Generation of Airway Epithelial Cell Air-Liquid Interface Cultures from Human Pluripotent Stem Cells
Summary
Recent advances in human induced pluripotent stem cell differentiation protocols allow for the stepwise derivation of organ-specific cell types. Here, we provide detailed steps for the maintenance and expansion of iPSC-derived airway basal cells and their differentiation into a mucociliary epithelium in air-liquid interface cultures.
Abstract
Diseases of the conducting airway such as asthma, cystic fibrosis (CF), primary ciliary dyskinesia (PCD), and viral respiratory infections are major causes of morbidity and mortality worldwide. In vitro platforms using human bronchial epithelial cells (HBECs) have been instrumental to our understanding of the airway epithelium in health and disease. Access to HBECs from individuals with rare genetic diseases or rare mutations is a bottleneck in lung research.
Induced pluripotent stem cells (iPSCs) are readily generated by “reprogramming” somatic cells and retain the unique genetic background of the individual donor. Recent advances allow for the directed differentiation of iPSCs to lung epithelial progenitor cells, alveolar type 2 cells, as well as the cells of the conducting airway epithelium via basal cells, the major airway stem cells.
Here we outline a protocol for the maintenance and expansion of iPSC-derived airway basal cells (hereafter iBCs) as well as their trilineage differentiation in air-liquid interface (ALI) cultures. iBCs are maintained and expanded as epithelial spheres suspended in droplets of extracellular matrix cultured in a primary basal cell medium supplemented with inhibitors of TGF-ß and BMP signaling pathways. iBCs within these epithelial spheres express key basal markers TP63 and NGFR, can be purified by fluorescence activated cell sorting (FACS), and when plated on porous membranes in standard ALI culture conditions, differentiate into a functional airway epithelium. ALI cultures derived from healthy donors are composed of basal, secretory and multiciliated cells and demonstrate epithelial barrier integrity, motile cilia, and mucus secretion. Cultures derived from individuals with CF or PCD recapitulate the dysfunctional CFTR-mediated chloride transport or immotile cilia, the respective disease-causing epithelial defects.
Here, we present a protocol for the generation of human cells that can be applied for modeling and understanding airway diseases.
Introduction
Chronic pulmonary diseases account for a large burden of morbidity and mortality worldwide1. Conditions that affect the conducting airways, such as asthma, cystic fibrosis (CF), primary ciliary dyskinesia (PCD), and viral infections represent both common and rarer diseases, acquired and genetic, that contribute to this worldwide burden. The major functions of the conducting airways are to: 1) act as a conduit for the laminar flow of air, and 2) provide mucociliary clearance of pathogens and debris. Secretory, multiciliated, and basal cells represent the major epithelial cell types of the conducting airway. Rarer epithelial cell types include ionocytes, tuft cells, and neuroendocrine cells, and have been reviewed elsewhere2.
Broadly, a major barrier to understanding mechanisms of disease and advancing therapeutic approaches has been a consistent lack of human primary tissue for the use in preclinical models. HBECs are generally considered the gold-standard in vitro model of human airway epithelial biology and have played key roles in CF research in particular3. However, they are typically isolated either from bronchoscopic biopsies, from lung tissue after surgery, or from lungs rejected for transplantation purposes. Access to HBECs from individuals with genetic diseases, including the research priorities of CF and PCD, is infrequent and unpredictable. Overcoming this bottleneck to patient-derived airway epithelial cells is needed. iPSCs are readily generated from any individual, they retain the unique genetic background of the donor, and have a remarkable ability to proliferate and survive long term in cell culture conditions4,5,6. By recapitulating key embryologic steps, iPSCs undergo "directed-differentiation" into organ-specific cell types. We and others have developed protocols to generate iPSC-derived lung progenitors, alveolar type 2 cells7,8 and the cells of the conducting airway including airway basal cells9,10,11,12.
The airway basal cell is the stem cell of the conducting airway13. In previously published work, our group has generated iPSC-derived airway epithelial cells, including a subset (iBCs) that expresses canonical basal cell markers including TP63, KRT5, and NGFR. Following cues from adult basal cell biology, inhibition of dual SMAD pathways (TGF-β and BMP) leads to upregulation of NGFR+ iBCs11,14. NGFR+ iBCs are resuspended as single cells in droplets of extracellular matrix and cultured in a basal cell medium. They self-renew to maintain an iBC population and form epithelial spheroids; however, a proportion also differentiates into secretory cells in this format (referred to as 3-D culture). In the following protocol, we detail the steps to maintain and expand these iBCs in 3-D culture, as well as the steps required to generate a functional 2-D mucociliary ALI culture.
The initial steps of this differentiation protocol have been published previously and will not be reviewed here11,12. This manuscript will center on the expansion and purification of iBCs and their subsequent differentiation into functional secretory and multiciliated cells in ALI culture.
Protocol
Each of the following steps should be performed using sterile technique in a biosafety level 2 laminar flow hood. All media should be warmed to room temperature (22 °C) prior to being added to cells, except when specifically mentioned otherwise. Each centrifugation step should be performed at room temperature (approximately 22 °C). Figure 1 outlines the schematics of the protocol.
1. Preparation of required media
NOTE: See Supplementary File 1 for details.
- Basal Cell Medium
- Prepare base medium according to the manufacturer's instructions.
- To every 50 mL of the base medium, add 5 µL of A83-01 (10 mM), 5 µL of DMH1 (10 mM), 50 µL of Y-27632 (10mM), and 100 µL of Primocin (50 mg/mL). Hereafter, this media is termed basal cell medium.
- Store at 4 °C for up to 1 month.
- ALI Differentiation Medium
- Prepare the medium according to the manufacturer's instructions.
- To every 50 mL of ALI Differentiation Medium, add 100 µL of Primocin (50 mg/mL).
- Store protected from light at 4 °C for up to 1 month.
- Sort Buffer
- For every 50 mL of Sort Buffer, combine 47.5 mL of Hanks' Balanced Salt Solution, 1 mL of FBS, 200 µL of EDTA (500 mM), 1.25 mL of HEPES Buffer (1 M), 50 µL of Y-27632 (10 mM), and 100 µL of Primocin (50 mg/mL).
- Filter sterilize using a 0.4 µm pore size.
- Store at 4 °C for up to 1 month.
- Cryopreservation Medium
- For every 50 mL of Cryopreservation Medium, combine 45 mL of Basal Cell Medium and 5 mL of dimethyl sulfoxide (DMSO).
- Filter sterilize using a 0.4 µm pore size.
- Store at 4 °C for up to 1 month.
2. Thawing of cryopreserved iBCs
- Thaw (on ice) a sufficient volume of 3-D growth factor reduced extracellular matrix (hereafter 3-D matrix). Keep on ice until ready for use.
NOTE: When thawing one vial (containing 250,000 cells), thaw 100-200 µL of 3-D matrix. - Thaw a vial of previously cryopreserved single-cell suspensions of iBCs by incubating in a 37 °C water or bead bath just until there is no visible frozen media (1-2 min).
- Using a 5 mL serological pipette, add cell suspension to a 15 mL conical tube.
- Add 6-10 mL of DMEM/F12 dropwise to the cell suspension, gently mix, and centrifuge at 300 x g for 5 min to pellet the cells.
- Aspirate the supernatant and resuspend the cell pellet in 1 mL of Basal Cell Medium with a P1000 micropipette. Remove a 10 µL aliquot and perform a cell count.
- Centrifuge the cells at 300 x g for 5 min. Aspirate the supernatant and resuspend the cells at a density of 4,000 cells/µL in previously thawed 3-D matrix with a P1000 micropipette.
NOTE: It is critical to avoid adding bubbles to 3-D matrix. Pipette the matrix slowly and carefully. - With a P200 micropipette, add one droplet (25 or 50 µL) to the base of each well of a 12-well tissue culture treated plate. If starting with 250,000 frozen cells, expected number of droplets at this step is between 3-4 (25 µL droplets), depending on the cell viability.
- Incubate the plate at 37 °C for approximately 15 min, and then add enough Basal Cell Medium to each well to fully submerge the droplet (1.5 mL for 50 µL; 1 mL for 25 µL), using a 5 mL serological pipette. Return the plate to a humidified incubator.
- Feed cells every 2 days using fresh Basal Cell Medium. Add fresh medium to the side of the well with 5 mL serological pipette, taking care not to disturb the droplet of cells.
NOTE: The density of cells plated in this stage is 10-fold higher than routine passaging of iBCs in section 3.
3. Dissociation of spheroids and expansion of iBCs in 3-D culture
- Thaw (on ice) a sufficient volume of 3-D matrix. Keep on ice until ready for use.
NOTE: The volume of 3-D matrix required will vary on how many cells the user will require. - Approximately 5-7 days later, aspirate the medium from each well and with a P1000 micropipette, add 1 mL of Dispase II (1 U/mL) directly onto the spheroids to dissociate from the droplet of the matrix.
- Place the plate in 37 °C incubator for 10-15 min.
- Using a P1000 micropipette, pipette the Dispase up and down 1-2 times, breaking up large clumps of 3-D matrix. Return the plate to 37 °C for an additional 30-40 min, allowing the matrix to dissolve completely.
- When the droplet of 3-D matrix is no longer visible under the light microscope, add the freely floating spheroids to a 15 mL conical using a 5 mL serological pipette tip. Add DMEM/F12 for a final volume of 10 mL per conical and centrifuge at 200 x g for 3 min to pellet the spheroids.
- Aspirate the supernatant and add 1 mL of 0.05% trypsin (37 °C) for every initial droplet dissociated (e.g., if starting with four droplets, add 4 mL trypsin to the conical flask).
- Incubate at 37 °C, triturating frequently (every 2-3 min).
- Evaluate with the light microscope every few minutes. Once the majority (>90%) of spheroids have been dissociated to single cells, add 10% Fetal Bovine Serum (in DMEM/F12) at a 1:1 volume ratio to the trypsin.
- Filter the cells through a 40 µm cell strainer and centrifuge at 300 x g for 5 min.
- Perform a cell count and evenly resuspend the cells at a density of 400 cells/µL of thawed 3-D matrix. Avoid introducing air bubbles. Resuspend as many droplets as needed for downstream application. Repeat step 2.6-2.9 for each well.
4. Evaluation and purification of NGFR+ iBCs
- After 10-14 days of the most recent passage, dissociate the spheroids to a single cell suspension as in steps 3.2-3.5 and perform a cell count.
NOTE: Different iPSC lines behave differently; therefore, the range of 10-14 days may vary for different iBCs. Please refer to Figure 2 for typical appearances of 3-D iBCs after 1, 4, 8, and 14 days, when they are adequate for NGFR+ cell sorting. Sorting earlier than recommended (e.g., day 8 as in Figure 2C), results in an overall lower cell number with less frequent NGFR expression. Sorting later than recommended (e.g., greater than 14 days after the most recent passage) leads to poor post-sort cell survival. - Resuspend the cells at a density of 1 x 106 cells/100 µL in Sort Buffer (main population). Transfer a small aliquot (25-50 µL) to a separate tube (minor population).
- Add conjugated anti-NGFR antibody (1:100 dilution) to the main cell population.
- Add isotype control antibody (1:200 dilution) to the minor cell population.
- Protect the cells from light and keep on ice for 30 min, intermittently (every 5-10 min) triturating the cells to prevent pelleting.
- After 30 min, add the Sort Buffer in a 1:1 ratio to each tube of cells. Centrifuge cells at 300 x g for 5 min.
- Aspirate the supernatant, resuspend the pelleted cells in the Sort Buffer at a density of 10 x 106 cells/mL, and add live or dead cell stain.
- Using appropriate NGFR+ gating strategy (based on isotype control, see Figure 3), sort enough live NGFR+ cells for necessary downstream applications.
NOTE: If using iBCs with fluorescent reporters (i.e., NKX2-1GFP TP63tdTomato), it is recommended to sort for "triple positive" cells (i.e., NKX2-1GFP+, TP63tdTomato+, NGFR+), with appropriate gate selection and compensation (Figure 3). During this expansion of iBCs, a proportion of cells differentiate into secretory cells and very small proportion of multiciliated cells may also be detected. Both of these populations are NGFR-.
5. Generation of mucociliary ALI cultures
- Prepare 6.5 mm porous membrane inserts by adding a 200 µL matrix to the apical chamber (human embryonic stem cell-qualified matrix or recombinant human laminin-521) per the manufacturer's instruction.
- Place at 37 °C for at least 2 h before needed.
- Aspirate the coating matrix from the apical chamber of the insert. Add 500 µL of the Basal Cell Medium to the basolateral chamber.
- Resuspend at least 30,000 sorted NGFR+ cells (from step 4.5) in 100-200 µL Basal Cell Medium and transfer to the apical chamber using a P200 micropipette. Repeat for as many wells as desired (and cells available). Place the plate in humidified 37 °C incubator.
- In 2-3 days, aspirate apical and basolateral chambers and feed with fresh Basal Cell Medium (500 µL to apical, 100 µL to basolateral).
- Monitor the apical chamber daily with light microscopy. When cells are >80% confluent (typically 3-7 days), replace Basal Cell Medium with ALI Differentiation Medium (not pre-warmed) in both apical and basolateral chambers. When working with ALI Differentiation Medium, protect from light by keeping media containers wrapped in foil and by turning off overhead lights when able.
- The next day, aspirate the medium from the apical chamber, thus exposing the apical surface to air.
- Replace the ALI Differentiation Medium media in the basolateral chamber every 2-3 days.
- Monitor the culture appearance every 1-2 days. Carefully aspirate any accumulated liquid from the apical chamber, without disturbing the cell layer.
- Assess the transepithelial electrical resistance (TEER) for epithelial integrity.
NOTE: TEER can be assessed in the days following air exposure to give a readout of epithelial layer integrity. The ideal time to measure TEER has not been established, though values consistent with high quality mucociliary differentiation are typically >500 x cm2. - If it is necessary to remove debris or mucus, gently add 100 µL PBS (without Ca2+ or Mg2+) to the apical chamber. Incubate at 37 °C for 10 min, and then carefully aspirate PBS.
- After 7-10 days of air exposure, motile cilia typically appear and can be seen using light microscopy. Depending on the planned experiment and readout, cells can be analyzed after 14-28 days of air exposure.
NOTE: If fluorescent reporter cells have been used, and if a live-cell fluorescent microscope is available, cells can be tracked with light microscopy as well as reporter fluorescence.
6. Optional (but recommended) cryopreservation of iBCs
- After 10-14 days of the most recent 3-D culture passage, dissociate the spheroids to single-cell suspension as in steps 3.2-3.5. Perform a cell count.
- Resuspend in Cryopreservation Medium at a density of 250,000 cells/500 µL in a cryovial.
- Place cryovial(s) in a container to ensure a steady drop in temperature (1 °C/min) and transfer to -80 °C for 24-48 h, followed by transfer to -150 °C for long term storage.
NOTE: Repeat cryopreservation as well as cryopreservation of previously NGFR+ sorted cells have been performed, but these have not been adequately assessed for cell viability and the ability to form functional ALI cultures. Especially with extended cell passaging, it is recommended to assess for the karyotype as this has been noted to vary in iPSCs and iPSC-derived cells.
Representative Results
Following this protocol, 200,000 cryopreserved iBCs (previously confirmed to have a normal 46XY karyotype)11 were thawed and expanded in 3-D culture. Five days later, the resulting spheroids were dissociated, counted, and passaged again for further expansion. Approximately 480,000 cells were obtained and re-suspended in 3-D matrix (12 x 50 µL droplets, density 400 cells/µL). Fresh Basal Cell Medium was applied every 2-3 days. Ten days later, the cells were once again dissociated and counted. A total of 19.7 x 106 cells were harvested and prepared for FACS. 106 cells were stained with the APC-conjugated IgG1κ isotype control and the remaining 18.7 x 106 cells were stained with the APC-conjugated anti-NGFR antibody for 30 min protected from light (Figure 3).
NGFR+ gating was performed after comparison to the isotype control cells and was set purposely to collect the highest expressing NGFR+ cells (Figure 3). With this gating technique, 28% of live, single cells were NGFR+. While more cells were available, 750,000 cells were collected for downstream culture. Sorted cells were resuspended in Basal Cell Medium at a concentration of 50,000 cells/100 µL. 50,000 cells were then seeded onto each 6.5 mm porous membrane insert, which had been coated with human recombinant laminin-521 (2 µg/200 µL) per the manufacturer's guidelines. 500 µL Basal Cell Medium was added to the basolateral chamber of each insert and the plate was placed in a 37 °C humidified incubator. Three days later, the media in the apical chamber was aspirated and the cells were ~90% confluent by light microscopy (Figure 4B). The media from the basolateral chamber was aspirated; ALI Differentiation Medium was added to the apical (100 µL) and basolateral (500 µL) chambers. The next day, the media from the apical chamber was aspirated.
Over the following 21 days, the cells were evaluated by light microscopy periodically and fed with fresh ALI Differentiation Medium (basolateral chamber only) every 2-3 days. Individual cells were initially easily identifiable (Figure 4A), had an elongated and spindle-shaped appearance, and formed a loosely packed monolayer (Figure 4B). Over the subsequent days to weeks, the cells formed a tightly packed, highly cellular, epithelial layer, and after 7-10 days there was the clear emergence of beating cilia and mucus production. TEER of the samples were calculated and similar to primary cell controls (range from 700-1600 Ω x cm2)11. Subsequent fixation (day 21-28) with paraformaldehyde and immunolabeling for canonical airway epithelial cell markers was performed for MUC5AC and acetylated-α-tubulin, among others (Figure 4C). Overall, with our observation of motile cilia, mucus production as well as confirmatory immunostaining of multiciliated and secretory cells which is similar to that of primary HBECs, we concluded that we successfully generated airway epithelial cells from induced pluripotent stem cells.
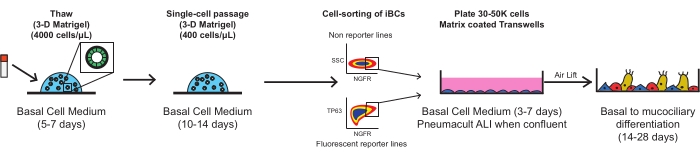
Figure 1: Overall schematic of protocol. Cryopreserved iBCs are thawed, expanded, and FACS purified prior to plating on porous membrane inserts, where they differentiate into a functional mucociliary epithelium. Please click here to view a larger version of this figure.
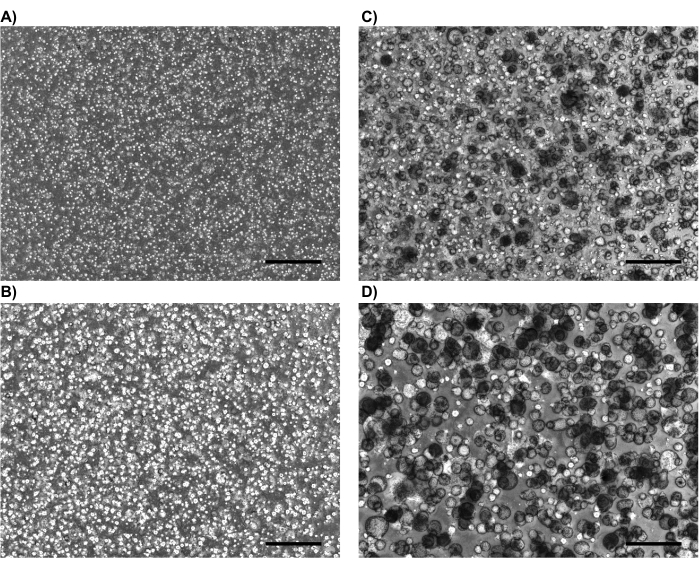
Figure 2: Representative phase contrast images. Representative phase contrast images demonstrating usual appearance of iBCs in 3-D culture after (A) 1 day, (B) 4 days, (C) 8 days, and (D) 14 days (just pre-NGFR sort). Scale bars represent 500 µm. Please click here to view a larger version of this figure.
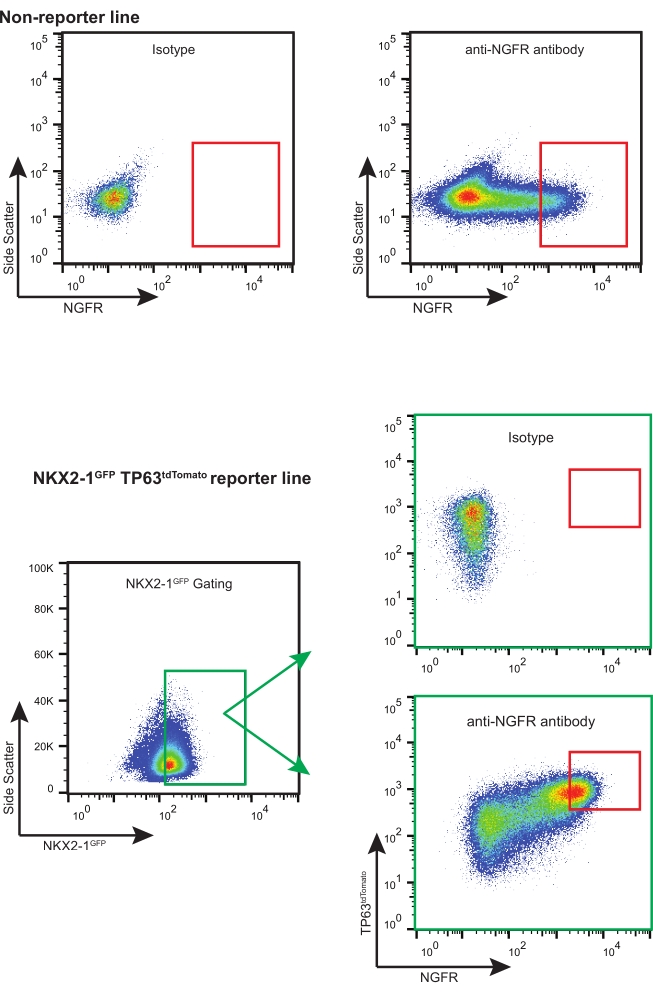
Figure 3: Representative FACS plots. Representative FACS plots for non-reporter and fluorescent-reporter iBCs. Examples of isotype controls are shown and were used to select for the highest expressing NGFR+ cells. Fluorescent reporter-containing iPSC lines are "triple sorted" for NKX2-1GFP+ TP63tdTomato+ NGFR+ cells. Please click here to view a larger version of this figure.
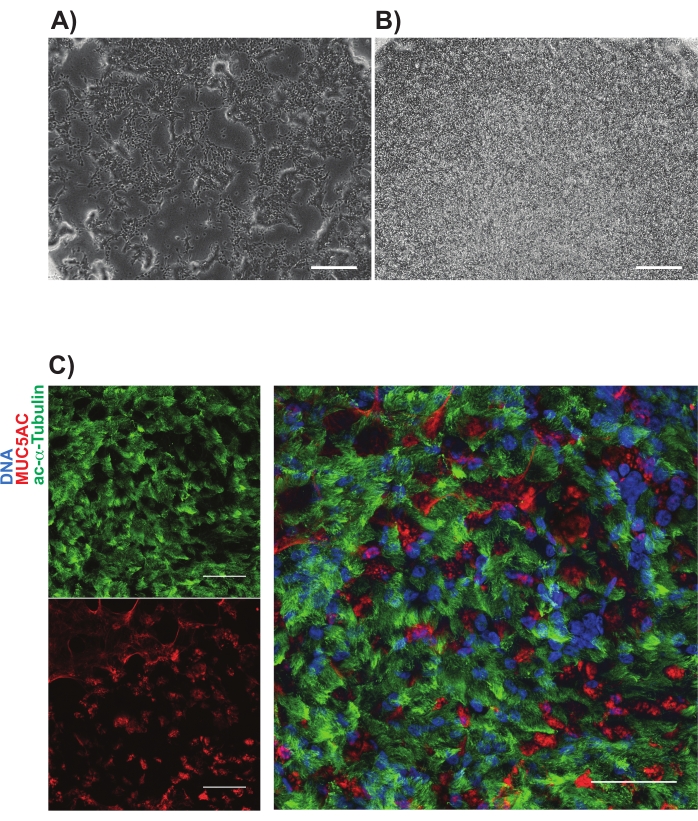
Figure 4: Representative images of iBC cultures on porous membranes. Phase contrast images are shown (A) 1 day and (B) 3 days after plating. Representative immunolabeling of mucociliary cultures shown in (C); acetylated-alpha tubulin (green) and MUC5AC (red). Scale bars represent 25 µm. Please click here to view a larger version of this figure.
Supplementary File 1: Media component table. Please click here to download this File.
Discussion
HBECs are the gold-standard cell-type to study diseases of the airway epithelium. Due to their limitations (including accessibility and difficulty to genetically manipulate), we have generated a protocol for the derivation of iBCs and ALI cultures. These cells can be derived from any donor and retain their unique genetic background, thus allowing for basic developmental studies, disease modeling, and novel therapeutic development.
While each individual step of the described protocol is necessary, there are several that deserve extra mention. Firstly, at each step that requires dissociation of spheroids into single cells, it is critical to avoid excessive pipetting; enzymatic digestion (with dispase or trypsin) promotes better cell survival, while excessive pipetting leads to significant cell death. Secondly, when purifying NGFR+ iBCs, utilization of the isotype control is crucial and we recommend selecting for the highest expressing NGFR+ cells with FACS (Figure 3). This approach results in optimal ALI cultures with appropriate mucociliary differentiation. Finally, the preparation and seeding of porous membranes precisely as documented in the protocol is fundamental for ALI culture survival. While we typically seed 30,000-60,000 cells per insert, we have had success with as few as 20,000 cells. While successful cultures can be generated using the human embryonic stem cell-qualified matrix coating, we have more recently used human recombinant laminin-521 with a significantly higher durability of the ALI cultures.
Very rarely, iPSC differentiations may fail to upregulate an adequate percentage of NGFR+ iBCs. In this instance, serial passaging of iBCs (in 3-D culture) can result in an increased NGFR frequency over time. Limitations of this protocol include the time, cost, and expertise needed to generate these cells. Additionally, we recognize that many researchers are interested in the less common cell types of the airway epithelium (e.g., ionocytes, neuroendocrine cells). While we have detected some of these rarer cell types, as in primary HBEC cultures, they are not reproducibly identified, likely due to incomplete knowledge regarding the developmental cues required to generate these cells.
As it is written, the above protocol begins with the thawing of already cryopreserved iBCs. The details prior to this cryopreservation are previously described and beyond the scope of this manuscript11,12.
Our airway epithelial ALI culture method allows for the generation of functional airway epithelial cells from nearly any donor. This greatly increases the accessibility to precious genetically controlled airway epithelial cells that may be used for disease modeling, drug screening, future cell-based therapies, as well as to improve the understanding of the developmental patterning within the airway epithelium.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank the members of the Hawkins, Kotton, and Davis laboratories for their helpful input over the years regarding this and other projects. We also are indebted to Brian Tilton (BU Cell Sorting Director) for his dedication and technical expertise, and we are grateful to Greg Miller and Marianne James of the Boston University Center for Regenerative Medicine (CReM) for their support and technical expertise in maintenance and characterization of patient-specific iPSCs, supported by NIH grants NO1 75N92020C00005 and U01TR001810. This work was supported by NIH grants U01HL148692, U01HL134745, U01HL134766, and R01HL095993 to D.N.K, R01HL139876 to B.R.D, R01 HL139799 to F.H., and Cystic Fibrosis Foundation (CFF) grants CFF 00987G220 and CFF WANG20GO to D.N.K, CFF DAVIS15XX1, DAVIS17XX0, DAVIS19XX0 to B.R.D, CFF SUZUKI19XX0 to S.S, CFF BERICA2010 to A.B., and CFF HAWKIN20XX2 to F.H.
Materials
| 12-well tissue culture treated plate | Corning | 3515 | flat bottom |
| 3-D growth factor reduced Matrigel | Corning | 356230 | |
| A83-01 | ThermoFisher Scientific | 293910 | |
| Cell culture inserts (Transwell) | Corning | 3470 | 6.5mm diameter; 0.4μm pore size |
| Dimethyl sulfoxide (DMSO) | Sigma | D8418 | |
| Dispase II, Powder | ThermoFisher Scientific | 17105-041 | |
| DMH1 | ThermoFisher Scientific | 412610 | |
| Dulbecco's Modified Eagle Medium/Nutrient Mixture (DMEM):F-12 | ThermoFisher Scientific | 11330-032 | |
| Dulbecco's Phosphate Buffered Saline (PBS) (1X) | Gibco | 14190-144 | |
| Ethylenediaminetetraacetic acid disodium salt solution (EDTA) | Sigma | E7889 | |
| Fetal Bovine Serum (FBS) | Fisher | SH3007003E | |
| Hanks' Balanced Salt Solution (HBSS) | ThermoFisher Scientific | 14175-079 | |
| HEPES | Sigma | H3375 | |
| hESC-qualified Matrigel | Corning | 354277 | |
| Mouse IgG1kappa isotype control, APC-conjugated | Biolegend | 400122 | |
| Mouse monoclonal anti-human CD271/NGFR, APC-conjugated | Biolegend | 345108 | |
| PneumaCult-ALI Medium (ALI Differentiation Medium) | StemCell Technologies | 05001 | |
| PneumaCult-Ex Plus Medium (Basal Cell Base Medium) | StemCell Technologies | 05040 | |
| Primocin | InvivoGen | ant-pm-2 | |
| rhLaminin-521 | ThermoFisher Scientific | A29248 | Final Concentration: 10ug/mL |
| Trypsin-EDTA Solution, 0.05% | Invitrogen | T3924 | |
| Y-27632 | Tocris | 1254 |
Referências
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. Respiratory Medicine. 8 (6), 585-596 (2020).
- Alysandratos, K. -. D., Herriges, M. J., Kotton, D. N. Epithelial stem and progenitor cells in lung repair and regeneration. Annual Review of Physiology. 83, 529-550 (2021).
- Fulcher, M. L., Gabriel, S., Burns, K. A., Yankaskas, J. R., Randell, S. H. Well-differentiated human airway epithelial cell cultures. Methods in Molecular Medicine. 107, 183-206 (2005).
- Takahashi, K., Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126 (4), 663-676 (2006).
- Takahashi, K., et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 131 (5), 861-872 (2007).
- Berical, A., Lee, R. E., Randell, S. H., Hawkins, F. Challenges facing airway epithelial cell-based therapy for cystic fibrosis. Frontiers in Pharmacology. 10, 74 (2019).
- Jacob, A., et al. Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells. Nature Protocols. 14 (12), 3303-3332 (2019).
- Gotoh, S., et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports. 3 (3), 394-403 (2014).
- Huang, S. X. L., et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nature Biotechnology. 32 (1), 84-91 (2013).
- Mou, H., et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 10 (4), 385-397 (2012).
- Hawkins, F. J., et al. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell. 28 (1), 79-95 (2021).
- Suzuki, S., et al. Differentiation of human pluripotent stem cells into functional airway basal stem cells. STAR Protocols. 2 (3), 100683 (2021).
- Rock, J. R., et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America. 106 (31), 12771-12775 (2009).
- Mou, H., et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 19 (2), 217-231 (2016).

