACD can be studied using animal models of contact hypersensitivity (CHS)14.
The CHS model induced by a reactive, low-molecular-weight, organic hapten, 2,4-dinitrofluorobenzene (DNFB), is a commonly used murine model that has been utilized in the study of the pathology as well as potential therapeutic interventions of ACD15,16. Thus, this T cell-dependent model could be applied to study the generation of the Th-GM subset in allergic disease. Here, we induced a murine model of CHS with DNFB, analyzed the generation of Th-GM in lesions and draining lymph nodes, and found that the Th-GM subset was mainly expanded upon reexposure to the same hapten. This suggests that the Th-GM subset could be essential for ACD development and represents a specific therapeutic target in ACD.
Protocol
All mice utilized in this protocol were on the C57BL/6 genetic background, kept under specific pathogen-free conditions, and provided with food and water ad libitum. All experiments were approved by the animal welfare ethical review body of West China Medical Center, Sichuan University (20210302059).
1. Reagent and material preparation
- 0.5% DNFB solution as a sensitizer
- For sensitization of 10 mice, prepare 1.1 mL of acetone/olive oil 4:1 (v/v) mixture: mixing 880 µL of acetone and 220 µL of olive oil to allow 0.1 mL of excess volume to account for any minor volume losses. Add 5 µL of DNFB to 1.1 mL of homogenized acetone/olive oil 4:1 mixture.
NOTE: Prepare freshly on the day of sensitization. The acetone/olive oil mixture should be prepared in a fume hood.
- For sensitization of 10 mice, prepare 1.1 mL of acetone/olive oil 4:1 (v/v) mixture: mixing 880 µL of acetone and 220 µL of olive oil to allow 0.1 mL of excess volume to account for any minor volume losses. Add 5 µL of DNFB to 1.1 mL of homogenized acetone/olive oil 4:1 mixture.
- 0.2% DNFB solution as challenger
- To challenge 10 mice, add 0.5 µL of DNFB in 0.25 mL of a homogenized acetone/olive oil 4:1 mixture described in step 1.1. Use the acetone/olive oil 4:1 mixture as a vehicle when challenging the mice after sensitization.
NOTE: The acetone/olive oil mixture should be prepared in a fume hood.
- To challenge 10 mice, add 0.5 µL of DNFB in 0.25 mL of a homogenized acetone/olive oil 4:1 mixture described in step 1.1. Use the acetone/olive oil 4:1 mixture as a vehicle when challenging the mice after sensitization.
- Chloral hydrate as anesthetic
- Dissolve 4 g of chloral hydrate in 50 mL of phosphate-buffered saline (PBS) to make an 8% working solution. Filter and store at 4 °C for up to 3 months. Use 5 µL per g of body weight for a mouse.
- 50x collagenase IV stock
- Dissolve 100 mg of collagenase IV in 1 mL of basic RPMI 1640 medium (not supplemented with antibiotics or fetal bovine serum [FBS]) to make a 100 mg/mL stock. Store at -20 °C in 100 µL aliquots for up to 6 months.
- 1,000x DNase I stock
- Dissolve 5 mg of DNase I in 1 mL of 0.15 M NaCl to make a 5 mg/mL stock. Store at -20 °C in 100 µL aliquots for up to 6 months.
- Stop buffer
- Dissolve 1.681 g of EDTA in 50 mL of basic RPMI 1640 medium to make a 100 mM stock. Store at 4 °C for up to 2 weeks.
- Wash medium
- Dissolve 0.372 g of EDTA to a final concentration of 2 mM and 5 mL of FBS to a final concentration of 1% with 500 mL of PBS. Filter and store at 4 °C for up to 1 month.
- Staining buffer
- Dissolve 0.372 g of EDTA to a final concentration of 2 mM and 10 mL of FBS to a final concentration of 2% with 500 mL of PBS. Filter and store at 4 °C for up to 1 month.
- Restimulation reagents
- Dissolve 1 mg of phorbol 12-myristate 13-acetate (PMA) in 20 mL of DMSO to make a 50 µg/mL stock (500x) and store it at -20 °C in 50 µL aliquots.
- Dilute 250 µL of ionomycin in solution (4 mg/mL) in 7.75 mL of DMSO to make a 500 µg/mL stock (500x) and store it at -20 °C in 50 µL aliquots.
- Aliquot 1 mL of protein transport inhibitor solution (containing brefeldin A) in 50 µL and store the aliquots at 4 °C.
2. Induction of CHS in mice
- On day -5, anesthetize the mice by injecting 400 mg/kg chloral hydrate intraperitoneally.
- Using a pet shaver, shave a 2 cm × 2 cm area on the mouse abdomen.
NOTE: Avoid scratching the mouse skin; keep the integrity of the cutaneous barrier intact. - Smear 100 µL of 0.5% DNFB solution onto the shaved abdomens gently and evenly with a micropipette with disposable tips. Hold the mice 5-10 s to allow some of the solvent to evaporate.
- On day -4, repeat steps 2.1-2.3 for another sensitization.
NOTE: Two sensitization events work better than one. - On day 0, challenge the right ear of the mice with 20 µL of 0.2% DNFB using a micropipettor with disposable tips, and treat the left ear with the same volume of acetone/olive oil 4:1 mixture as the vehicle.
NOTE: Challenge the dorsal and ventral sides of the ear with equal volumes of DNFB or vehicle. - In the next 3 days, measure the ear thickness of the right and left ears of mice daily using a dial thickness gauge, and calculate the increase in ear thickness: right ear thickness – left ear thickness.
NOTE: A gradual increase in ear thickness could be observed in 0.2% DNFB-challenged mouse ears compared with vehicle-treated ears.
3. Sample collection of CHS mice
- On day 3, anesthetize the mice by an intraperitoneal injection of 400 mg/kg chloral hydrate. Wait for 5-10 min for the mouse to be in a state of unconsciousness and perform a gentle toe pinch on both rear feet to confirm that the mice are deeply anesthetized.
- Take a photo of each ear per mouse using a camera. Score the incrustation (scaling on the ear) and redness of the ear (erythema on the ear) independently to evaluate the severity of inflammation on a scale from 0 to 5: 0, none; 1, slight; 2, moderate; 3, marked; 4, very marked; 5, most severe.
- Euthanize the mouse by cervical dislocation. Cut off the whole ear and dissect the parallel draining lymph nodes of mice using sterile sharp scissors and forceps. Place the tissues into one well of a 6-well plate placed on ice containing 5 mL of prechilled PBS for enzymatic or physical dissociation to generate single-cell suspensions (Supplemental Figure S1A and Supplemental Figure S2A).
- For histological analysis, fix the ear specimens in 4% paraformaldehyde, dehydrate, and embed them in paraffin for immunohistochemical staining, as described elsewhere17.
4. Preparation of single-cell suspension from ears
- Split the ventral and dorsal sides of the ear by pinching and tearing the cut ends with two curved forceps (Supplemental Figure S1B). Place them in a well of a 6-well plate containing 4.5 mL of digestion buffer, ensuring that those tissues are completely immersed in the digestion buffer (Supplemental Figure S1C).
NOTE: The digestion buffer was composed of RPMI 1640 containing 10% FBS + 25 mM HEPES + 2 mg/mL collagenase IV + 5 µg/mL DNase I. - Incubate the ear leaflets in a 37 °C cell culture incubator for 20 min. Cut the ear leaflets with scissors as small as possible to facilitate tissue digestion (Supplemental Figure S1D), and put the sample back in the incubator for another 40 min.
NOTE: Efficient digestion can be achieved within 40-50 min. Overdigestion at 37 °C affects cell viability. - Add 0.5 mL of stop buffer per well to neutralize the enzymatic activities of collagenase IV and DNase I. Perform the next steps on ice.
- Transfer the ear tissue fragments with a pair of curved forceps to a 50 mL centrifuge tube containing a 70 µm cell strainer and pipette the whole volume of medium into the same cell strainer.
- Disrupt the tissues using the top end of a 1 mL syringe plunger. Press in circular movement against the 70 µm cell strainer until only white connective tissues remain (Supplemental Figure S1D).
- Rinse the cell strainers twice with 1,000 µL of wash medium. Transfer the entire volume of the well into a 50 mL centrifuge tube through a 40 µm cell strainer. Keep the tube on ice.
5. Preparation of single-cell suspensions from draining lymph nodes (dLNs)
- Transfer the lymph nodes with a pair of curved forceps to a 70 µm cell strainer on top of the well of a 6-well plate (Supplemental Figure S2B). Dissociate the lymph nodes using the top end of a 1 mL syringe plunger. Press in circling movements against the 70 µm cell strainer until only white debris remains (Supplemental Figure S2C).
- Rinse the cell strainer twice with 1,000 µL of wash medium. Transfer the entire volume of the well to a 5 mL tube. Keep the tubes on ice.
6. Restimulation of the Th-GM subset with 12-myristate 13-acetate (PMA)/ionomycin in the presence of a protein transport inhibitor
- Centrifuge the cells isolated from the ear and the draining lymph node samples for 8 min at 400 × g at 4 °C, and discard the supernatant. Resuspend the pellet with 1,000 µL of wash medium gently.
- Collect a small portion of each sample to count the total number of cells derived from the ear and draining lymph node using a hemocytometer. Add 0.04% trypan blue to measure cell viability.
NOTE: Cell viability should be more than 60% for subsequent T cell stimulation and analysis. Cell density could be 1-5 × 106/mL. - Centrifuge the cells in step 6.1 and discard the supernatant. Gently resuspend the pellet with 1,000 µL of RPMI 1640 medium containing 2% FBS, and seed 1 mL of the single-cell suspension (106 cells/mL) in 12-well flat-bottom tissue culture plates.
- Add 2 µL of phorbol 12-myristate 13-acetate (PMA) (final concentration 100 ng/mL), 2 µL of ionomycin (final concentration 1,000 ng/mL), and 1 µL of protein transport inhibitor (containing Brefeldin A) to each well in the 12-well plate containing 1 mL of the single-cell suspension above and incubate at 37 °C for 4 h.
7. Analysis of Th-GM subsets generated in vivo by cell surface and intracellular staining
- Transfer the stimulated cells to 1.5 mL centrifuge tubes using a pipette. Centrifuge the centrifuge tubes for 8 min at 400 × g at room temperature, and discard the supernatant.
- Wash the cell pellet once with 1 mL of staining buffer and pellet the cells by centrifugation, as described in step 7.1. Resuspend the cells at 1-10 × 106/mL in 0.5 mL of staining buffer.
- Add 0.5 µL of viability dye stock solution (see the Table of Materials) to 0.5 mL of cell suspension and vortex immediately. Incubate the mixture for 10 min at room temperature in the dark. Wash the cells twice with 1 mL of staining buffer, and repeat the centrifugation in step 7.1 to pellet the cells.
- Resuspend the pellet in 0.1 mL of staining buffer. Add 1 µL of anti-CD4 antibody (FITC-conjugated), 1 µL of anti-CD44 antibody (APC-conjugated), and 1 µL of anti-CD62L antibody (PE/Cyanine7-conjugated) to three 0.1 mL aliquots of the cell suspension. Vortex immediately and incubate at room temperature for 15 min, protected from light.
- Repeat the washes and centrifugation in step 7.3.
- Resuspend the pellet in 200 µL of IC fixation buffer and incubate for 20 min in the dark. Meanwhile, prepare permeabilization buffer (1x) by diluting 1 part of permeabilization buffer (10x) with 9 parts of distilled water. Wash the cells twice with 1 mL of permeabilization buffer (1x) by centrifugation at 600 × g for 5 min at room temperature.
- Resuspend the pellet in 0.1 mL of permeabilization buffer (1x) with antibodies against GM-CSF (PE-conjugated, 1 µL/test), IFN-γ (BV711-conjugated, 1 µL/test), IL-17A (BV421-conjugated, 1 µL/test), and IL-22 (PerCP/Cyanine5.5-conjugated, 5 µL/test) and incubate for 30 min at room temperature in the dark.
- Repeat the washes and centrifugation in step 7.6.
- Resuspend the cell pellet in 200 µL of PBS and transfer the suspension to a round-bottom test tube. Run the stained cells in a flow cytometer.
8. Gating strategy for identifying the Th-GM subset
- Analyze the flow cytometry data (see the Table of Materials for details about the software used) using the gating strategy illustrated in Supplemental Figure S1. For the software used here, download and set it up in the computer.
- Import the flow cytometry data by clicking on File |Open | Import FCS Files into the software, and annotate the group and name of each sample by selecting the Rename tab.
- Choose the lymphocytes on an FSC versus SSC plot (FSC low SSC low, P1) to exclude debris found in the lower-left corner and myeloid cells with large size and high granularity by clicking the Density Plot, and show the X-axis of this plot as FSC-A and the Y-axis as SSC-A.
- Double-click the events in P1 to generate another density plot, and separate the single cells (shown as a correlated line, P2) from cell aggregates by selecting the cell height and area in this plot (X-axis as FSC-A vs Y-axis as FSC-H).
- Double-click the events in P2 to generate another density plot, and show the X-axis of this plot as FSC-A and Y-axis as SSC-A. Gate the viable cell populations using FVS 780– events (P3).
- Double-click the events in P3 to generate the next density plot (the X-axis as CD4-A and the Y-axis as SSC-A), and distinguish the CD4 T cells by choosing the CD4+ population (P4).
- Double-click the events in P4 to generate another density plot (the X-axis as CD44-A and the Y-axis as CD62L-A), and define the effector T helper cells by choosing the upper-right population of this plot (CD62L– CD44+, P5).
9. Statistical analysis
- Perform statistical analyses, comparing two groups using an unpaired t-test. *P < 0.05, ** P < 0.01, *** P < 0.001 and **** P < 0.0001 (mean ± SD).
Representative Results
DNFB-induced CHS (contact hypersensitivity) in mice
To induce CHS in mice, the mice were sensitized and challenged with DNFB applied to the ear skin, as illustrated in Figure 1A. Ear thickness, an indicator of epidermal spongiosis, was markedly increased in DNFB-challenged mice compared to vehicle-treated mice (Figure 1B, 70 vs 3 µm at day 1, 203 vs 7.5 µm at day 2, 276 vs 5 µm at day 3). Seventy-two hours after the challenge, the right ear of the mice displayed signs of erythema, incrustation, and thickening, while the vehicle-treated ear did not show any signs of inflammation (Figure 1C). The incrustation and redness scores of mouse ears in the DNFB-challenged group were higher than those in vehicle-treated ears (Figure 1D).
Increased epidermal thickening and marked inflammatory infiltration after DNFB challenge
Analysis of H&E-stained sections from the DNFB-treated skin showed increased epidermal thickening in ear skin compared to vehicle-treated skin (Figure 2A,B). The immunohistochemistry results showed that the infiltration of CD45+ (total hematopoietic cells), Ly6G+ (neutrophils), and F4/80+ (macrophages) cells in the ears was significantly higher in DNFB-treated skin than in vehicle-treated tissues (Figure 2C,D). Taken together, DNFB induced a typical symptom of ACD in mice.
Th-GM cell subset generation in the DNFB sensitization/challenge immunological model
We hypothesized that hapten-specific naïve Th precursor cells differentiate into GM-CSF-expressing effector cells during hapten sensitization/challenge immune responses. To test this hypothesis, Th cell differentiation was investigated in DNFB-sensitized/challenged or DNFB-sensitized/vehicle challenged ears with intracellular staining by flow cytometry (Supplemental Figure S3). After the challenge, there were approximately 4,000 CD4+ T cells and 1,898 effector CD4+ T cells per mouse on average (23.8-fold and 38.7-fold increases compared to vehicle-treated mice, respectively, Supplemental Figure S4). The production of GM-CSF+ IFN-γ– IL-17A– IL-22– in effector Th cells, that is, Th-GM, was more than 3-fold higher in DNFB-sensitized/challenged ears (25.6% in contrast to 8.3% in vehicle-challenged ears, Figure 3A,B). There was a nearly 10-fold expansion in the frequency of effector CD4 T cells in DNFB-challenged ears versus vehicle-challenged skins, indicating vigorousness of Th cell activation upon hapten challenge (Figure 4A,B). The changes in the Th-GM cell subset in the skin infiltrates were partially mirrored in the dLNs, although this increase was not as remarkable as that in the tissues (3.7-fold, Figure 4C,D). Thus, the Th-GM cell subset was successfully boosted in this hapten sensitization/challenge immunological model.
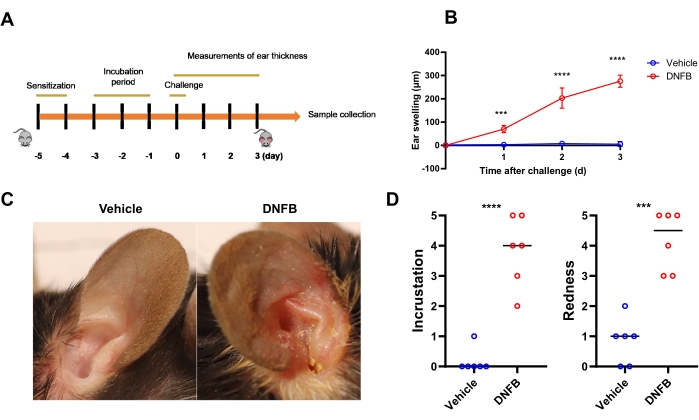
Figure 1: DNFB-induced dermatological inflammation in mice phenotypically resembles contact dermatitis in human. (A) Schematic illustration of the proposed DNFB experiment. C57BL/6J mice were sensitized by smearing DNFB on the abdomen on days -5 and -4, challenged with DNFB or vehicle on the ear on day 0, and assessed on days 1, 2, and 3. (B) Ear swelling in vehicle-treated or DNFB-challenged mice (n = 6 per group). (C) Representative photographs of whole ears in vehicle- or DNFB-challenged mice at day 3. (D) Incrustation and redness scores in ears challenged with DNFB or vehicle are shown (n = 6 per group). *** P < 0.001 and **** P < 0.0001 (mean ± SD). Data are representative of three independent experiments with 6 samples in each group. Abbreviation: DNFB = 2,4-dinitrofluorobenzene. Please click here to view a larger version of this figure.
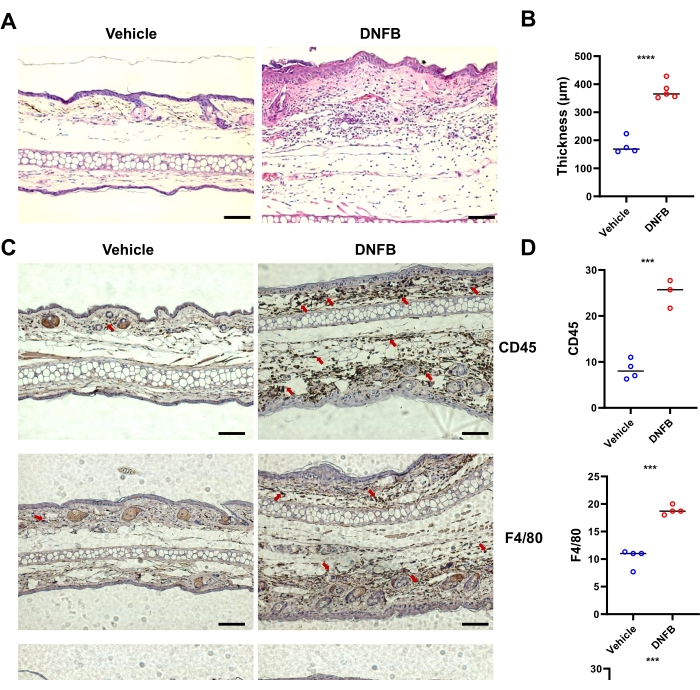
Figure 2: Typical alteration of histological inflammation in mice induced by DNFB sensitization/challenge. (A) H&E-staining of the ear skin from individual mice representative of the indicated groups. (B) Epidermal thickening measured on images of H&E staining shown by bar graph. (C) Immunohistochemistry of CD45, F4/80, and Ly6G in the ears of mice challenged with DNFB or vehicle. Red arrows indicate positively stained cells. (D) Quantification of CD45-, F4/80-, or Ly6G-positive cells by counting brown staining from three or four blind different sections per mouse. *** P < 0.001, and **** P < 0.0001 (mean ± SD). Data are representative of two independent experiments with 3-5 mice in each group. Scale bar = 1,000 µm (A, C). Abbreviations: DNFB = 2,4-dinitrofluorobenzene; H&E = hematoxylin and eosin. Please click here to view a larger version of this figure.
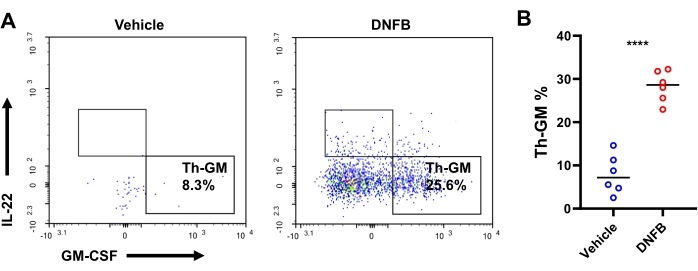
Figure 3: Th-GM subset in the ear skin of mice. (A) Flow cytometry of the Th-GM subset in the ear skin of mice challenged with DNFB or vehicle assessed 3 days after challenge. (B) Frequencies of Th-GM within effector CD4+ T cells. Each symbol represents an individual mouse. *** P < 0.001 and **** P < 0.0001 (mean ± SD). Data are representative of 6 samples in each group. Abbreviations: DNFB = 2,4-dinitrofluorobenzene; GM-CSF = granulocyte-macrophage colony-stimulating factor; Th-GM = GM-CSF-producing T helper. Please click here to view a larger version of this figure.
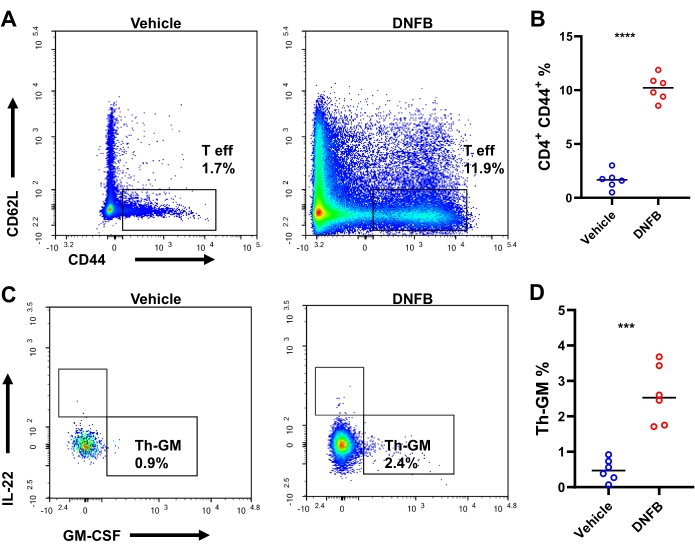
Figure 4: Th-GM subset in the ear draining lymph nodes of mice. (A) Flow cytometry of effector CD4+ T cells in the ear draining lymph nodes of mice challenged with DNFB or vehicle assessed 3 days after challenge. (B) Frequencies of effector CD4+ T cells within CD4+ T cells. (C) Flow cytometry of the Th-GM subset in the ear draining lymph nodes of mice challenged with DNFB or vehicle assessed 2 days after challenge. (D) Percentage of the Th-GM subset within effector CD4+ T cells in ear draining lymph nodes. *** P < 0.001 and **** P < 0.0001 (mean ± SD). Data are representative of 6 samples in each group. Abbreviations: DNFB = 2,4-dinitrofluorobenzene; GM-CSF = granulocyte-macrophage colony-stimulating factor; Th-GM = GM-CSF-producing T helper; T eff = effector CD4+ T cells. Please click here to view a larger version of this figure.
Supplemental Figure S1: The processing of mouse ear skin. (A) An ear cut off from a C57BL/6J mouse. (B) Splitting the dorsal and ventral sides of the ear. (C) Immerging the dorsal and ventral sides into digestion buffer. (D) Small ear leaflets with scissors. (E) Hair and white connective tissues left on the cell strainer after dissociation. Please click here to download this File.
Supplemental Figure S2: Processing of mouse auricular lymph nodes. (A) Dissecting auricular lymph nodes from a C57BL/6J mouse. The red arrow indicates the location of the auricular lymph nodes. (B) The auricular lymph node was transferred to a 70 µm cell strainer on top of the well of a 6-well plate before mincing. (C) The debris left on the cell strainers after mincing. Abbreviation: DNFB = 2,4-dinitrofluorobenzene. Please click here to download this File.
Supplemental Figure S3: Gating strategy used to analyze the Th-GM subset in auricular lymph nodes. First, the lymph cells were gated based on granularity and size (FSC low SSC low, P1), and singlets were selected in the FSC-A vs. FSC-H plot (P2). Then, dead cells were eliminated by gating on the FVS780– population (P3). Third, effector CD4 T cells were identified as the CD4+- CD44+ population (P5). Finally, the Th-GM subset was distinguished by intracellular cytokine expression, that is, IFN-γ– IL-17A– IL-22– GM-CSF+ (P7). Abbreviations: GM-CSF = granulocyte-macrophage colony-stimulating factor; Th-GM = GM-CSF-producing T helper; IFNγ = interferon-gamma. Please click here to download this File.
Supplemental Figure S4: Composition of total GM-CSF-expressing effector CD4 T cells in the tissue of mice after challenge. (A) Proportion (%) of GM-CSF-expressing effector Th cells among total GM-CSF+ CD4+ CD44+ cells. (B) Flow cytometry of GM-CSF, IFN-γ, and IL-17A expression gated in live CD4+ CD44+ T cells in the ears of mice after challenge. Abbreviations: GM-CSF = granulocyte-macrophage colony-stimulating factor; Th-GM = GM-CSF-producing T helper; IFNγ = interferon-gamma. Please click here to download this File.
Discussion
This protocol provides a simple in vivo assay to analyze the generation and expansion of the Th-GM cell subset. It is essential to utilize a T cell-mediated disease model in mice initiated by haptens or antigens, mimicking that activation in human. DNFB is a small-molecule hapten that is more economical and time-saving than peptide or protein antigens for triggering the T cell immune response in vivo18,19. During the course of the disease, we observed the largest magnitude of the ear swelling reaction challenged with DNFB compared to induction with other haptens, including 2,4,6-trinitrochlorobenzene (TNCB), oxazalone (OXA), or fluorescein isothiocyanate (FITC), at least in mice with a C57BL/6J genetic background (data not shown), although the other three reagents above were reported to generate contact sensitivity in mice14. Investigators must determine the appropriate mouse strain and haptens for full induction of disease.
A low yield of cells from the ears is one of the most frequently encountered problems when working with mouse tissues of a smaller size. This could be caused by to incomplete enzymatic digestion due to ineffective enzymatic activity of collagenase types or any expired reagents used for digestion. To overcome this, we used collagenase type IV for ear skin digestion as it was superior to other collagenases, including collagenase I, in efficiently dissociating inflamed ears. The higher viability of cells isolated from ears is beneficial for intracellular cytokine accumulation upon restimulation with PMA/ionomycin/protein transport inhibitor. Additionally, the concentration of calcium in RPMI 1640 medium used for restimulation can be adjusted to 1.5 mM with sterile 1 M calcium chloride stock, and the restimulation time can be extended to 6 h to achieve maximum production of cytokines in T cells20.
Multiparametric flow cytometry allows the accurate identification and quantification of the Th-GM cell subset in the context of autoimmune reactions. When acquiring data using a cytometer, unstained and single-stained samples were set up to determine appropriate PMT voltages and adjust compensations. For extended analysis of immune cells using flow cytometry, readers can refer to the recognized guidelines for the use of flow cytometry 21 for more information about compensation adjustment, antibody staining settings, and gating strategies. Notably, the Th-GM subset is deemed to be parallel to other traditional lineages and identified as GM-CSF+ IFN-γ– IL-17A– IL-22– in effector CD4 T cells (Supplemental Figure S1). This gating strategy should be applied to analyze the flow cytometry data for the investigation of Th-GM cell biology.
In summary, this paper presents a protocol to analyze the novel Th-GM lineage in DNFB-induced murine contact hypersensitivity using flow cytometry. This method can be used to further study the biology of Th-GM cells and pharmacological research of therapeutic strategies centered on GM-CSF in various conditions, such as allergic contact dermatitis.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81602763, 81803142, 82003347), the Excellent Researcher Program of China Postdoctoral Science Foundation (No. 2017T100700), and the Regular Researcher Program of China Postdoctoral Science Foundation (No. 2016M592673). The authors would like to thank Yan Wang and Meng-Li Zhu (Core Facilities of West China Hospital, Sichuan University) for technical support of flow cytometry in this study.
Materials
| 2,4-dinitrofluorobenzene | BT REAGENT | P0001746 | CAS NO: 70-34-8 |
| Acetone | CHRON CHEMICALS | / | 67-64-1 |
| anti-CD4 antibody | Biolegend | 300506 | 1:100 Diluted |
| anti-CD44 antibody | Biolegend | 103012 | 1:100 Diluted |
| anti-CD62L antibody | Biolegend | 104417 | 1:100 Diluted |
| anti-GM-CSF antibody | BD Bioscience | 554507 | 1:100 Diluted |
| anti-IFN-γ antibody | Biolegend | 505836 | 1:100 Diluted |
| anti-IL-17A antibody | BD Bioscience | 563354 | 1:100 Diluted |
| anti-IL-22 antibody | Biolegend | 516411 | 5 µL/test |
| CD45 | Biolegend | 103101 | 1:200 Diluted |
| Chloral hydrate | CHRON CHEMICALS | / | 302-17-0 |
| Dial thickness gauge (0.01 mm type) | PEACOCK | G-1A | / |
| DMSO | LIFESCIENCES | D8371 | 67-68-5 |
| EDTA Na2 | Solarbio | E8030 | 6381-92-6 |
| F4/80 | Biolegend | 123102 | 1:200 Diluted |
| Fixable Viability Stain 780 | BD Bioscience | 565388 | 1:1,000 Diluted, viability dye |
| Flow cytometer | BD Bioscience | BD FACS ARIA II SORP | / |
| GraphPad Prism | GraphPad Software | Prism 7 | Software for statistics and graphing |
| Intracelluar Fixtation and Permeablization Buffer Set | Thermo Fisher | 88-8824-00 | prepared freshly |
| Ionomycin | Sigma-Aldrich | 407951 | CAS NO: 56092-81-0 |
| Ly6G | Biolegend | 127602 | 1:200 Dilutied |
| NovoExpress | Agilent | / | Software for flow cytometry data analysis; https://www.agilent.com.cn/zh-cn/product/research-flow-cytometry/flow-cytometry-software/novocyte-novoexpress-software-1320805 |
| Olive oil | YUANYE BIO | S30503 | 8001-25-0 |
| PMA | Sigma-Aldrich | P8139 | CAS NO: 16561-29-8 |
| Protein Transport Inhibitor (Containing Brefeldin A) | BD Bioscience | 555029 | 1 µL/mL |
Referências
- Rasouli, J., et al. A distinct GM-CSF(+) T helper cell subset requires T-bet to adopt a TH1 phenotype and promote neuroinflammation. Science Immunology. 5 (52), (2020).
- Galli, E., et al. GM-CSF and CXCR4 define a T helper cell signature in multiple sclerosis. Nature Medicine. 25 (8), 1290-1300 (2019).
- Komuczki, J., et al. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1beta. Immunity. 50 (5), 1289-1304 (2019).
- Sheng, W., et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Research. 24 (12), 1387-1402 (2014).
- Herndler-Brandstetter, D., Flavell, R. A. Producing GM-CSF: a unique T helper subset. Cell Research. 24 (12), 1379-1380 (2014).
- Lu, Y., Fu, X. Y., Zhang, Y. In vitro differentiation of mouse granulocyte-macrophage-colony-stimulating Factor (GM-CSF)-producing T Helper (THGM) Cells. Journal of Visualized Experiments: JoVE. (139), e58087 (2018).
- Hu, Y., et al. Interleukin-1beta-induced IRAK1 ubiquitination is required for TH-GM-CSF cell differentiation in T cell-mediated inflammation. Journal of Autoimmunity. 102, 50-64 (2019).
- Reynolds, G., et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Annals of the Rheumatic Diseases. 75 (5), 899-907 (2016).
- Al-Mossawi, M. H., et al. Unique transcriptome signatures and GM-CSF expression in lymphocytes from patients with spondyloarthritis. Nature Communation. 8 (1), 1510 (2017).
- Wicks, I. P., Roberts, A. W. Targeting GM-CSF in inflammatory diseases. Nature Reviews Rheumatology. 12 (1), 37-48 (2016).
- Scheinman, P. L., et al. Contact dermatitis. Nature Reviews Disease Primers. 7 (1), 38 (2021).
- Pesonen, M., Koskela, K., Aalto-Korte, K. Contact urticaria and protein contact dermatitis in the Finnish Register of Occupational Diseases in a period of 12 years. Contact Dermatitis. 83 (1), 1-7 (2020).
- Vocanson, M., Hennino, A., Rozieres, A., Poyet, G., Nicolas, J. F. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 64 (12), 1699-1714 (2009).
- Gaspari, A. A., Katz, S. I., Martin, S. F. Contact hypersensitivity. Current Protocols in Immunology. 113, 1-7 (2016).
- Manresa, M. C. Animal models of contact dermatitis: 2,4-dinitrofluorobenzene-induced contact hypersensitivity. Methods in Molecular Biology. 2223, 87-100 (2021).
- Kim, J. H., et al. CD1a on Langerhans cells controls inflammatory skin disease. Nature Immunology. 17 (10), 1159-1166 (2016).
- Canene-Adams, K. Preparation of formalin-fixed paraffin-embedded tissue for immunohistochemistry. Methods in Enzymology. 533, 225-233 (2013).
- Achuthan, A., et al. Cytokine-induced acute inflammatory monoarticular arthritis. Methods in Molecular Biology. 1784, 215-223 (2018).
- Stromnes, I. M., Goverman, J. M. Active induction of experimental allergic encephalomyelitis. Nature Protocols. 1 (4), 1810-1819 (2006).
- Kruisbeek, A. M., Shevach, E., Thornton, A. M. Proliferative assays for T cell function. Current Protocols in Immunology. , (2004).
- Cossarizza, A., et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). European Journal of Immunology. 49 (10), 1457 (2019).

.
