Inducing Polyp Bail-out in Coral Colonies to Obtain Individualized Micropropagates for Laboratory Experimental Use
Summary
Polyp bail-out is a process induced by acute stress, in which coral polyps digest the tissue connecting them to their colony and detach from it to live as individuals. The present protocol describes how to induce coral micropropagation by bail-out using hypersaline or calcium-free seawater treatments.
Abstract
Corals are colonial animals formed by modular units called polyps. Coral polyps are physiologically linked and connected by tissue. The phenomenon of polyp bail-out is a process induced by acute stress, in which coral polyps digest the tissue connecting them to the rest of the colony and ultimately detach from the skeleton to continue living as separate individuals. Coral biologists have acknowledged the process of polyp bail-out for years, but only recently the micropropagates generated by this process have been recognized as a model system for coral biology studies. The use of polyp bail-out can create a high number of clonal units from a single coral fragment. Another benefit is that single polyps or patches of polyps can be easily visualized under a microscope and maintained in highly standardized low-cost environments such as Petri dishes, flasks, and microfluidic chips. The present protocol demonstrates reproducible methods capable of inducing coral micropropagation and different approaches for maintaining the single polyps alive in the long term. This methodology was capable of successfully cultivating polyps of the coral species Pocillopora verrucosa for up to 8 weeks after bail-out, exhibiting the practicality of using individual coral polyps for coral research.
Introduction
Scleractinian or reef-building corals are cnidarians capable of forming carbonate skeletons, creating reefs, and structurally complex ecosystems that can be found from deep to shallow water environments1. Tropical coral reefs host high biodiversity and provide essential ecosystem services, such as coastal protection and fisheries maintenance2. Most shallow-water reef-building corals rely on a mutualistic relationship with algae of the family Symbiodiniaceae, which provide the energy that corals require to build their skeletons. The symbiosis between the coral and the algae can be broken by environmental stress, causing coral bleaching3,4,5,6. Recent temperature anomalies have caused major coral bleaching events around the world, leading to mass coral mortality and permanent reef degradation7,8,9,10,11. As this phenomenon is based on the expulsion of symbionts by post heat stress-associated cellular mechanisms, such as apoptosis, autophagy, and exocytosis, coral bleaching can be described as a cellular process that has ecosystem-scale consequences5,6,12, which means having in vitro cultures of coral cells or tissues would be applicable to study this phenomenon closely.
Due to the importance of coral reefs and the major threats they have been facing, particularly in the past two decades2, corals have become the focus of research for protection and restoration purposes worldwide13. However, the development of approaches and experimental systems that are reliable, reproducible, and offer minimal environmental impact to study corals is a major struggle in this field.
Micropropagation is defined as the in vitro proliferation of an organism's genotype by culturing its biological material in controlled vessels14,15. The culturing of cells, tissues, and organs has been crucial for plant and animal biology over recent decades. It allows the mass reproduction of organisms in laboratories, the rapid assessment of different treatments (such as drugs and pharmaceuticals), and the direct study of cell function14,15,16,17. In general, in vitro models have been useful for complementing and deepening the studies of different organisms under better-controlled physical and chemical conditions. Due to the advantages of in vitro culturing techniques, different animal cell and tissue culture technologies have been developed, optimized, and used as important tools in many research fields, where multiple cell lines have been studied and commercialized for numerous applications16,17,18.
Many advancements in the knowledge of cell and tissue culture have been made since the first animal tissue culture in 188217, such as the use of natural and synthetic media, the invention of established cell lines, and the development of 3D media to cultivate a multitude of cell types in a better way16,17,18,19. However, the field of cell biology has mostly focused on a select group of model organisms, while many taxa still do not have well-established in vitro cultures of cells, tissues, or organs20. For instance, in coral research, no immortalized cell lines have been extensively used for research, constraining coral cell research to the use of primary cell cultures. These cultures have viability limited to a few weeks21, with no studies recording the survival of individual cells from all coral tissues for more than 13 days until the beginning of 202122. The first report of sustainable coral cell lines to be published was with Acropora tenuis cells that lived up to 6 months, and the utility of these cells for future research remains to be explored23.
To overcome the limitations in culturing coral cell cultures and to maintain a laboratory culture that preserves the overall tissue organization of corals, the use of isolated polyps has recently been proposed as a model for coral biology research24,25. Polyps are the anatomical units of corals, and each of them has a mouth located in the center of their oral disk and is connected to other polyps by the coenosarc in its aboral region26. The separation of live polyps occurs naturally by the process of polyp bail-out, in which acute stress causes the digestion of the coenosarc between the polyps, which can then detach from the colony's skeleton25,27,28. This phenomenon has been reported to occur in a variety of taxa, including octocorals29,30,31, black corals32, and scleractinian corals25,27,28,32,33, and has been linked to multiple environmental stressors, such as lack of calcium in water24,34, increased acidity35, hyperosmotic conditions25,27,32,36, high temperatures36,37, starvation33, air exposure25,30, and insecticide contamination28,38. Polyp bail-out has been, for example, reported in pocilloporid corals19, which are widely distributed across the world and are commonly used as models in coral research. Species belonging to this group, such as Pocillopora damicornis and Styllophora pistillata, have generated approximately 30-40 micropropagates from a 5 mm fragment25. This number emphasizes the advantage of using polyp bail-out as a method for coral micropropagation, as it creates the possibility of generating many genetically identical individuals from a small piece of coral. The use of isolated polyps for research also has the same advantages as cell cultures regarding the possibility of being cultured in controlled lab environments, such as flasks and Petri dishes. Additionally, microfluidic platforms to maintain live polyps have demonstrated that these micropropagates can be kept in relatively cheap and easy-to-reproduce environments, with controlled water flow, surface, and temperature24,25. These microfluidics platforms can also be used to visualize live coral structures under a microscope directly24,25.
In the present article, we summarize and demonstrate the techniques that have been developed to isolate individual coral polyps from their colonies, showing how to maintain them in laboratory conditions for long-term culture. The methods discussed include polyp bail-out through hyperosmotic conditions by evaporation and pumping high-salinity seawater and incubation in calcium-free seawater.
Protocol
For the present study, a colony belonging to the Pocillopora verrucosa coral species was collected from the Al Fahal reef (22.305118 N; 38.964568 E) by SCUBA by diving using a hammer and chisel. The genus of the colony was identified morphologically, and its species was classified as P. verrucosa based on previously published work, including Pocillopora from the Red Sea, indicating that, from a genetic point of view, the species present in this area is P. verrucosa39,40. Al Fahal reef is not part of a protected environmental area, and no special permits were needed for coral collection. The colony was kept in a 300 L aquarium for a month before being fragmented and having its polyps "bailed-out". The aquarium was kept at 26 °C with two aquarium heaters, three pumps, and two light sources (see Table of Materials), maintaining a 12 h light cycle. The temperature of the aquarium was maintained by connecting each one of the two heaters to a temperature controller. Light emission was programmed to start at 6 AM and finish at 6 PM, producing an irradiance curve that peaked at 12 PM with 230 µmol photons m−2s−1.
1. Polyp bail-out by high salinity after water evaporation
NOTE: This method was adapted from Shapiro et al.25. If using different species than Pocillopora verrucosa, the size of the polyps should be taken into account before determining the size of the fragment to be cut.
- Cut small fragments (less than 1 cm in length) from a coral colony using diagonal cutting pliers (see Table of Materials).
NOTE: Adapt the cutting tools according to the coral species being used. Diagonal pliers are useful for cutting corals with slim branches. In the current study, one fragment was cut for each treatment, but the number and size of fragments can vary depending on the number of polyps desired. The size of the cut fragments must not exceed the depth of the water after evaporation, so the corals stay completely inside the water after the process is done. - Place the cut fragments into small Petri dishes filled with 12 mL of seawater to which the coral colony has been acclimated to. This can be artificial seawater (see Table of Materials) or filtered seawater at the same salinity as the coral colony was inserted into before fragmentation.
NOTE: It is important to cover the fragment completely with water. If 12 mL in a Petri dish is not enough to cover the cut fragment, optimize the container and the volume accordingly. In the present study, the water utilized was collected from the Red Sea, and its salinity was 40 PSU, measured by a multiparameter meter (see Table of Materials). - Leave the plate open for ~24 h at ambient temperature so that the water can evaporate and its salinity can gradually increase. When tissue digestion is observable between polyps, proceed to Step 1.4.
NOTE: After the incubation time has passed, the salinity of the water must be approximately 40% higher than in the beginning, and the polyps need to be ready to be detached from the skeleton. - Using a 1 mL transfer pipette, create a gentle flow close to the coral tissue. The flow will slowly help the complete detachment of the polyps that have already digested the tissue around them from the skeleton.
NOTE: When the water flow is created with the pipette, separate polyps or groups of polyps must come off the skeleton as flakes. If a cloudy substance comes off instead, it indicates tissue death or disintegration, which means the corals were incubated for too long or in a too stressful condition (in this case, high salinity). It is very important to make a gentle flow of water to detach the polyps, as a stronger flow may cause physical damage to them. - Slowly exchange the water in the Petri dish with isosmotic water (use the same water as in Step 1.2.) using a pipette. A 50% water exchange over 10 min is enough to acclimate the polyps back to a non-stressful salinity condition.
NOTE: As coral colonies of the pocilloporid species Pocillopora verrucosa from the Red Sea were used, tests were carried out to determine under which specific conditions the corals from this unique environment would bail-out. It is important to highlight the high salinity of the Red Sea when compared to most other reef environments.
2. Polyp bail-out by high salinity seawater supply
NOTE: This method was adapted from Chuang et al.27.
- Prepare 3 L of high-salinity seawater by adding NaCl to seawater until the salinity increases by 85%, measured by a salinity probe.
NOTE: In the current study, a 74 PSU high-salinity seawater was prepared from 40 PSU seawater from the Red Sea. - Cut small fragments of coral as described in Step 1.1.
- Place the cut fragments into a 10 L container filled with 3 L of isosmotic seawater (in this case, seawater with non-stressful salinity for the corals, the same as used in Step 1.2) connected to a peristaltic pump (see Table of Materials).
NOTE: For better maintenance of the coral health, temperature controllers, air pumps, and lights can be added to simulate the same aquarium conditions as the colony was previously exposed to. During the incubation in the present work, the coral fragments were kept in containers at 26 °C, under a 12 h daily light cycle. The water movement and homogenization of temperature were maintained by air pumps. - Fill the container containing coral fragments with the high-salinity seawater prepared in Step 2.1 using the peristaltic pump for 24 h at a 126 mL/h rate. Add a total of 3 L of water at an increasing salinity of approximately 40%.
NOTE: High-salinity water can be produced simply by adding NaCl to seawater until reaching the intended salinity. - Create a gentle water flow with a pipette to release the polyps from the skeleton (Step 1.4.).
- Slowly exchange the water in which the polyps are contained for isosmotic water (Step 1.5.). Next, proceed to Step 4 or Step 5.
3. Polyp bail-out by calcium-free seawater incubation
NOTE: This method was adapted from Pang et al.24.
- Prepare calcium-free artificial seawater (CaFSW) solution by adding 26.29 g of NaCl, 0.872 g of KCl, 2.16 g of MgSO4, 11.94 g of MgCl2, 3.42 g of Na2SO4, and 0.286 g of NaHCO3 (see Table of Materials) to 1 L of deionized water.
NOTE: This solution was adjusted from previous protocols to be better suited for corals adapted to a salinity of 40 PSU. For corals adapted to other salinity conditions, use the same proportion of salts but adjust the total mass of solutes to obtain the salinity that is better suited to the corals in use. - Cut small fragments of corals as described in Step 1.1.
- Submerge the coral fragments in Petri dishes filled with the CaFSW prepared previously (Step 3.1.) and incubate them in orbital incubators at an 80 rpm rotation speed for 3 h.
NOTE: Again, it is important to cover the fragment completely with water. If a Petri dish is not enough to cover the fragment, optimize the container and the volume according to the experimental need. - Transfer the fragments to Petri dishes or 6-well plates filled with 20% Dulbecco's Modified Eagle Media (DMEM) and 100 mg/mL of ampicillin solution (see Table of Materials) prepared with 40 PSU artificial seawater. Incubate the fragments at 26 °C and 80 rpm, exchanging the media every day until tissue digestion is observable between the polyps and individual polyps start detaching from the skeleton.
NOTE: In most cases, tissue digestion is complete within 20 h of incubation in this media, and no exchanges are needed. - Transfer the polyps to sterilized seawater for initial recovery using a pipette. After 1 h of incubation, proceed to Step 4 or Step 5.
4. Polyp maintenance in Petri dishes
- Once the polyps are returned to filtered seawater with non-stressing salinity, select viable polyps by observing tissue integrity and movement caused by ciliary flow under a stereomicroscope.
- Place the selected polyps in a glass or plastic Petri dish and cover the Petri dish with a plankton net (200 µm mesh size, see Table of Materials) so that the polyps do not float away from the dish.
NOTE: The size of the mesh net can vary according to the size of the polyps. It is important to note that the nets must have pores that are smaller than the polyps and allow water exchange and light penetration. - Place the Petri dish inside an aquarium with the appropriate conditions for the coral species used.
NOTE: In the present study, the conditions were 40 PSU filtered seawater at 26 °C and a 12 h light cycle. - Open the Petri dishes at least once a week to renew the water and clean the dishes. This step is important to eliminate algae overgrowth that can compete with or damage the coral polyps by locally releasing toxic compounds.
5. Polyp maintenance in incubators
- Select viable polyps as described in Step 4.1.
- Place the polyps in 75 cm2 surface cell flasks filled with 50 mL of isosmotic seawater using transfer pipettes.
NOTE: In the current work, filtered seawater collected from the Red Sea was used for polyp maintenance. Water with the same characteristics as the one used in Step 1.2 can be used. - Close the flasks and move them to incubators. Set the incubators to 12 h light cycles at 26 °C and 40 rpm. Exchange 50% of the water volume every 4 days and transfer the content to clean flasks when/if they get full of algae or biofilm on the walls.
NOTE: The images were captured with a fully apochromatic zoom system equipped with an HD camera (see Table of Materials) for the representative results.
Representative Results
Polyp bail-out was induced in coral fragments belonging to a single colony of the species P. verrucosa following three different methods (Figure 1). Bail-out induced by high salinity after water evaporation was complete after 24 h of incubation of coral fragments in Petri dishes at ambient temperature filled with water initially at 40 PSU, which then reached a final salinity of 59 PSU once the process was over (Figure 2A–C,I). Bail-out by saltwater supply was also reached after 24 h of incubation in water initially at 40 PSU that reached a salinity of 52 PSU after 12 h and 59 PSU at the end of the process, after 24 h (Figure 2D–F,I). In both experiments, an increase in salinity was responsible for the induction of tissue digestion by the polyps.After 12 h, the high salinity condition caused the contraction of the polyps in conjunction with the gradual thinning of the coenosarc, ultimately causing the final detachment of the polyps after 24 h. The bail-out induction through incubation in calcium-free seawater was complete after a 3h incubation in CaFSW followed by a 20h incubation in the 20% DMEM media (Figure 2G–H). The tissue was detached from the skeleton in all three methods after pushing it away with a pipette until individualized coral polyps (Figure 3A–C) and "tissue balls" were generated.
After the detachment, the polyps from all three methods were collected and allowed to recover in seawater before being allocated to Petri dishes covered with nets or cell flasks. The polyps obtained from the evaporation and the water supply methods were maintained in Petri dishes inside aquariums and survived for 6 weeks and 8 weeks, respectively (Figure 3D and Figure 3F). These micropropagates retained the usual anatomy of polyps, presenting tentacles, basal disks, and mouths1. The polyps obtained through the incubation in calcium-free seawater had a short life span, surviving up to 1 day, after which their tissue dissociated. Polyps obtained from the seawater evaporation method kept in cell culture flasks inside incubators survived for up to 3 weeks without dissociation of tissues (Figure 3F). In all cases, even though the polyps could not attach to the substrate, they were visually healthy and maintained their color, with zooxanthellae cells still being visible inside their tissues1.
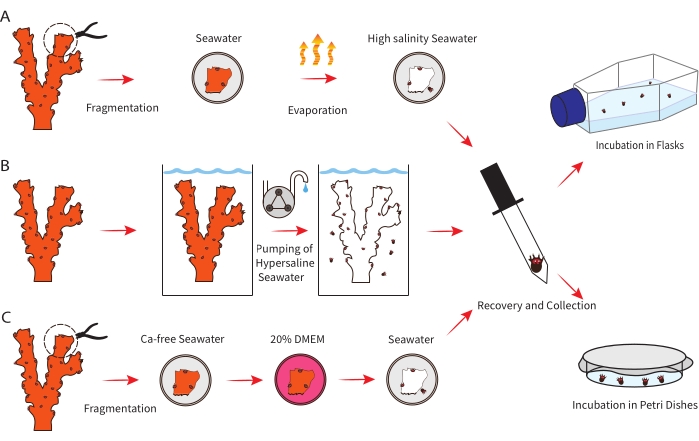
Figure 1: Schematic representation of the three different tested methodologies for polyp bail-out induction (left) followed by the illustration of two methods to maintain the acquired polyps in lab conditions (right). (A) Representation of the methodology for polyp bail-out by water evaporation. (B) Representation of the methodology for polyp bail-out by high salinity seawater supply. (C) Representation of the methodology for polyp bail-out by calcium-free seawater incubation. Please click here to view a larger version of this figure.
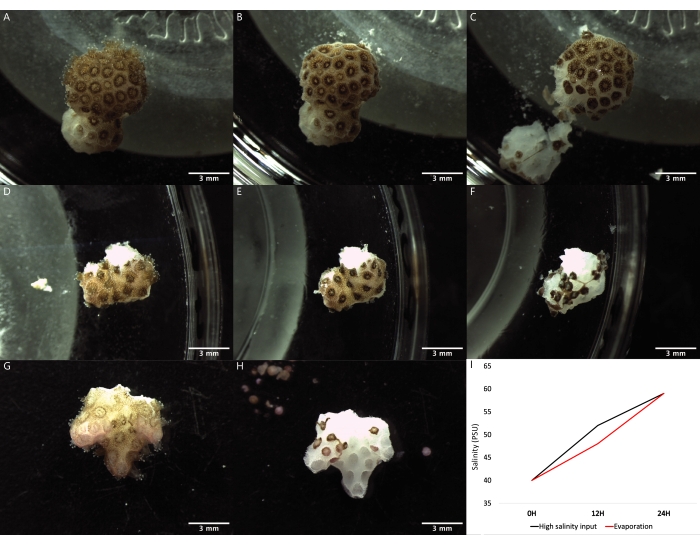
Figure 2: Images of the polyp bail-out process induced by three different methodologies using fragments of the coral species P. verrucosa. (A–C) A coral fragment at 0 h, 12 h, and 24 h after incubation, respectively, in a Petri dish using the water evaporation method. (D–F) A coral fragment at 0 h, 12 h, and 24 h after incubation, respectively, using the high salinity seawater supply method. (G,H) Coral fragments exposed to the calcium-free seawater incubation method before and after, respectively. The incubations in calcium-free artificial seawater were for 3 h and in 20% DMEM for 21 h. (I) Graphical representation of the salinity values in PSU of the seawater over time during the water evaporation and high-salinity seawater supply bail-out induction methods. Please click here to view a larger version of this figure.
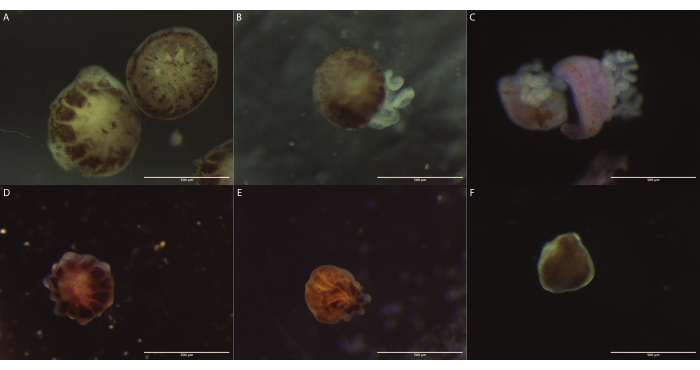
Figure 3: Images of P. verrucosa polyps obtained from the three demonstrated bail-out induction procedures. (A–C) The images of the polyps obtained from the evaporation, saltwater supply, and calcium-free seawater methods, respectively, were captured immediately after they were detached from the skeleton. (D) The image of a coral polyp obtained from the evaporation method after surviving 6 weeks in a Petri dish. (E) The image of a coral polyp obtained from the saltwater supply method after surviving 8 weeks in a Petri dish. (F) The image of a coral polyp obtained from the evaporation method after surviving 3 weeks in a cell culture flask. Please click here to view a larger version of this figure.
Discussion
The polyps survival rate after being submitted to bail-out processes and the time needed for the process to be completed vary among previously reported research25,33,41, which is possibly explained by the different experimental approaches applied in each study. For instance, different coral species, or even corals from the same species but acclimated to different environmental conditions (e.g., corals from the Red Sea), present different thresholds to salinity levels. The method of bail-out selected and the laboratory/aquarium conditions also play important roles in the results. In some cases, the maintenance of coral micropropagates under laboratory conditions has surpassed the survival time of coral cell cultures by reaching months of survival in azooxanthellate33,41 and zooxanthellate25 corals. The time for the polyp bail-out process to be complete has also varied in different studies, ranging from a few hours25,27,30 to weeks35 of incubation exposed to the stressor responsible for causing bail-out. Another variable to be taken into account when studying polyp bail-out is the recovery of the polyps after exposure to the acute stress that triggered their release. It is still debatable if polyps after bail-out are in a good enough condition to be used as models to study coral biology. The recovery of their tissues after the degradation of the coenosarc is a matter of concern when using these micropropagates. However, polyps in many studies, including the present, have been able to present zooxanthellae cells inside their tissues and external morphologies with intact oral-aboral polarization and tentacles weeks after bail-out25,27,32,36. Previous studies have also found that, after being relieved from acute stress, released coral polyps exposed to highly saline or heated seawater have been able to recover the expression of genes related to processes such as apoptosis, proteolysis, and cell division to levels similar to the ones found before bail-out32,36 and even to increase the expression of genes related to tissue healing36.
Concerning the difference in survival between methods, it is important to highlight that this time can vary among different experiments even if the same techniques are used, and it can be related to the health of the fragments used and the proper maintenance of the polyps after the bail-out process. In the case of the bail-out through calcium-free seawater incubation, the polyp survival was limited to 1 day. Thus, it can be concluded that the method is not well suited for the long-term survival of the species studied, or a better adaptation of the technique for P. verrucosa corals from the Red Sea must be made. The reported results showed a longer survival time was obtained with the methods based on the gradual increase in salinity, when the polyps were subjected to the dripping of high-salinity water. This method can deliver a more controlled increase in salinity than the evaporation method, at the same time as not being responsible for an increase in the concentration of other substances found in the seawater, including the coral's metabolic waste, which is potentially toxic for the organism. For all these reasons, this method has been suggested as a safer alternative for maintaining healthy polyps27. Although this method has been hypothesized to be safer for polyp health and capable of producing polyps that live longer, a fact that was corroborated in this current publication, additional surveys are needed to confirm it. Both high salinity-induced bail-out experiments demonstrated a complete detachment of polyps after the salinity reached 59 PSU in 24 h. If salinity is increased past the level at which the bail-out is complete, further stress will be caused to the polyps, reducing their chance of surviving and recovering from the acute stress treatment. Therefore, it is not recommended to maintain the polyps longer in such salinity levels. When performing the bail-out induction method by exposure to calcium-free seawater, a complete detachment was obtained from a 3 h incubation in calcium-free artificial seawater, meaning further exposure to this medium is also not recommended.
To address the methods that were more adequate for the study of coral polyps in laboratory/in vitro surveys, this study focused only on three procedures that took close to 24 h for the bail-out process to be complete and were used in studies that involved the long-term maintenance of coral polyps from scleractinian corals. Other methods reported to take significantly longer than this time were not engaged. The settlement of polyps to a substrate was not attempted in this study, which focused on producing polyps that could be transferred to different environments or easily collected for analyses using disposable pipettes. The results demonstrate that polyps from the coral species P. verrucosa were kept alive, with associated zooxanthellae cells, healthy visual status, and a preserved gross external anatomical structure, for up to 8 weeks, even without attachment to a substrate. These results indicate that more biological replicates can be generated from single coral fragments using some of the techniques demonstrated in this study. Such biological replicates can be kept in controlled environments (such as Petri dishes and cell flasks) and maintained in laboratory conditions for month-long experiments and used for several purposes.
Since the first incidental descriptions of polyp bail-out42,43, new protocols have been established to find more standardized methods for inducing polyp release and maintaining such polyps alive, which can be used for future research applications. These include investigating different aspects associated with the coral holobiont physiology44 and host-microbiome interactions45, the molecular mechanisms involved in coral bleaching5,25, and the health, resilience, and protection of the coral holobiont12,13,46,47. Moreover, released coral polyps can be used for applications outside the realm of research and have been suggested to be useful for creating propagules that can attach to a substrate and grow, potentially creating multiple coral individuals that can be used for restoration purposes once standardized protocols for bail-out become widespread28. Overall, although more in-depth experiments using bailed-out polyps should be performed to standardize the methodology, it has been shown that polyp bail-out is a reproducible approach that can be applied as a tool in coral research for several purposes.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Adam Barno and Francisca Garcia for their support in the experiments and monitoring of the coral polyps. We also thank the KAUST Coastal & Marine Resources Core Lab for their assistance regarding the aquarium maintenance and infrastructure. The study was funded by KAUST grant number BAS/1/1095-01-01.
Materials
| 5560 Conductivity/Temperature Probe | YSI | 5560 | Conductivity probe used with the ProQuatro Multiparameter meter |
| Ace 5 in. Alloy Steel Diagonal Pliers | Ace Hardware | 2004083 | Used to cut coral fragments |
| Ampicillin sodium salt | Sigma-Aldrich | A9518 | Used in DMEM medium. |
| DMEM (1x) Dulbecco's Modified Eagle Medium | Gibco | 41965-039 | Used for incubating coral fragments in the calcium-free polyp bail-out method |
| Fisherbrand Petri Dish, Stackable Lid 60 mm x 15 mm Sterile, Polystyrene | Thermo Fisher Scientific | FB0875713A | Petri dish used for bail-out by evaporstion and for keeping polyps inside an aquarium. |
| Heizer Titanrohr Heizstab SW MW 600 Watt | Schego | 548 | Heaters used in aquarium |
| Leica Application Suite Version 4.2 | Leica Microsystems | NA | Software used for image capture in demonstrative results |
| Leica IC80 HD | Leica Microsystems | 12730216 | Camera used to take demonstrative results pictures |
| Leica MDG33 | Leica Microsystems | 10 450 123 | Stereoscope stand used to take demonstrative results pictures |
| Leica Z6 APO | Leica Microsystems | NA | Macroscope used to take demonstrative results pictures |
| Magnesium Chloride | Thermo Fisher Scientific | 7487-88-9 | Used for preparing calcium-free artificial seawater. |
| Magnesium Sulfate Anhydrous | Sigma-Aldrich | 7791-18-6 | Used for preparing calcium-free artificial seawater. |
| Masterflex I/P Easy-Load Pump Head for Precision Tubing, White PPS Housing, SS Rotor | Masterflex | HV-77602-10 | Peristaltic pump head. |
| Masterflex L/S Precision Modular Drives with Benchtop Controller | Masterflex | EW-07557-00 | Peristaltic pump drive used for pumping high salinity seawater. Can be substituted for any peristaltic pump capable of mainaining water flow as described in protocol. |
| Masterflex L/S Precision Pump Tubing, Platinum-Cured Silicone, L/S 16; 25 ft | Masterflex | HV-96410-16 | Tubing for peristaltic pump. |
| Millex 33 mm PVDF 0.22 µm Sterile RUO | Sigma-Aldrich | SLGVR33RS | Used to filter artificial sea water. |
| Nunc EasYFlask 75 cm2 Nunclon Delta Surface | Thermo Fisher Scientific | 156499 | Flask usually used for cell culture used for polyp culture. |
| Orbital shaker, Advanced 5000, VWR | VWR | 444-2916 | Shaker used inside incubator. |
| Percival Incubator – I-22VL | Percival | NA | Incubator used for maintaing corals kept in cell flasks. |
| Plankton net 200 µm mesh size | KC Denmark | NA | Used for covering petri dishes containing coral polyps. |
| Potassium Chloride | VWR Chemicals | 7447-40-7 | Used for preparing calcium-free artificial seawater. |
| ProQuatro Multiparameter Meter | YSI | 606950 | Used for measuring salinity thoughout the protocol |
| RADION XR15 G5 PRO | Ecotech | NA | Lights used in aquarium |
| Red Sea Salt Premium grade, moderate Alkalinity |
Red Sea | NA | Used to prepare 40 PSU artifical sea water. |
| Sodium Bicarbonate | Sigma-Aldrich | 144-55-8 | Used for preparing calcium-free artificial seawater. |
| Sodium Chloride | Sigma-Aldrich | S3014 | Used for preparing calcium-free artificial seawater. |
| Sodium Sulfate Anhydrous | VWR Chemicals | 7757-82-6 | Used for preparing calcium-free artificial seawater. |
| TRD 112 thermostat | Schego | NA | Thermostat used in aquarium |
| Turbelle Nanostream 6025 | Tunze | 6025 000 | Pumps used in aquarium |
Referências
- Goffredo, S., Dubinsky, Z. . The Cnidaria, Past, Present and Future: The World of Medusa and her Sisters. , (2016).
- Knowlton, N., et al. Rebuilding coral reefs: a decadal grand challenge. International Coral Reef Society and Future Earth Coasts. , 56 (2021).
- Brown, B. Coral bleaching: causes and consequences. Coral Reefs. 16 (1), 129-138 (1997).
- Douglas, A. Coral bleaching–how and why. Marine Pollution Bulletin. 46 (4), 385-392 (2003).
- Nielsen, D. A., Petrou, K., Gates, R. D. Coral bleaching from a single cell perspective. The ISME Journal. 12 (6), 1558-1567 (2018).
- Oakley, C. A., Davy, S. K. . Coral Bleaching: Patterns, Processes, Causes and Consequences. Coral Bleaching Book. , 189-211 (2018).
- Donner, S. D., Heron, S. F., Skirving, W. J. Future scenarios: a review of modelling efforts to predict the future of coral reefs in an era of climate change. Coral Bleaching. , 159-173 (2009).
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research. 50 (8), 839-866 (1999).
- Hughes, T. P., et al. Global warming impairs stock-recruitment dynamics of corals. Nature. 568 (7752), 387-390 (2019).
- Moore, J. A., et al. Unprecedented mass bleaching and loss of coral across 12 of latitude in Western Australia in 2010-11. PLoS One. 7 (12), 51807 (2012).
- Duarte, G. A., et al. Heat waves are a major threat to turbid coral reefs in Brazil. Frontiers in Marine Science. 7, 179 (2020).
- Santoro, E. P., et al. Coral microbiome manipulation elicits metabolic and genetic restructuring to mitigate heat stress and evade mortality. Science Advances. 7 (33), (2021).
- Voolstra, C. R., et al. Extending the natural adaptive capacity of coral holobionts. Nature Reviews Earth & Environment. 2 (11), 747-762 (2021).
- Debergh, P. C., Read, P. E. . Micropropagation. 1, 1-13 (1991).
- Nitish, K., Reddy, M. P. In vitro plant propagation: a review. Journal of Forest and Environmental Science. 27 (2), 61-72 (2011).
- Bokhari, M., Carnachan, R. J., Cameron, N. R., Przyborski, S. A. Novel cell culture device enabling three-dimensional cell growth and improved cell function. Biochemical and Biophysical Research Communications. 354 (4), 1095-1100 (2007).
- Yao, T., Asayama, Y. Animal-cell culture media: history, characteristics, and current issues. Reproductive Medicine and Biology. 16 (2), 99-117 (2017).
- Merten, O. W. Introduction to animal cell culture technology-past, present and future. Cytotechnology. 50 (1-3), 1 (2006).
- Ravi, M., Paramesh, V., Kaviya, S. R., Anuradha, E., Solomon, F. D. P. 3D cell culture systems: advantages and applications. Journal of Cellular Physiology. 230 (1), 16-26 (2015).
- Goldstein, B., King, N. The future of cell biology: emerging model organisms. Trends in Cell Biology. 26 (11), 818-824 (2016).
- Lecointe, A., et al. Scleractinian coral cell proliferation is reduced in primary culture of suspended multicellular aggregates compared to polyps. Cytotechnology. 65 (5), 705-724 (2013).
- Nowotny, J. D., Connelly, M. T., Traylor-Knowles, N. Novel methods to establish whole-body primary cell cultures for the cnidarians Nematostella vectensis and Pocillopora damicornis. Scientific Reports. 11 (1), 1-9 (2021).
- Kawamura, K., Nishitsuji, K., Shoguchi, E., Fujiwara, S., Satoh, N. Establishing sustainable cell lines of a coral, Acropora tenuis. Marine Biotechnology. 23, 373-388 (2021).
- Pang, A. -. P., Luo, Y., He, C., Lu, Z., Lu, X. A polyp-on-chip for coral long-term culture. Scientific Reports. 10 (1), 1-9 (2020).
- Shapiro, O. H., Kramarsky-Winter, E., Gavish, A. R., Stocker, R., Vardi, A. A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nature Communications. 7 (1), 1-10 (2016).
- Tambutté, S., et al. Coral biomineralization: From the gene to the environment. Journal of Experimental Marine Biology and Ecology. 408 (1-2), 58-78 (2011).
- Chuang, P. -. S., Mitarai, S. Signaling pathways in the coral polyp bail-out response. Coral Reefs. 39 (6), 1535-1548 (2020).
- Schweinsberg, M., Gösser, F., Tollrian, R. The history, biological relevance, and potential applications for polyp bail-out in corals. Ecology and Evolution. 11 (13), 8424-8440 (2021).
- Rakka, M., et al. First description of polyp bail-out in cold-water octocorals under aquaria maintenance. Coral Reefs. 38 (1), 15-20 (2019).
- Wells, C. D., Tonra, K. J. Polyp bail-out and reattachment of the abundant Caribbean octocoral Eunicea flexuosa. Coral Reefs. 40 (1), 27-30 (2021).
- Coppari, M., et al. Unveiling asexual reproductive traits in black corals: polyp bail-out in Antipathella subpinnata. Coral Reefs. 39 (6), 1517-1523 (2020).
- Chuang, P. S., Ishikawa, K., Mitarai, S. Morphological and genetic recovery of coral polyps after bail-out. Frontiers in Marine Science. 8, 280 (2021).
- Serrano, E., Coma, R., Inostroza, K., Serrano, O. Polyp bail-out by the coral Astroides calycularis (Scleractinia, Dendrophylliidae). Marine Biodiversity. 48 (3), 1661-1665 (2018).
- Kopecky, E. J., Ostrander, G. K. Isolation and primary culture of viable multicellular endothelial isolates from hard corals. In Vitro Cellular & Developmental Biology-Animal. 35 (10), 616-624 (1999).
- Kvitt, H., et al. Breakdown of coral colonial form under reduced pH conditions is initiated in polyps and mediated through apoptosis. Proceedings of the National Academy of Sciences. 112 (7), 2082-2086 (2015).
- Gösser, F., Raulf, A., Mosig, A., Tollrian, R., Schweinsberg, M. Signaling pathways of heat-and hypersalinity-induced polyp bail-out in Pocillopora acuta. Coral Reefs. 40 (6), 1713-1728 (2021).
- Fordyce, A. J., Camp, E. F., Ainsworth, T. D. Polyp bail-out in Pocillopora damicornis following thermal stress. F1000Research. 6, 687 (2017).
- Wecker, P., et al. Exposure to the environmentally-persistent insecticide chlordecone induces detoxification genes and causes polyp bail-out in the coral P. Damicornis. Chemosphere. 195, 190-200 (2018).
- Schmidt-Roach, S., Miller, K. J., Lundgren, P., Andreakis, N. With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zoological Journal of the Linnean Society. 170 (1), 1-33 (2014).
- Gélin, P., Postaire, B., Fauvelot, C., Magalon, H. Reevaluating species number, distribution and endemism of the coral genus Pocillopora Lamarck, 1816 using species delimitation methods and microsatellites. Molecular Phylogenetics and Evolution. 109, 430-446 (2017).
- Capel, K. C. C., Migotto, A., Zilberberg, C., Kitahara, M. V. Another tool towards invasion? Polyp "bail-out" in Tubastraea coccinea. Coral Reefs. 33 (4), 1165 (2014).
- Kawaguti, S. Materials for the study of reef-building corals III. Science of the South Sea (Kagaku Nanyo). 5, 95-106 (1942).
- Goreau, T. F., Goreau, N. I. The physiology of skeleton formation in corals. II. Calcium deposition by hermatypic corals under various conditions in the reef. Biological Bulletin. 117, 239-250 (1959).
- Swain, T. D., et al. Physiological integration of coral colonies is correlated with bleaching resistance. Marine Ecology Progress Series. 586, 1-10 (2018).
- Sweet, M., et al. Insights into the cultured bacterial fraction of corals. mSystems. 6 (3), 01249 (2020).
- Peixoto, R. S., Rosado, P. M., Leite, D. C. d. A., Rosado, A. S., Bourne, D. G. Beneficial microorganisms for corals (BMC): proposed mechanisms for coral health and resilience. Frontiers in Microbiology. 8, 341 (2017).
- Peixoto, R. S., et al. Coral probiotics: premise, promise, prospects. Annual Review of Animal Biosciences. 9, 265-288 (2021).

