Zygote Microinjection for Creating Gene Cassette Knock-in and Flox Alleles in Mice
Summary
The present protocol describes zygote microinjection of CRISPR-Cas9 and donor DNA to efficiently produce gene cassette knock-in and floxed mice.
Abstract
CRISPR-Cas technology has enabled the rapid and effortless generation of genetically modified mice. Specifically, mice and point mutant mice are readily produced by electroporation of CRISPR factors (and single-stranded oligo DNA donors) into the zygote. In contrast, gene cassette (>1 kb) knock-in and floxed mice are mainly generated by microinjection of CRISPR factors and double-stranded DNA donors into zygotes. Genome editing technologies have also increased the flexibility of genetically modified mice production. It is now possible to introduce the intended mutations in the target genomic regions in a number of beneficial inbred mouse strains. Our team has produced over 200 gene cassette knock-in mouse lines, and over 110 floxed mouse lines by zygote microinjection of CRISPR-Cas9 following requests from several countries, including Japan. Some of these genome editing used BALB/c, C3H/HeJ, and C57BL/6N inbred strains, however most used C57BL/6J. Unlike the electroporation method, genome editing by zygote microinjection in various inbred strains of mice is not that easy. However, gene cassette knock-in and floxed mice on single inbred genetic backgrounds are as critical as genetic humanized, fluorescent reporter, and conditional knockout mouse models. Therefore, this article presents the protocol for the zygote microinjection of CRISPR factors and double-stranded DNA donors in C57BL/6J mice for generating gene cassette knock-in and floxed mice. This article exclusively focuses on nuclear injection rather than cytoplasmic injection. In addition to zygote microinjection, we outline the timeline for the production process and peripheral techniques such as induction of superovulation and embryo transfer.
Introduction
Knock-in mice, in which the intended exogenous genes are introduced at the target loci, are widely used in many in vivo studies as gene humanized mice, fluorescent reporter mice, and Cre driver mice1,2. When knock-in mutations are induced by genome editing in mouse zygotes, single-stranded DNA (single-stranded oligo DNA donors, ssODN) or double-stranded DNA (dsDNA) is used as donor DNA2,3. ssODN is mainly used to knock-in relatively short gene fragments of less than 200 bp4. It is possible to knock-in fragments longer than 1 kb DNA using long ssODNs (lsODN)5,6, however their preparation is time-consuming. When dsDNA donors are used, gene cassette (>1 kb) knock-in mice can be generated without laborious donor DNA preparation7.
The principal advantage of using ssODNs is that electroporation8 can generate knock-in mice. However, dsDNA donors must be introduced directly into the nucleus by zygote microinjection. Simultaneous knock-in at two sites is required to create floxed mice, in which each loxP sequence is knocked-in upstream and downstream of the target gene. There are two ways to generate floxed mice by genome editing in mouse zygotes-using two independent ssODNs, each carrying a single loxP site, or using a single dsDNA (or lsDNA) with a floxed sequence; the former is very inefficient9,10,11. Genome editing using dsDNA donors is the simplest approach to produce gene cassette knock-in and floxed mice if the environment is conducive to zygote microinjection.
Initially, mixtures of Cas9 mRNA and sgRNA or DNA vectors encoding sgRNA and Cas9 are used for genome editing in mouse zygotes12,13. Since high-quality and stable Cas9 proteins are now available at a low cost, the introduction of crRNA-tracrRNA-Cas9 ribonucleoprotein (RNP) into mouse zygotes14 has become popular. Recently, knock-in mice have been generated with high efficiency by introducing the crRNA-tracrRNA-Cas9 RNP and donor DNA into both pronuclei of mouse zygotes in a cell cycle phase where knock-in is most likely to occur15. Therefore, the present protocol describes techniques for producing various types of knock-in mice by this method.
There are many useful inbred strains of laboratory mice16. Genetic modification within the inbred background can disregard the influence of genetic background on the phenotype. This article describes a method for inducing gene cassette knock-in and floxed mutations in the zygote of the most commonly used inbred mouse line, the C57BL/6J17. Further, the timeline for the generation of genetically modified mice and the peripheral techniques used are also discussed, including the induction of superovulation and embryo transfer.
Protocol
All animal experiments were conducted humanely with approval from the Institutional Animal Experiment Committee of the University of Tsukuba, according to the Regulations for Animal Experiments of the University of Tsukuba and the Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science, and Technology of Japan. C57BL/6J mice of both sexes, 10-25 weeks old, were used as the zygote donor. ICR mice (older than 10 weeks) were used as the recipient mice. The mice were obtained from commercial sources (see Table of Materials).
1. Confirmation of crRNA cleavage activity in a cell-free system
- Dissolve 2 nmol of crRNA (dry) and 20 nmol of tracrRNA (dry) in 25 µL and 250 µL of RNase-free water, respectively (see Table of Materials).
NOTE: CRISPR target sequences that were predicted to have high cleavage activity and as few off-targets as possible are selected using CRISPOR (see Table of Materials). In the case of gene cassette knock-in, target the sequence across the insertion site. The exon(s) to be floxed were selected using the KOnezumi (see Table of Materials). - Amplify the DNA fragment (approximately 0.5-1 kb) containing the target site by genomic PCR9. Purify this PCR amplicon with the common PCR product extraction kit (see Table of Materials).
- Prepare 10 µL of CRISPR mixture (100 ng/µL of crRNA, 150 ng/µL of tracrRNA, and 1 ng/µL of Cas9 in the Cas9 Nuclease Reaction Buffer, see Table of Materials). To construct the CRISPR-Cas9 complex, place the CRISPR mixture in a heat block at 37 °C for 30 min.
- Add the total volume (10 µL) of CRISPR mixture to the PCR product (10 µL, 20 ng/µL) described in step 1.2 and incubate at 37 °C for 1 h. Add 1 µL of RNase (500 ng/µL) to degrade the crRNA and tracrRNA at 37 °C for 30 min, as RNAs must be absent during electrophoresis. Perform electrophoresis of the above samples using loading dye containing sodium dodecyl sulfate (2% w/v).
NOTE: In rare cases, crRNAs with no cleavage activity are observed. In such cases, it is recommended to redesign the genome editing design.
2. Preparation of the mixture of crRNA, tracrRNA, Cas9 protein, and donor DNA for zygote microinjection
- Prepare CRISPR solution (50 ng/µL of crRNA, 200 ng/µL of tracrRNA, and 200 ng/µL of Cas9 in RNase-free water). Prepare a donor DNA solution (20 ng/µL of self-constructed plasmid DNA vector).
- Filter the donor DNA solution through a sterile syringe filter with a 0.22 µm pore size. Mix equal volumes of CRISPR solution and filtered donor DNA solution. Ensure that the final concentration of each is 25 ng/µL of crRNA, 100 ng/µL of tracrRNA, 100 ng/µL of Cas9, and 10 ng/µL of donor DNA. This mixture will hereafter be referred to as RNPD.
NOTE: When cutting two sites, such as during floxed mice production, the concentration of each crRNA must be 25 ng/µL. The donor DNA is a circular plasmid DNA and can be purified with a common mini prep spin column kit (see Table of Materials). The prepared solution must be placed on ice and used for micro-injection as soon as possible.
- Filter the donor DNA solution through a sterile syringe filter with a 0.22 µm pore size. Mix equal volumes of CRISPR solution and filtered donor DNA solution. Ensure that the final concentration of each is 25 ng/µL of crRNA, 100 ng/µL of tracrRNA, 100 ng/µL of Cas9, and 10 ng/µL of donor DNA. This mixture will hereafter be referred to as RNPD.
3. Obtaining mice zygotes by natural mating
- Inject 5 IU of pregnant mare's serum gonadotropin (PMSG, see Table of materials) subcutaneously into C57BL/6J female mice (10-25 weeks old) 3 days before microinjection. Approximately 46-48 h later, administer 5 IU of human chorionic gonadotropin (hCG, see Table of materials) intraperitoneally. Co-house the PMSG- and hCG-treated female mice with male C57BL/6J mice and check the vaginal plugs the following morning.
NOTE: Approximately 15-25 zygotes can be obtained from one female C57BL/6J mouse. The timeline is shown in Figure 1. - Prepare two 35 mm Petri dishes for zygote culture, as shown in Figure 2. Place them in the incubator (37 °C, 5% CO2) until use. These can be stored overnight.
- Euthanize the female mice with confirmed vaginal plugs by cervical dislocation and, using small scissors, incise through the abdominal skin and muscle layer from the midline of the lower abdomen to below the ribs.
NOTE: Follow local animal ethics committee recommendations for euthanasia. - Collect the oviducts and place them in a 20 µL drop of M2 medium containing 0.75 mg/mL of hyaluronidase (see Table of Materials) on the lid of the Petri dish. Pick up one oviduct with fine tip forceps and perfuse about 50 µL of M2 medium with hyaluronidase through the fimbriae.
NOTE: At this time, the zygotes are loosely surrounded by numerous cumulus cells. - After 30 s, pick up morphologically normal zygotes with a small amount of medium using a glass capillary and wash them by transferring them to fresh M16 medium droplets. Transfer the washed zygotes into a Petri dish (Figure 2) for pooling.
NOTE: The timeline is shown in Figure 1. Morphologically normal zygotes have a male and female pronucleus and two polar bodies that have not severely compromised the spherical shape15. The morphology of the zygote varies considerably among strains and species. - Repeat steps 3.3 and 3.4 until all zygotes are retrieved. About 100 zygotes can be pooled in one M16 drop in the culture dish. Store the dish in the incubator (37 °C, 5% CO2) until microinjection.
4. Preparation of the microinjection needle
- Pull some glass pipettes using the programmable pipette puller (see Table of Materials).
NOTE: Microinjection needles must have a thin tip and long taper. If the puller has a program for making needles for intracytoplasmic sperm injection, the same program can be used to make needles for microinjection. - Break the tip of the microinjection needle by hitting the glass ball attached to the microforge tip. Ensure that the tip size of the microinjection needle is around 1 µm in diameter. Check the tip size under a microscope at 1000x magnification.
- Bend the microinjection needle at about 2-3 mm from the tip using a microforge.
NOTE: The angle of the bend depends on the setup of the micromanipulator. Too much bending will reduce the effectiveness of the piezo pulse.
5. Zygote microinjection
- Inject 1-1.5 cm of the operation liquid (inertia body that is needed for the piezo effect to make holes as an alternative to mercury, see Table of Materials) into the center of the microinjection needle and attach it to the manual microinjector holder with the piezo actuator.
- Further, attach the holding needle to the opposite manual microinjector holder. Move the operation liquid to the microinjection needle tip with gradual pressure. Fix both the manual microinjector holders on the micromanipulator.
NOTE: The operation liquid coming out of the tip of the microinjection needle can be observed under a microscope at 50x magnification. After adjusting the amount of liquid emitted, the splashing of liquid can be observed when the piezo pulses are applied, confirming that the piezo pulses are effective. If this cannot be confirmed, re-prepare the microinjection needle.
- Further, attach the holding needle to the opposite manual microinjector holder. Move the operation liquid to the microinjection needle tip with gradual pressure. Fix both the manual microinjector holders on the micromanipulator.
- Prepare an injection chamber with three droplets: (1) 10 µL of M2 medium; (2) 5 µL of 12% polyvinylpyrrolidone (PVP) in M2 medium; and (3) a 5 µL mixture of crRNA, tracrRNA, Cas9 protein, and the Donor DNA (RNPD) solution. Cover the droplets with mineral oil in the same manner as step 3.2 and set the injection chamber on the inverted microscope stage.
- Under the inverted microscope at a 50x magnification, lower the microinjection pipette into the 12% PVP drop. Aspirate and dispense 12% PVP to clean the inside of the microinjection needle tip and transfer it to the RNPD droplet.
- Put the manual microinjector under negative pressure and suck up the RNPD solution into the microinjection needle. Wait for a few minutes until a sufficient volume is aspirated; under a microscope magnified 50x, fill the entire inside of the microinjection needle with liquid.
- Stop the intake and allow the RNPD solution to be expelled gradually by setting the manual microinjector to positive pressure. Move the tip of the microinjection needle into the oil.
NOTE: Turn the knob of the manual microinjector counterclockwise for inhalation. To stop inhalation, rotate the knob clockwise and gradually increase the pressure while observing under a microscope. The RNPD solution will be gradually drained. Liquid level movement in the microinjection needle allows confirmation of the outflow.
- Stop the intake and allow the RNPD solution to be expelled gradually by setting the manual microinjector to positive pressure. Move the tip of the microinjection needle into the oil.
- Put 50 zygotes in the M2 droplet and gently lower the holding pipette into the same droplet. Transfer the microinjection needle to the M2 drop containing zygotes. Switch to a 20x objective and focus on the tip of the microinjection pipette.
NOTE: Put as many zygotes that can be injected in 10 min. - Hold a zygote and focus on the pronucleus by moving the micromanipulator. Insert the microinjection needle into the zygote and bring the tip close to the pronucleus. Once the tip reaches the pronucleus membrane, apply the piezo pulse (intensity: 6-10, speed: 1) to pierce.
- When the microinjection needle punctures the pronucleus, inject the RNPD solution, and observe the swelling of the pronucleus. Once the pronucleus is fully inflated, quickly pull out the needle. Perform the same procedure on both the female and male pronuclei.
NOTE: The inflation of the pronucleus by injection can be clearly seen under the microscope. The degree of this inflation is difficult to verbalize but can be confirmed by referring to previous reports15.
- When the microinjection needle punctures the pronucleus, inject the RNPD solution, and observe the swelling of the pronucleus. Once the pronucleus is fully inflated, quickly pull out the needle. Perform the same procedure on both the female and male pronuclei.
- Move the injected zygote to another location within the M2 droplet to identify the zygotes before and after injection.
- Repeat steps 5.5-5.6 until all the zygotes in the M2 droplet have been injected. After the injection, transfer the zygotes into a fresh M16 dish.
- Select the survived zygotes 10 min after injection. Remove the lysed zygotes which are damaged by the injection and distribute survived zygotes to each droplet in the M16 dish. Each droplet must contain 20-24 zygotes for one pseudopregnant female. This reduces the time the M16 dish is exposed to the environment outside the incubator during embryo transfer.
NOTE: Under optimal injection conditions, the survival rate is 90%-95%. This depends on the size of the needle tip, the strength of the piezo pulse, and the outflow pressure of the RNPD solution.
6. Embryo transfer into the oviduct
- For obtaining pseudopregnant mice, mate each female ICR mouse in the proestrus phase with a vasoligated male ICR mouse. Check the plugs the following day.
NOTE: Approximately 15 pseudopregnant mice are obtained from 20 mating pairs. - Administer three types of mixed anesthetic agents (0.2 mg/kg of medetomidine, 4.0 mg/kg of midazolam, and 2.5 mg/kg of butorphanol, see Table of Materials) subcutaneously to the pseudopregnant female mice. Confirm appropriate anesthesia by a decrease in respiratory rate and disappearance of the tail-pinch and hindlimb pedal withdrawal reflexes. Apply ophthalmic ointment to lubricate the eyes as per local animal ethics committee recommendations.
- After razor-shaving the dorsal region of the mouse in the prone position and disinfecting the surgical area three times, alternating between an iodine or chlorhexidine-based scrub and alcohol, incise the dorsal skin approximately 1 cm along the spine with small dissecting scissors.
- Shift the dorsal incision wound to one side ventrally, incise the muscle layer through which the ovary can be seen, grasp the fat above the ovary with tweezers, and withdraw the ovary, oviduct, and uterus.
- Secure the fat with a serrefine clamp (see Table of Materials). Place the reproductive tissues on sterile gauze to avoid direct contact with the coat.
- In the following order, place a small amount of culture medium (M2 or M16), some air bubbles as a marker, and the zygotes in a pipette for transfer.
NOTE: Transfer approximately 10-12 zygotes per oviduct. - Make an incision using a micro shear (see Table of Materials) between the ampulla and the fimbriae of the oviduct (just long enough to allow the glass pipette to enter), introduce the zygotes into the incision, and simultaneously confirm that the air bubble has been introduced.
- Perform the implantation into the other oviduct similarly (step 6.6).
- Suture the outer skin with autoclips (see Table of Materials).
NOTE: Do not suture the incised muscle layer unless the incision wound is unavoidably large. The ideal size of the incised muscle layer should be less than 2-3 mm. If the incision is greater than 5 mm, the muscle layer should be sutured using a sterilized suture needle and thread (see Table of Materials).
7. Animal recovery
- Administer atipamezole hydrochloride (0.3 mg/kg) subcutaneously to mice.
NOTE: Follow local animal ethics committee recommendations for post-operative analgesia. - Then, place the mice in cages and keep them warm on a hot plate at 37 °C.
- After the mice are awakened, keep them warm for about 2 h. After confirming no abnormal behavior of the mice, return the cages to the breeding rack.
Representative Results
The production outcomes of gene cassette knock-in and floxed mice using this protocol are shown in Table 1 and Table 2. The target genes were not stated because each genome-edited mouse line is currently being used in an independent, unpublished project. Instead, the target chromosomal regions were described.
The genotyping analyses were performed using a previously reported method18. In this genotyping method, mice in which the donor DNA is inserted into unintended chromosomal sites are not counted as positive individuals, even if the desired genome editing is induced. Hence, the production efficiency of the founder mice that are truly useful for actual purposes was shown, rather than the efficiency of the genome editing itself.
The median production efficiency of 13 independent gene cassette knock-in mice was 20.8%, with a maximum of 39.5% and a minimum of 7.9% (Table 1). This efficiency was considered to be sufficiently practical. Any lethal embryonic genes were not targeted during the gene cassette knock-in. The median birth rate under this non-lethal gene targeting condition was 34.0% (maximum 43.3% and minimum 15.9%). Since this is comparable to the birth rate in embryo transfer without genetic manipulation, we considered that the toxicity of the introduced genome editing elements and the physical damage of zygote microinjection was unlikely to affect embryonic development.
The median production efficiency of the 10 independent floxed mice was 7.7%, with a maximum of 20.7% and a minimum of 2.1% (Table 2). Although the functions of several target genes were unknown, the median birth rate was 30.2%, with a maximum of 43.8% and a minimum of 17.3%. This rate was comparable to that of gene cassette knock-in mice, suggesting that the micro-injection operation has a negligible effect on the embryonic development of floxed mice.
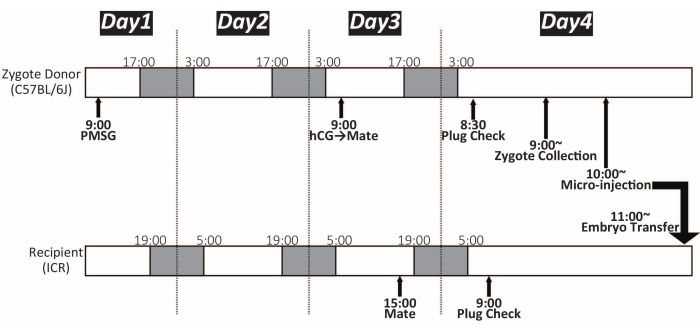
Figure 1: Timeline of zygote microinjection. White and dark color shows the light and dark cycle, respectively. The mice were maintained under a 14 h light/10 h dark cycle. PMSG: pregnant mare's serum gonadotropin; hCG: human chorionic gonadotropin Please click here to view a larger version of this figure.
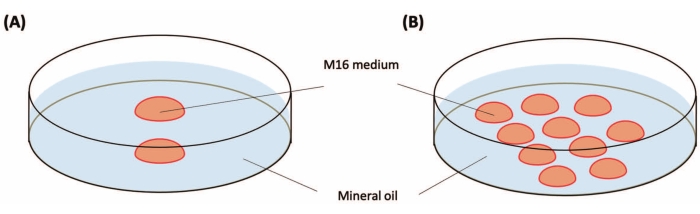
Figure 2: Preparation of culture dishes. (A) Culture dish for the zygotes before the injection. Place two drops of M16 medium (20-25 µL for each) in a 35 mm plastic Petri dish. (B) Culture dish for the zygotes after the injection. Place 10 drops (10-20 µL for each drop) of M16 in a 35 mm plastic Petri dish. Drops are covered with mineral oil (3 mL). Please click here to view a larger version of this figure.
| Targeted Chromosomal Region | Number of | Knock-in insertion length (kb) | Homology arm length (kb) | ||||||
| Embryos | Newborns | Examined (weanings) | Knock-in W/O rTG | rTGc | |||||
| injected | transferred | 5' arm | 3' arm | ||||||
| 2qE2 | 349 | 331 | 114 (34.4%)a | 114 | 9 (7.9%)b | 5 (4.4%)d | 1.0 | 1.0 | 1.0 |
| 2qE2 | 231 | 215 | 78 (36.3%)a | 74 | 15 (20.3%)b | 23 (31.1%)d | 2.2 | 1.0 | 1.1 |
| 5qG2 | 288 | 262 | 90 (34.4%)a | 81 | 19 (23.5%)b | 18 (22.2%)d | 1.6 | 2.0 | 2.0 |
| 6qB1 | 278 | 259 | 58 (22.4%)a | 46 | 11 (23.9%)b | 11 (23.9%)d | 4.2 | 1.2 | 1.0 |
| 6qE3 [ROSA26] | 295 | 277 | 97 (35.0%)a | 23 | 2 (8.7%)b | 4 (17.4%)d | 6.5 | 1.1 | 3.0 |
| 6qE3 [ROSA26] | 246 | 219 | 87 (39.7%)a | 83 | 22 (26.5%)b | 18 (21.7%)d | 5.8 | 1.1 | 3.0 |
| 7qF5 | 279 | 268 | 116 (43.3%)a | 114 | 45 (39.5%)b | 44 (38.6%)d | 1.4 | 3.1 | 1.0 |
| 11qB4 | 405 | 353 | 56 (15.9%)a | 47 | 8 (17.0%)b | 12 (25.5%)d | 1.7 | 1.0 | 0.8 |
| 11qD | 310 | 294 | 100 (34.4%)a | 98 | 15 (15.3%)b | 76 (77.6%)d | 1.0 | 1.8 | 2.4 |
| 12qE | 368 | 320 | 86 (26.9%)a | 84 | 12 (14.3%)b | 19 (22.6%)d | 3.4 | 1.1 | 1.3 |
| 12qF1 | 315 | 265 | 76 (28.7%)a | 72 | 17 (23.6%)b | 28 (38.9%)d | 1.0 | 1.1 | 1.1 |
| 14qE5 | 256 | 215 | 48 (22.3%)a | 48 | 11 (22.9%)b | 21 (43.8%)d | 3.1 | 1.0 | 0.9 |
| 14qE5 | 287 | 285 | 85 (29.8%)a | 72 | 15 (20.8%)b | 19 (26.4%)d | 2.6 | 1.0 | 0.9 |
Table 1: Knock-in mice production by micro-injection. The table shows the birth rate and the efficiency of producing mice that carry the intended knock-in allele without (W/O) random integration (rTG) of the donor DNA. It was possible to obtain mice with the intended knock-in allele in all projects. a: Number of Newborns/Number of transferred embryos; b: Number of knock-in alleles carried mice/number of examined mice; c: it is not certain if there is an intended allele; d: Number of rTG allele carried mice/number of examined mice; rTG: random integration of donor vector. W/O: without.
| Targeted Chromosomal Region | Number of | floxed length (kb) | Homology arm length (kb) | |||||
| Embryos | Newborns | Examined (weanings) | floxed W/O rTG | |||||
| injected | transferred | 5' arm | 3' arm | |||||
| 3qC | 325 | 313 | 93 (29.7%)a | 87 | 9 (10.3%)b | 1.3 | 1.2 | 1.1 |
| 4qB1 | 210 | 200 | 36 (18.0%)a | 29 | 6 (20.7%)b | 1.6 | 1.2 | 0.9 |
| 4qC4 | 272 | 256 | 112 (43.8%)a | 100 | 5 (5.0%)b | 2.4 | 1.0 | 1.4 |
| 5qF | 143 | 137 | 40 (29.2%)a | 40 | 6 (15.0%)b | 1.8 | 1.0 | 1.1 |
| 5qF | 255 | 227 | 80 (35.2%)a | 76 | 2 (2.6%)b | 1.3 | 1.1 | 0.9 |
| 9qE3.1 | 289 | 261 | 80 (30.7%)a | 77 | 9 (11.7%)b | 1.2 | 1.2 | 1.2 |
| 10qB3 | 284 | 269 | 88 (32.7%)a | 78 | 3 (3.8%)b | 1.3 | 1.1 | 0.9 |
| 10qC1 | 243 | 231 | 40 (17.3%)a | 38 | 4 (10.5%)b | 2.1 | 1.0 | 1.3 |
| 14qE5 | 390 | 372 | 139 (37.4%)a | 135 | 5 (3.7%)b | 1.1 | 0.8 | 0.9 |
| 17qA3.3 | 383 | 374 | 99 (26.5%)a | 95 | 2 (2.1%)b | 2.1 | 0.6 | 0.8 |
Table 2: Floxed mice production by micro-injection. The table shows the birth rate and the efficiency of producing mice that carry the intended flox allele without (W/O) random integration (rTG) of the donor DNA. It was possible to obtain mice with the intended flox allele in all projects. a: Number of newborns/number of transferred embryos; b: number of flox allele carried mice/number of examined mice. rTG: random integration of or donor vector; W/O: without.
Discussion
In the present study, fresh (not frozen-thawed) C57BL/6J mouse zygotes, obtained from natural mating, were used for genome editing mouse production. By injecting crRNA-tracrRNA-Cas9 and donor DNA (RNPD) into both pronuclei of these zygotes in a cell cycle phase where knock-in is most likely to occur, gene cassette knock-in and floxed mice were generated with a high birth rate and sufficient genome editing efficiency. The current study strengthens the extensibility of the SPRINT-CRISPR method15, where it ensures sufficient knock-in efficiency even in the C57BL/6J genetic background, and can be applied to the production of floxed mice.
Electroporation is a highly generalized method of producing genome-edited mice because of the low cost of the experimental devices and the ease of learning the technique8. Furthermore, the i-GONAD method19, in which genome editing is done with preimplantation embryos existing in the oviduct, does not require a recipient mouse. Compared to these methods, the present method presented here is costly and time-consuming when preparing and maintaining an experimental environment in terms of both hardware and software. However, once the experimental facilities are set up, complex gene cassette knock-in and floxed mice can be produced with only commercially available CRISPR elements (crRNA, tracrRNA, and Cas9 protein) and a simple, small amount of circular plasmid vector to be used as donor DNA. This means that many complex genome-edited mouse lines can be produced without the need for time-consuming (or relatively expensive) lsODN5,6 or adeno-associated virus vectors20 to prepare.
Unlike the original SPRINT-CRISPR method15, fresh (frozen-thawed) zygotes were used. When obtaining fresh zygotes by natural mating, the technical errors can be ignored that may occur during in vitro fertilization or freezing and thawing. Also, the embryo manipulation process can be completed in approximately half a day (Figure 1) following the current approach. On the other hand, some limitations of the present method include the requirement of many male mice, the loose control of the fertilization timing, and the limited ability to completely predict the number of zygotes for microinjection. Compared to the original SPRINT-CRISPR method, this method may have a higher birth rate (Table 1). Despite this, it cannot be concluded that the present method is better in terms of birth rate because of the differences in the experiment conductors, target genes, genetic backgrounds, and timing of embryo confirmation.
As shown in Table 1, a large number of mice in most of the gene cassette knock-in projects had random integration alleles. Random integration must be eliminated because it can lead to unintended disruption of endogenous genes and ectopic expression of transgenes. The challenge for the future is to find experimental conditions that reduce the number of random integration events while maintaining sufficient knock-in efficiency.
Almost all mouse zygote genome editing using genome editing effectors that result in DNA double-strand breaks is likely to induce unintended mutations at on-target sites9,21. The results of these unintended on-target mutations are not described here because we are not in a situation in which the next generation of mice can be managed in order to present clear evidence of them. The best way to rule out the concern of confusion caused by unintended on-target mutagenesis is to obtain the next generation of mice by mating founders with wild-type mice rather than intercrossing founders. In principle, the N1 mice resulting from this cross are wild-type (WT) on one side of the allele, so if the intended knock-in (KI) allele is detected, they are WT/KI heterozygous. Therefore, the on-target mutagenesis is not a critical problem in the laboratory mouse, which has a relatively fast life cycle and is a prolific animal. However, further improvement will be necessary when this technology is applied to relatively large mammals.
It has been confirmed that this technology can produce gene cassette knock-in mice in inbred strains other than C57BL/6J, but a sufficient number of projects are not secured, and the data are highly variable. For this reason, this information is not included in the present study. It is believed that further studies on this subject will be necessary to advance mouse genetics and disease model research.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by Scientific Research (B) (19H03142: to SM), Scientific Research (A) (20H00444: to FS), Scientific Research (A) (21H04838: to SM), and Scientific Research on Innovative Areas "Platform of Advanced Animal Model Support" (16H06276: to ST) from the Ministry of Education, Culture, Sports, Science, and Technology. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. We are grateful to Ryoichi Mori for his helpful discussion about genome-editing design.
Materials
| Atipamezole Hydrochloride | ZENOAQ | – | |
| Autoclip Wound Clip | BD | 427631 | |
| Autoclip Wound Clip Applier | BD | 427630 | |
| Butorphanol | Meiji Seika Pharma | – | |
| C57BL/6J mice | Jackson Laboratory Japan | – | older than 10 weeks old |
| Calibrated Pipet, 100ul | Drummond Scientific Company | 2-000-100 | for collecting zygote and embryo transfer |
| Cas9 | Thermo Fisher Scientific | A36499 | |
| Cas9 Nuclease Reaction Buffer | NEB | B7203 | |
| CellTram 4r oil | Eppendorf | 5196000030 | |
| CRISPOR | http://crispor.tefor.net/ | web tool for genome editing experiments with the CRISPR-Cas9 system | |
| crRNA | IDT | – | |
| Gel/PCR Extraction Kit | FastGene | FG-91302 | |
| hCG | ASKA Animal Health | – | |
| Hyaluronidase | Merck Sigma-Aldrich | H3884 | |
| ICR mice | Jackson Laboratory Japan | – | older than 10 weeks old, weight 28 G or more |
| Inverted microscope | Leica | ||
| KOnezumi | https://www.md.tsukuba.ac.jp/LabAnimalResCNT/KOanimals/konezumi.html | a web application for automating gene disruption strategies to generate knockout mice | |
| M16 medium | Merck Sigma-Aldrich | M7292 | |
| M2 medium | Merck Sigma-Aldrich | M7167 | |
| Medetomidine | ZENOAQ | – | |
| Micro shears | Natsume Seisakusho | MB-54-1 | |
| Microforge | Narishige | MF-900 | for fire polishing of holding pipette and bending the microinjection needle |
| Micromanipulator units | Narishige | ||
| Micropipette puller | Sutter Instrument | P-1000 | programmable pipette puller |
| Midazolam | Maruishi Pharmaceutical | – | |
| MILLEX-GV 0.22 µm filter | Merck Millipore | SLGV033R | |
| Mineral oil | Nacalai | 26114-75 | for zygote culture and injection chamber |
| Petri dish (35mm, untreated) | Iwaki | 1000-035 | for zygote culture |
| PIEZO micromanipulator | PRIME TECH | PMM-150 | |
| Plasmid Mini Kit | FastGene | FG-90502 | mini prep spin column kit |
| PMM Operation Liquid | PRIME TECH | KIT-A | operation liquid for microinjection |
| PMSG | ASKA Animal Health | – | |
| Polyvinylpyrrolidone | Merck Sigma-Aldrich | P5288 | |
| RNase | QIAGEN | 19101 | |
| RNase Free Water | IDT | – | tracrRNA Accessory Reagents |
| Serrefine clamp | Natsume Seisakusho | C-18 | |
| Sodium dodecyl sulfate | SIGMA | 151-21-3 | |
| Suture needle with thread | Natsume Seisakusho | C11-60B2 | |
| Thin wall borosilicate glass without filament |
Sutter Instrument | B100-75-10 | for microinjection needle and holding pipette |
| tracrRNA | IDT | – |
Referências
- Suzuki, H., et al. Generation of bicistronic reporter knockin mice for visualizing germ layers. Experimental Animals. 68 (4), 499-509 (2019).
- Le, H. T., et al. Generation of B6-Ddx4(em1(CreERT2)Utr) , a novel CreERT2 knock-in line, for germ cell lineage by CRISPR/Cas9. Genesis. 58 (7), 23367 (2020).
- Osawa, Y., et al. EXOC1 plays an integral role in spermatogonia pseudopod elongation and spermatocyte stable syncytium formation in mice. Elife. 10, 59759 (2021).
- Funato, H., et al. Forward-genetics analysis of sleep in randomly mutagenized mice. Nature. 539 (7629), 378-383 (2016).
- Yoshimi, K., et al. ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nature Communications. 7, 10431 (2016).
- Miura, H., Gurumurthy, C. B., Sato, T., Sato, M., Ohtsuka, M. CRISPR/Cas9-based generation of knockdown mice by intronic insertion of artificial microRNA using longer single-stranded DNA. Scientific Reports. 5, 12799 (2015).
- Murata, K., et al. Efficient induction of proximity-dependent labelling by biotin feeding in BMAL1-BioID knock-in mice. The Journal of Biochemistry. 170 (4), 453-461 (2021).
- Kaneko, T., Mashimo, T. Simple genome editing of rodent intact embryos by electroporation. PLoS One. 10 (11), 0142755 (2015).
- Kuno, A., et al. DAJIN enables multiplex genotyping to simultaneously validate intended and unintended target genome editing outcomes. PLOS Biology. 20 (1), 3001507 (2022).
- Miyasaka, Y., et al. CLICK: one-step generation of conditional knockout mice. BMC Genomics. 19 (1), 318 (2018).
- Gurumurthy, C. B., et al. Reproducibility of CRISPR-Cas9 methods for generation of conditional mouse alleles: a multi-center evaluation. Genome Biology. 20 (1), 171 (2019).
- Yang, H., et al. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 154 (6), 1370-1379 (2013).
- Hasegawa, Y., et al. Generation of CRISPR/Cas9-mediated bicistronic knock-in ins1-cre driver mice. Experimental Animals. 65 (3), 319-327 (2016).
- Aida, T., et al. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biology. 16, 87 (2015).
- Abe, T., Inoue, K. I., Furuta, Y., Kiyonari, H. Pronuclear Microinjection during S-phase increases the efficiency of CRISPR-Cas9-assisted knockin of large DNA donors in mouse zygotes. Cell Reports. 31 (7), 107653 (2020).
- Lilue, J., et al. Sixteen diverse laboratory mouse reference genomes define strain-specific haplotypes and novel functional loci. Nature Genetics. 50 (11), 1574-1583 (2018).
- Birling, M. C., et al. A resource of targeted mutant mouse lines for 5,061 genes. Nature Genetics. 53 (4), 416-419 (2021).
- Mizuno-Iijima, S., et al. Efficient production of large deletion and gene fragment knock-in mice mediated by genome editing with Cas9-mouse Cdt1 in mouse zygotes. Methods. 191, 23-31 (2021).
- Ohtsuka, M., et al. i-GONAD: a robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biology. 19 (1), 25 (2018).
- Mizuno, N., et al. Intra-embryo gene cassette knockin by CRISPR/Cas9-mediated genome editing with adeno-associated viral vector. iScience. 9, 286-297 (2018).
- Burgio, G., Teboul, L. Anticipating and identifying collateral damage in genome editing. Trends in Genetics. 36 (12), 905-914 (2020).

