Brain Mapping Using a Graphene Electrode Array
Summary
We present a graphene array-based brain mapping procedure to reduce the invasiveness and improve spatiotemporal resolution. Graphene array-based surface electrodes exhibit long-term biocompatibility, mechanical flexibility, and suitability for brain mapping in a convoluted brain. This protocol allows for constructing multiple forms of sensory maps simultaneously and sequentially.
Abstract
Cortical maps represent the spatial organization of location-dependent neural responses to sensorimotor stimuli in the cerebral cortex, enabling the prediction of physiologically relevant behaviors. Various methods, such as penetrating electrodes, electroencephalography, positron emission tomography, magnetoencephalography, and functional magnetic resonance imaging, have been used to obtain cortical maps. However, these methods are limited by poor spatiotemporal resolution, low signal-to-noise ratio (SNR), high costs, and non-biocompatibility or cause physical damage to the brain. This study proposes a graphene array-based somatosensory mapping method as a feature of electrocorticography that offers superior biocompatibility, high spatiotemporal resolution, desirable SNR, and minimized tissue damage, overcoming the drawbacks of previous methods. This study demonstrated the feasibility of a graphene electrode array for somatosensory mapping in rats. The presented protocol can be applied not only to the somatosensory cortex but also to other cortices such as the auditory, visual, and motor cortex, providing advanced technology for clinical implementation.
Introduction
A cortical map is a set of local patches representing response properties to sensorimotor stimuli in the cerebral cortex. They are a spatial formation of neural networks and enable prediction for perception and cognition. Therefore, cortical maps are useful in evaluating neural responses to external stimuli and processing sensorimotor information1,2,3,4. Invasive and noninvasive methods are available for cortical mapping. One of the most common invasive methods involves the use of intracortical (or penetrating) electrodes for mapping5,6,7,8.
Assessing the on-demand high-resolution cortical maps using penetrating electrodes has faced several obstacles. The method is too laborious to obtain a decent map and too invasive to implement for clinical use, prohibiting further development. More recent technologies such as electroencephalography (EEG), positron emission tomography (PET), magnetoencephalography (MEG), and functional magnetic resonance imaging (fMRI) have gained popularity because these are less invasive and reproducible. However, given their prohibitive costs and poor resolution, they are used in a limited number of cases9,10,11. Recently, flexible surface electrodes with superior signal reliability have attracted considerable attention. Graphene-based surface electrodes demonstrate long-term biocompatibility and mechanical flexibility, providing stable recordings in a convoluted brain12,13,14,15,16. Our group has recently developed a graphene-based multichannel array for high-resolution recording and site-specific neurostimulation on the cortical surface. This technology allows us to keep track of the cortical representations of sensory information for an extended period.
This article describes the steps involved in acquiring a brain map of the somatosensory cortex using a 30-channel graphene multielectrode array. To measure brain activity, a graphene electrode array is placed on the subdural area of the cortex, while the forepaw, forelimb, hind paw, hindlimb, trunk, and whiskers are stimulated with a wooden stick. The somatosensory-evoked-potentials (SEPs) are recorded for somatosensory areas. This protocol can also be applied to other brain areas, such as the auditory, visual, and motor cortex.
Protocol
All animal-handling procedures were approved by the Institutional Animal Care and Use Committee of the Incheon National University (INU-ANIM-2017-08).
1. Animal preparation for surgery
NOTE: Use Sprague Dawley Rat (8-10 weeks old) without the sex bias for this experiment.
- Anesthetize the rat with 90 mg/kg ketamine and 10 mg/kg xylazine cocktail intraperitoneally. To maintain the desired depth of anesthesia throughout the surgery, provide a supplemental 45 mg/kg ketamine and 5 mg/kg xylazine cocktail when the rat shows signs of waking up.
- Confirm that the rat is under deep anesthesia and regularly check body reflections such as toe pinch, tail pinch, and corneal reflex.
- Shave the fur between the eyes and the back of the ears using a trimmer.
- Apply an ophthalmic ointment onto the eyes to prevent them from drying out.
2. Surgery for cortical surface exposure
- Fix the rat head on the stereotaxic apparatus with a stereotaxic adaptor. To maintain the body temperature of 37 °C during the surgery, place the rat on a temperature-controlled heating pad.
- Sterilize the shaved area with alternating scrubs of alcohol and povidone-iodine three times.
- Use forceps to grasp the scalp firmly and inject 0.1 mL of lidocaine (2%) with a syringe directly into the scalp to induce local anesthesia in the surgery area.
- Make a 2-3 cm long midline incision with a scalpel and pull apart the scalp to expose the skull.
- Clamp the scalp with mosquito forceps to expose the skull.
- Scratch the surface of the skull with forceps to remove the periosteum.
- Blunt dissect the muscles over the occipital skull to expose the cisterna magna above the axis on the top of the spinal cord.
- Incise the cisterna magna with the blade to drain the cerebrospinal fluid and put a sterile gauze inside the incision of the cisterna magna to absorb the cerebrospinal fluid constantly to prevent brain edema and minimize inflammation.
- Using a pencil, mark on the skull a rectangular window measuring 3 mm in the anteroposterior axis and 6 mm in the right lateral direction from the bregma of the right hemisphere.
NOTE: Marking must secure a 1 mm distance from the midline to avoid superior sagittal sinus rupture. - Drill the marked area according to the stereotaxic coordinate and remove the skull with a bone rongeur.
- To remove the dura mater, bend the tip of the 26 G needle to 90°, create a hole in the dura mater, lift the dura mater, insert forceps into that hole, and tear it with forceps.
- Place saline-wetted gauze on the somatosensory cortex to prevent it from drying out.
3. Preparation of graphene electrode array connected to the recording system
- Prepare a graphene electrode array with an omnetics connector.
- Detach the graphene multielectrode array without causing damage by applying the saline solution.
- Remove the outer covering of the reference and ground wires from the connector.
- Connect the head stage with the graphene electrode array to the connector.
- Plug the interface cable linked to the head stage into the recording system.
- Secure the graphene electrode array complex into the stereotaxic arm.
- To capture neural signals from all channels, position the array on the somatosensory cortex without any bending, following the predetermined stereotaxic coordinates.
- Place a reference wire underneath the tissue behind the occipital bone and connect the ground wire to the grounded optical table.
4. Physical stimulation and recording SEPs for mapping
- Open the neural signal recording software.
- Set the recording software environment: (1) set the sampling rate for SEPs and notch filter (60 or 50 Hz, a frequency of household electrical power) to remove the noise from the power line.
- For whisker mapping, bend the whisker with a fine stick.
- Constantly poke the forepaw, forelimb, hind paw, hindlimb, and trunk with a wooden stick for body mapping.
- Record neural signals in the data acquisition system for the indicated time.
5. Animal euthanasia
- After all the recording procedures, sacrifice the rats with anesthesia using >5% isoflurane and perform cervical dissection.
6. SEP measurement for cortical mapping
- Open MATLAB code-named read_Intan_RHS2000_file.m for signal analysis.
NOTE: read_Intan_RHS2000_file.m can be downloaded from "https://intantech.com/downloads.html?tabSelect=Software”. - Click the Run button, select the recording file with the ".rhs" filename extension, and wait for the file to be processed and read.
- Enter the command "plot (t, amplifier_data("channel number",:))" to create a 2D line plot of the recording data, find the SEPs, and calculate the amplitude of SEPs in all channels.
NOTE: Enter the channel number at "channel number." For example, "plot(t, amplifier_data(1,:))" creates channel 1's 2D line plot. In addition, when the experimenter calculates the amplitude of the response, choose the response recorded from each channel. - Obtain data by coloring the grid with a different hue according to the amplitude of the SEPs.
NOTE: MATLAB command "imagesc" helps obtain a topographic map more quickly.
Representative Results
This protocol describes how a graphene multichannel array is mounted on the surface of the brain. The somatosensory map was constructed by acquiring neural responses to physical stimuli and calculating the amplitude of the response. Figure 1 shows the schematic of this experiment.
Figure 2A presents the structural characteristics of a graphene electrode array. There are thru-holes of the substrate between the electrodes. These holes help the electrode firmly contact the cortical surface (Figure 2B). The strong adhesion of the electrode to the cortex helps record neural signals with less noise.
Figure 2C (left) shows the location-dependent neural responses acquired by stimulating the whiskers, trunk, paws, and limbs coded in different colors. A rat homunculus, the miniature body of the rat, is drawn with the actual ratio of each color size in the somatosensory cortex map (Figure 2C, right).
Figure 2D presents stimuli-specific responses with colors associated with each body part. The responses are recorded through a graphene electrode array placed on the surface of the cortex. Using the data recorded from the graphene array, the amplitude of SEPs is calculated to obtain the amplitude-dependent somatosensory map.
Sensory stimulus-induced local field potentials enable the construction of the somatosensory map. The response size to each body stimulus poses rodent homunculus. Each color represents a different body part (Figure 3).
The acquired cortex map using this protocol reveals the specific regions within the somatosensory cortex that respond to the whiskers, forepaws, forelimbs, hind paws, hindlimbs, and trunks. It provides insights into the extent of the involvement of the cortical area in processing physical stimulus information for each body part.
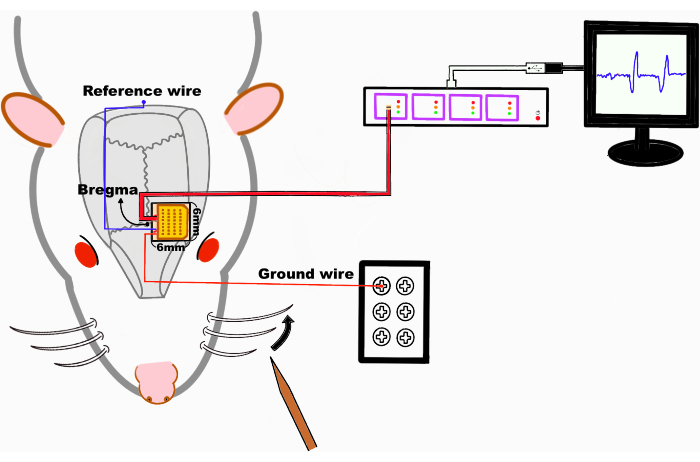
Figure 1: Schematic of the experiment setup. The graphene-based electrode array is attached to the somatosensory cortex, and the whiskers or other body parts are stimulated by gentle touch. The thick red line represents the cable, and the thin red and blue lines represent the ground and reference wires. The black dot indicates the bregma. The data acquisition system is connected to the computer via USB. Please click here to view a larger version of this figure.
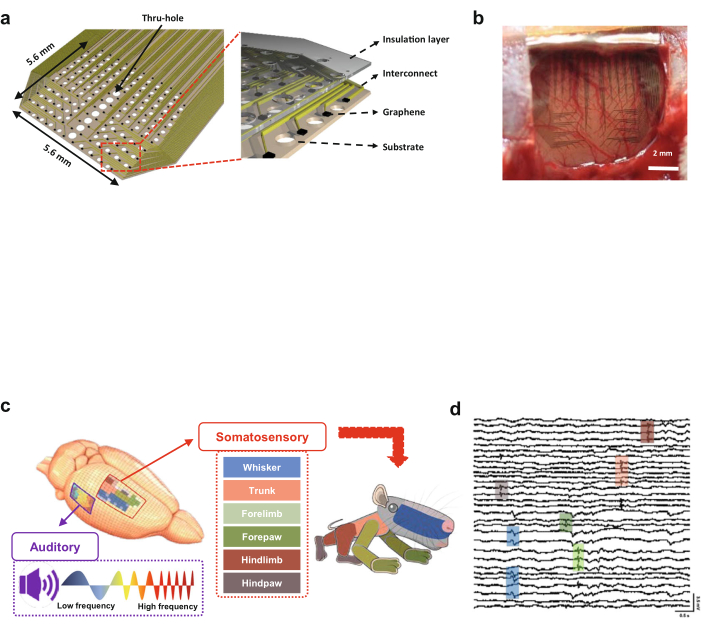
Figure 2: Graphene-based microelectrode array for brain mapping on the cortical surface. (A) Schematic of the graphene-based electrode array. (B) Optical image of the graphene electrode array on the cortical surface. (C) Rat's auditory and somatosensory cortices. Two maps of auditory and somatosensory areas responding to auditory stimuli with various frequency tones and physical stimuli applied to each body part. (D) The 30-channel (excluding the reference and ground electrodes) recording of the graphene-electrode array on the cortical surface. Box colors correlate with the geographical locations of the cortical surface. The figures are adapted and modified from Lee et al. (2021).4 Please click here to view a larger version of this figure.
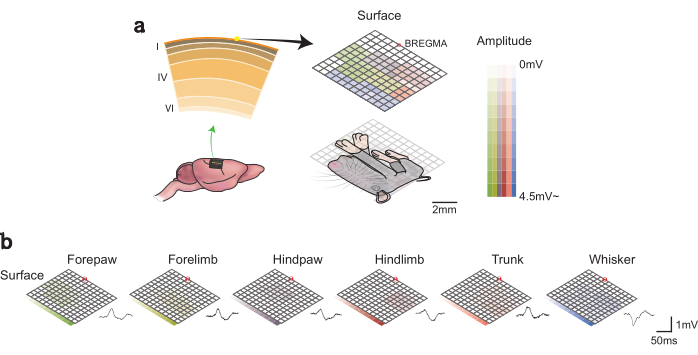
Figure 3: Somatosensory map. (A) Location of neural recordings across cortical layers (left). A cortical surface map determined using a graphene electrode array. A color-coded somatosensory map constructed using the response amplitudes and overlapped with the homunculus (right). (B) Recorded cortical SEPs and maps following the stimulation of each body part. This figure is adapted and modified from Lee et al. (2021).4 Please click here to view a larger version of this figure.
Discussion
The presented protocol provides an in-depth, step-by-step process that explains how to access and map the somatosensory responses of rats using a graphene electrode array. The protocol-acquired data are SEPs that provide somatosensory information that is synaptically linked to each body part.
Several aspects of this protocol should be considered. When extracting cerebrospinal fluid to prevent brain edema and mitigate inflammation, it is crucial for the experimenter not to damage the brainstem located in front of the cisterna magna.
Facial whiskers provide tactile sensory information about the surroundings, such as a dark and narrow environment. Accordingly, rodent whiskers are well-developed enough to sense an object through the deflection directions, stimulus intensity, and location of the stimulated whiskers. The somatosensory cortex responds to the bending direction, intensity, and location of each whisker differently18,19. Therefore, all whiskers are stimulated with constant intensity and direction in this protocol.
This protocol cannot record signals evoked in deep brain structures as our graphene electrode array is mounted on the cortical surface. Thus, the experimenter cannot identify how the columnar network is hierarchically organized concerning neural responses.
This protocol is superior to previous recording methods because the graphene electrode array is less invasive, adaptable, and biocompatible12,13,14,15,16. Furthermore, the graphene electrode array has >30 channels for recording signals, thus enabling faster cortical mapping than a single or tetrode electrode. This protocol can be further applied to other cortical areas whenever required15,20. The experimenter can place the electrode array on the auditory or visual cortex to extract auditory and visual information as the auditory or visual maps. Finally, this method can be implemented for chronic implantation and diagnosis of neural diseases, such as stroke, epilepsy, tinnitus, and Parkinson's disease.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by Incheon National University (International Cooperative) for Sunggu Yang.
Materials
| 1mL syringe | KOREAVACCINE CORPORATION | injecting the drug for anesthesia | |
| 3mL syringe | KOREAVACCINE CORPORATION | injecting the drug for anesthesia | |
| Bone rongeur | Fine Science Tools | 16220-14 | remove the skull |
| connector | Gbrain | Connect graphene electrode to headstage | |
| drill | FALCON tool | grind the skull | |
| drill bits | Osstem implant | grind the skull | |
| Graefe iris forceps slightly curved serrated | vubu | vudu-02-73010 | remove the tissue from the skull or hold wiper |
| graphene multielectrode array | Gbrain | records signals from neuron | |
| isoflurane | Hana Pharm Corporation | sacrifce the subject | |
| ketamine | yuhan corporation | used for anesthesia | |
| lidocaine(2%) | Daihan pharmaceutical | local anesthetic | |
| Matlab R2021b | Mathworks | Data analysis Software | |
| mosquito hemostats | Fine Science Tools | 91309-12 | fasten the scalp |
| ointment | Alcon | prevent eye from drying out | |
| povidone | Green Pharmaceutical corporation | disinfect the incision area | |
| RHS 32ch Stim/Record headstage | intan technologies | M4032 | connect connector to interface cable and contain intan RHS stim/amplifier chip |
| RHS 6-ft (1.8m) Stim SPI interface cable | intan technologies | M3206 | connect graphene electrode to headstage |
| RHS Stim/Recording controller software | intan technologies | Data Acquisition Software | |
| RHS stimulation/ Recording controller | intan technologies | M4200 | |
| saline | JW Pharmaceutical | ||
| scalpel | Hammacher | HSB 805-03 | |
| stereotaxic instrument | stoelting | fasten the subject | |
| sterile Hypodermic Needle | KOREAVACCINE CORPORATION | remove the dura mater | |
| Steven Iris Tissue Forceps | KASCO | 50-2026 | remove the dura mater |
| surgical blade no.11 | FEATHER | inscise the scalp | |
| surgical sicssors | Fine Science Tools | 14090-09 | inscise the scalp and remove the dura mater |
| wooden stick | whisker stimulation | ||
| xylazine | Bayer Korea | used for anesthesia |
Referências
- Leergaard, T. B., et al. Rat somatosensory cerebropontocerebellar pathways: spatial relationships of the somatotopic map of the primary somatosensory cortex are preserved in a three-dimensional clustered pontine map. Journal of Comparative Neurology. 422 (2), 246-266 (2000).
- Craner, S. L., Ray, R. H. Somatosensory cortex of the neonatal pig: I. Topographic organization of the primary somatosensory cortex (SI). Journal of Comparative Neurology. 306 (1), 24-38 (1991).
- Benison, A. M., Rector, D. M., Barth, D. S. Hemispheric mapping of secondary somatosensory cortex in the rat. Journal of Neurophysiology. 97 (1), 200-207 (2007).
- Lee, M., et al. Graphene-electrode array for brain map remodeling of the cortical surface. NPG Asia Materials. 13 (1), (2021).
- Yang, S. C., Weiner, B. D., Zhang, L. S., Cho, S. J., Bao, S. W. Homeostatic plasticity drives tinnitus perception in an animal model. Proceedings of the National Academy of Sciences of the United States of America. 108 (36), 14974-14979 (2011).
- Yang, S., Zhang, L. S., Gibboni, R., Weiner, B., Bao, S. W. Impaired development and competitive refinement of the cortical frequency map in tumor necrosis factor-alpha-deficient mice. Cerebral Cortex. 24 (7), 1956-1965 (2014).
- Miyakawa, A., et al. Tinnitus correlates with downregulation of cortical glutamate decarboxylase 65 expression but not auditory cortical map reorganization. Journal of Neuroscience. 39 (50), 9989-10001 (2019).
- Yang, S., Su, W., Bao, S. Long-term, but not transient, threshold shifts alter the morphology and increase the excitability of cortical pyramidal neurons. Journal of Neurophysiology. 108 (6), 1567-1574 (2012).
- Beniczky, S., Schomer, D. L. Electroencephalography: basic biophysical and technological aspects important for clinical applications. Epileptic Disorders. 22 (6), 697-715 (2020).
- Kim, S. G., Richter, W., Uğurbil, K. Limitations of temporal resolution in functional MRI. Magnetic Resonance Medicine. 37 (4), 631-636 (1997).
- Cho, Z. H., et al. A fusion PET-MRI system with a high-resolution research tomograph-PET and ultra-high field 7.0 T-MRI for the molecular-genetic imaging of the brain. Proteomics. 8 (6), 1302-1323 (2008).
- Viventi, J., et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nature Neuroscience. 14 (12), 1599-1605 (2011).
- Masvidal-Codina, E., et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nature Materials. 18 (3), 280-288 (2019).
- Blaschke, B. M., et al. Mapping brain activity with flexible graphene micro-transistors. 2D Materials. 4 (2), 025040 (2017).
- Park, S. W., et al. Epidural electrotherapy for epilepsy. Small. 14 (30), 1801732 (2018).
- Lim, J., et al. Hybrid graphene electrode for the diagnosis and treatment of epilepsy in free-moving animal models. NPG Asia Materials. 15 (1), 7 (2023).
- Hermanns, H., et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. British Journal of Anaesthesia. 123 (3), 335-349 (2019).
- Tchoe, Y., et al. Human brain mapping with multithousand-channel PtNRGrids resolves spatiotemporal dynamics. Science Translational Medicine. 14 (628), (2022).
- Wilent, W. B., Contreras, D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nature Neuroscience. 8 (10), 1364-1370 (2005).
- Insanally, M. N., Köver, H., Kim, H., Bao, S. Feature-dependent sensitive periods in the development of complex sound representation. Journal of Neuroscience. 29 (17), 5456-5462 (2009).

