Development of Leishmania Species Strains with Constitutive Expression of eGFP
Summary
Here, we describe the methodology used for generating L. panamensis and L. donovani strains expressing the gene for eGFP as a stable integrated transgene using the pLEXSY system. Transfected parasites were cloned by limiting dilution, and clones with the highest fluorescence intensity in both species were selected for further use in drug screening assays.
Abstract
Protozoan parasites of the genus Leishmania cause leishmaniasis, a disease with variable clinical manifestations that affects millions of people worldwide. Infection with L. donovani can result in fatal visceral disease. In Panama, Colombia, and Costa Rica, L. panamensis is responsible for most of the reported cases of cutaneous and mucocutaneous leishmaniasis. Studying a large number of drug candidates with the methodologies available to date is quite difficult, given that they are very laborious for evaluating the activity of compounds against intracellular forms of the parasite or for performing in vivo assays. In this work, we describe the generation of L. panamensis and L. donovani strains with constitutive expression of the gene that encodes for an enhanced green fluorescent protein (eGFP) integrated into the locus that encodes for 18S rRNA (ssu). The gene encoding eGFP was obtained from a commercial vector and amplified by polymerase chain reaction (PCR) to enrich it and add restriction sites for the BglII and KpnI enzymes. The eGFP amplicon was isolated by agarose gel purification, digested with the enzymes BglII and KpnI, and ligated into the Leishmania expression vector pLEXSY-sat2.1 previously digested with the same set of enzymes. The expression vector with the cloned gene was propagated in E. coli, purified, and the presence of the insert was verified by colony PCR. The purified plasmid was linearized and used to transfect L. donovani and L. panamensis parasites. The integration of the gene was verified by PCR. The expression of the eGFP gene was evaluated by flow cytometry. Fluorescent parasites were cloned by limiting dilution, and clones with the highest fluorescence intensity were selected using flow cytometry.
Introduction
Protozoan parasites of the genus Leishmania cause leishmaniasis, a disease with a wide range of clinical manifestations. This disease is prevalent in 98 countries, and its annual incidence is estimated at 0.9 to 1.6 million cases1. Leishmania species that are pathogenic to humans are divided into two subgenera, namely L. (Leishmania) and L. (Viannia). Infection with some species belonging to the L. (Leishmania) subgenus, such as L. donovani and L. infantum, may result in visceral leishmaniasis (VL), which is fatal if left untreated2. Species belonging to the L. (Viannia) subgenus are associated with most cases of cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis (MCL) in Central and South America, particularly in Panama, Colombia, and Costa Rica, with L. panamensis being the main etiological agent of these clinical presentations3,4.
Existing anti-leishmania chemotherapy that includes drugs such as pentavalent antimonials, miltefosine, and amphotericin B is highly toxic and expensive. Furthermore, increased drug resistance during recent decades has been added to the factors that interfere with the effective treatment of patients worldwide5. Substantial differences have been demonstrated across species of the genus Leishmania in relation to drug susceptibility, especially between New and Old World species6,7. For these reasons, it is necessary to direct efforts to the identification and development of new anti-leishmania drugs, paying special attention to species-specific approaches. Studying large libraries of drug candidates with traditional methodologies is quite difficult, given that these methodologies are very laborious for evaluating the activity of compounds against intracellular amastigotes or for performing in vivo experiments8; therefore, it has been necessary to develop new techniques that reduce these disadvantages, including the implementation of reporter genes and development of high-content phenotypic screening assays9.
The use of reporter genes has shown the potential to increase the efficiency of the drug screening process as it facilitates the development of high-throughput and in vivo assays. Recombinant Leishmania parasites expressing several reporter genes have been generated by various research groups. Reporter genes, such as β-galactosidase, β-lactamase, and luciferase, have been introduced in several Leishmania species using episomal vectors, showing limited utility for drug screening in extra- and intra-cellular forms of the parasite10,11,12,13,14,15. These approaches have the limitation of requiring a strong selective pressure in culture to avoid the elimination of the episomal construct, as well as the use of additional reagents to reveal the activity of the reporter gene. Conversely, the green fluorescent protein (GFP) and its variant, the enhanced green fluorescent protein (eGFP), have been used in the generation of a large number of transgenic Leishmania strains for in vitro drug screening assays due to their flexibility and sensitivity, as well as the possibility of automating the screening process using flow cytometry or fluorometry15,16,17,18,19. Despite promising results, cultures of these transgenic strains were highly heterogeneous in their fluorescence levels, since the number of copies of the GFP gene was not the same in all the parasites. Furthermore, maintaining fluorescence required constant selective pressure on the parasites in culture, since the GFP gene was introduced in an episomal construct.
For the reasons stated before, many efforts have focused on developing new methodologies for producing stable recombinant strains. These efforts have mostly relied on the integration of reporter genes into ribosomal loci, taking advantage of the higher transcription rates of ribosomal genes20. Strains of L. infantum and L. amazonensis have been generated having integrated the genes coding for β-galactocidase21, IFP 1.4, iRFP22, and tdTomato23, and they have been evaluated for their usefulness in drug screening assays. Various groups have developed L. donovani strains that express GFP constitutively by integrating its coding gene into the 18S ribosomal RNA locus (ssu locus) through homologous recombination24,25; they showed stable and homogeneous GFP expression in the transfected population, including intracellular amastigotes24,25, and they were successfully implemented in drug screening assays24,25,26. Bolhassani et al.27 developed strains of L. major and L. infantum expressing GFP as an integrated transgene. They used the integration vector pLEXSY, originally designed for the transgenic expression of proteins in a system using L. tarentolae as the host28. The pLEXSY-GFP vector has shown to be very efficient for the generation of different Leishmania strains constitutively expressing GFP24,25,27,29,30. In these parasites, fluorescence is homogeneous and maintained in the intracellular forms, being able to be detected in footpad lesions of infected mice27.
In this work, we describe the methodology used for generating L. panamensis and L. donovani strains expressing the gene encoding for eGFP as an integrated transgene using the pLEXSY system. The strains generated through this process are used in our laboratory for performing drug-screening assays that evaluate the potential anti-leishmania activity of molecules of natural and synthetic origin.
Protocol
To keep the samples sterile, all steps involving parasite culture should be performed inside a biosafety level 2 (BSL-2) hood or according to local health and safety regulations. A graphical summary of this protocol can be found in Figure 1.
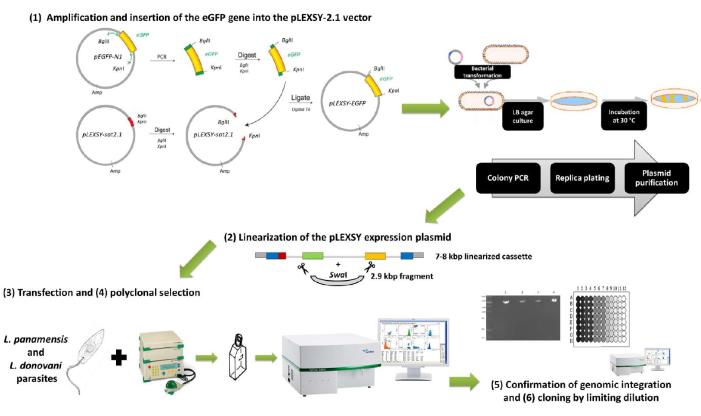
Figure 1: Summary scheme of the protocol for generating eGFP-expressing L. panamensis and L. donovani parasites using the pLEXSY-2.1 vector family. All six major sections described in the article are depicted here. 1) Amplification and insertion of the eGFP gene into the pLEXSY-2.1 vector: the target gene is amplified, adding the recognition sites for the enzymes KpnI and BglII, and both the eGFP amplicon and pLEXSY plasmid are sequentially digested with both enzymes for subsequent ligation with T4 ligase. 2) Linearization of the pLEXSY expression plasmid: the pLEXSY + eGFP construct is digested with SwaI for linearization and purification of the 7-8 kbp expression cassette. 3) Transfection and 4) polyclonal selection: Leishmania promastigotes are transfected with the expression cassette by electroporation and put back in culture for antibiotic selection of recombinant parasites. Parasite fluorescence is confirmed through flow cytometry. 5) Confirmation of genomic integration and 6) cloning by limiting dilution: integration of the pLEXSY-eGFP expression cassette is confirmed by diagnostic PCR, using a forward primer hybridizing to the expression cassette and a reverse primer hybridizing to a chromosomal sequence absent in the expression cassette. Transfected cultures could be enriched for fluorescent parasites by cloning by limiting dilution, and clones with the highest mean fluorescent intensities can be selected for further applications. Please click here to view a larger version of this figure.
1. Amplification and insertion of the eGFP gene into the pLEXSY-2.1 vector
- Amplification of the eGFP gene
NOTE: As this protocol uses the pLEXSY-2.1 vector family (see Table of Materials) for the constitutive expression of target proteins following the integration of the expression cassette into the chromosomal 18S rRNA locus (ssu) of Leishmania species, the first step is to introduce in the eGFP gene the sequences containing the restriction sites that allow its insertion into pLEXSY-2.1 vectors. In this case, as eGFP is only required to be expressed cytosolically, the restriction sites for the enzymes BglII and KpnI were added in the 5' and 3' ends of the eGFP gene, respectively. Cloning with KpnI results in the fusion of the target protein to a C-terminal polyhistidine tag of six residues, followed by a stop codon encoded in the pLEXSY-2.1 plasmid for further purification of the protein of interest. For this reason, the reverse primer sequence does not contain a stop codon.- Depending on the plasmid source for eGFP, analyze the target sequence using a restriction analysis tool such as NEBcutter (http://tools.neb.com/NEBcutter/index.php3)31 to make sure that it does not contain internal sites for the restriction enzymes used for cloning (BglII and KpnI) or for SwaI, which is the enzyme used for vector linearization prior to transfection.
- Design forward and reverse primers for eGFP amplification containing the BglII and KpnI restriction sequences. As an example, the eGFP source for this protocol was the pEGFP-N1-1X plasmid32, and the primer sequences were those reported by Bolhassani et al.27:
Forward primer (EGFP1): 5'-ATGATATCAAGATCTATGGTGAGCAAGGGC-3' (BglII restriction site in bold).
Reverse primer (EGFP2): 5'-GCTCTAGATTAGGTACCCTTGTACAGCTCGTC-3' (KpnI restriction site in bold). - Amplify the target gene using a high-fidelity polymerase to ensure the preservation of the coding sequence. As an example, for the primers EGFP1 and EGFP2, run the PCR cycling protocol as reported by Bolhassani et al.27. Run an initial denaturation of 2 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 57 °C, and 1 min at 72 °C. Run a final extension step of 10 min at 72 °C. Add the primers at a final concentration of 0.2 µM.
- Purify the fragment using a conventional PCR product purification kit, or standard sodium acetate and ethanol precipitation, as described elsewhere33.
- Insertion of the eGFP into the pLEXSY-2.1 expression vector
- Trim the ends of the eGFP-PCR product using BglII and KpnI, following the manufacturer's instructions.
- First, set up the reaction for the enzyme requiring the lowest salt concentration (in this case, KpnI), according to the manufacturer's instructions, and incubate at 37 °C for 1 h.
- Then, add 100 mM NaCl and 10 units of BglII and incubate for 15 min. Purify the reaction, as described in step 1.1.4.
- Digest the pLEXSY expression vector with BglII and KpnI, following the sequential digestion protocol described in step 1.2.1.
- Ligate the pLEXSY vector and eGFP gene with T4 ligase using a molar ratio of 1:3 (vector:insert). Briefly add 100 ng of digested pLEXSY and 28 ng of digested eGFP product to a 20 µL reaction containing ligase buffer at a final concentration of 1x and 3 units of T4 DNA ligase. Incubate overnight at 4 °C.
- Transform competent E. coli cells, such as XL-10, DH5α, or DH10B, with the ligation product obtained in step 1.2.3 using standard transformation protocols33. Plate the transformed cells in Luira-Bertani (LB)-ampicillin agar and incubate for 24 h at 30 °C to select recombinant clones (pLEXSY plasmids are more stable in E. coli at 30 °C than at 37 °C).
- Screen for the presence of the insert in the plasmids by colony PCR.
- Pick individual colonies, resuspend in 20 µL of nuclease-free water, heat at 95 °C for 15 min, and take 1 µL of the lysate as a DNA template for colony PCR. Use the forward primer for eGFP amplification, described in step 1.1.2 (in this example, EGFP1), and the primer A264 (5'-CATCTATAGAGAAGTACACGTAAAAG-3') as a reverse primer29.
- The primer A264 is designed to anneal 80 bp after the stop codon of the insert in pLEXSY-2.1 expression vectors. As an example, for the primer pair EGFP1 + A264, use a PCR cycling protocol consisting of an initial denaturation of 2 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 50 °C, and 1 min at 72 °C. Run a final extension step of 10 min at 72 °C. The primers are added at a final concentration of 0.2 µM.
NOTE: The expected product using this primer set is 859 bp long. The annealing sites for the primers EGFP1 and A264 are depicted in Figure 2.
- Prepare the purified plasmid DNA from a positive clone for subsequent transfection using a commercial plasmid isolation kit or standard alkaline precipitation33. Use 50 mL of an overnight culture at 30 °C for isolation of a minimum of 10 µg of plasmid/positive clone.
- Trim the ends of the eGFP-PCR product using BglII and KpnI, following the manufacturer's instructions.
2. Linearization of the pLEXSY expression plasmid
- Use at least 10 µg of the purified pLEXSY-eGFP plasmid for digestion with SwaI (recognition site: 5'-ATTTAAAT-3'). Digest for 3-4 h at 25 °C and heat-inactivate at 65 °C for 20 min.
- Run the digestion product in agarose gel electrophoresis to separate the resulting fragments. This digestion will generate two fragments, a 2.9 kbp fragment representing the elements necessary for replication and selection in E. coli, and a 7-8 kbp fragment representing the linearized expression cassette.
- Purify the expression cassette using a commercial agarose gel extraction kit.
3. Transfection of L. panamensis and L. donovani by electroporation
NOTE: Leishmania culture is performed as described elsewhere34. In this example, L. panamensis and L. donovani promastigotes were cultured in Schneider's insect medium, supplemented with 20% (v/v) fetal bovine serum (FBS) and 50 µg/mL gentamicin, and incubated at 26 °C.
- Grow the parasite cultures until there are enough log-phase or early stationary-phase promastigotes to use approximately 4 x 107 parasites per transfection. The maximum cell density per culture flask before reaching the stationary phase may vary depending on the Leishmania species and how well the strains are adapted to laboratory conditions. Pool the contents of multiple culture flasks if required.
- Centrifuge the parasite culture at 2,000 x g for 3 min at room temperature (RT).
- Resuspend the pellet in electroporation buffer at 4 °C (21 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, and 6 mM glucose; pH 7.5)27 to get a concentration of 1 x 108 parasites/mL. Put on ice for 10 min.
NOTE: The electroporation buffer must be sterilized by filtering before use. - In parallel, pre-chill tubes with 2-10 µg of linearized pLEXSY-eGFP construct in a maximum volume of 50 µL of water or 10 mM tris buffer (pH 8.0) and electroporation cuvettes of d = 2 mm.
- Add 350 µL of pre-chilled parasites to the tube with the linearized plasmid and transfer the entire 400 µL to the electroporation cuvette on ice. In parallel, electroporate the parasite cells without plasmid DNA as a negative control.
- Electroporate using one of these two protocols:
Exponential decay: 450 V, 450 µF, one pulse.
Time constant: 450 V, T = 3.5 ms, one pulse.
NOTE: These protocols were run using a commercial gene pulser (Table of Materials) with PC and CE modules. - Put the cuvette back on ice for 10 min and transfer the electroporated parasites to 5 mL of the appropriate culture medium. This example used Schneider's insect medium supplemented with 20% (v/v) FBS. Incubate at 26 °C for 20 h.
4. Polyclonal selection in culture
- Approximately 20 h post-electroporation, observe the cultures under a microscope. At least half of the parasite population should show visually good morphology and motility (drop-like promastigotes with oscillating flagellum moving through the media and/or forming active parasite aggregates). Add the appropriate selective antibiotic depending on the pLEXSY plasmid used. In this example, pLEXSY-sat2.1 is used, which contains streptothricine acetyltransferase as a selection marker. Therefore, use nourseothricin as a selective antibiotic at a 0.1 mg/mL concentration.
- Follow the cultures microscopically until a clear difference between the parasites electroporated with and without plasmid DNA is seen.
- Verify the parasite fluorescence through flow cytometry.
- Centrifuge 1 mL of the stationary-phase culture at 2,000 x g for 3 min at RT. Wash twice in PBS and resuspend the final pellet in 1 mL of PBS.
NOTE: The fluorescence of eGFP can be detected in the FL1 channel of most commercial cytometers. Gain settings for forward and side scatter, and the FL1 channel may vary between cytometers. For this example, a CyFlow space (Sysmex) cytometer was used. - Run the samples, collecting 20,000 events at a speed of 0.5 µL/s, and set the gain values for the forward scatter (FSC), side scatter (SSC), and fluorescence 1 (FL-1) channels as 225.0, 200.0, and 520.0, respectively. Run a control sample with non-fluorescent parasites to determine the parasite population in a FSC versus SSC dot-plot.
- Create a gate (G1) containing the parasite population and filter the FL-1 channel through that gate for determining the autofluorescence of promastigotes and setting a range gate (G2) for the FL-1 channel.
- Run the transfected parasites to verify if there is fluorescence. Record the percentage of parasites in G1 that are fluorescent and the mean of fluorescence intensity in G2.
- Centrifuge 1 mL of the stationary-phase culture at 2,000 x g for 3 min at RT. Wash twice in PBS and resuspend the final pellet in 1 mL of PBS.
5. Confirmation of genomic integration
NOTE: Integration of the pLEXSY-eGFP expression cassette can be confirmed by diagnostic PCR, using a forward primer hybridizing to the expression cassette (varies depending on the pLEXSY-2.1 vector used) and the reverse primer F3002 (5'-CTGCAGGTTCACCTACAGCTAC-3') hybridizing to a chromosomal ssu-flanking sequence absent in the expression cassette. In this example, the F2999 forward primer (5'-CCTAGTATGAAGATTTCGGTGATC-3') is used as it hybridizes in the pLEXSY-sat2.1 expression cassette. A schematic representation of the integrated expression cassette and primer annealing sites for diagnostic PCR is depicted in Figure 2.
- Purify the genomic DNA from 2-5 mL of a stationary-phase parasite culture by conventional phenol/chloroform extraction35 or with a commercial kit.
- Perform the diagnostic PCR for pLEXSY-sat2.1 using a cycling protocol consisting of an initial denaturation of 2 min at 94 °C, followed by 30 cycles of 30 s at 94 °C, 30 s at 53 °C, and 1 min at 72 °C. Run a final extension step of 10 min at 72 °C. The primers are added at a final concentration of 0.2 µM.
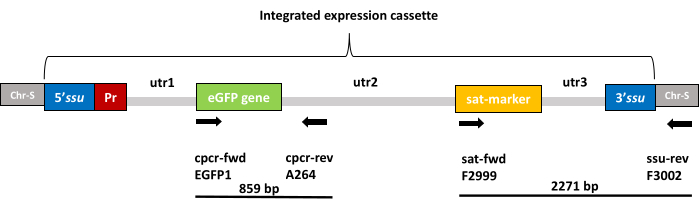
Figure 2: Schematic representation of the integrated expression cassette. Boxes labeled as Chr-S (gray) represent the adjacent chromosomal sequences of the 18S rRNA locus (ssu) into which the expression cassette is integrated. The integrated expression cassette consists of 5' and 3' sequences (blue) for homologous recombination into the ssu locus, an additional Leishmania ribosomal promoter (red) for enhanced protein production, the eGFP gene (green), a selection marker gene (yellow), and three untranslated regions (light gray), namely utr1, 2, and 3, which provide the splicing signals for post-transcriptional mRNA processing for optimized expression of eGFP and the selection marker in Leishmania parasites. Annealing sites for forward and reverse primers used for verification of the insert presence by colony PCR (step 1.2.5) are marked by the black arrows labeled as cpcr-fwd (EGFP1 primer) and cpcr-rev (A264 primer) respectively, which delimit an 859 bp product. The annealing sites for forward and reverse primers used for the verification of genomic integration by diagnostic PCR (step 5) are marked by the black arrows labeled as sat-fwd (F2999 primer) and ssu-rev (F3002 primer), respectively, which delimit a 2,271 bp product. Please click here to view a larger version of this figure.
6. Cloning by limiting dilution (optional)
- After confirmation of fluorescence through flow cytometry, prepare a dilution of recombinant parasites at a concentration of five promastigotes/mL36.
- Add 100 µL of this dilution into each well of a 96-well plate. In this manner, the plates are seeded at an average density of 0.5 promastigotes/well, which ensures that some wells receive a single parasite while minimizing the probability of having more than one parasite per well36.
- Leave the plate undisturbed for at least 12 h. Follow the plate microscopically at a minimum magnification of 100x until wells with parasite growth are detected.
- When a plate well is full of parasites, use the content to inoculate a 5 mL culture. This takes 1-2 weeks.
- When clone-seeded cultures reach the stationary phase, verify fluorescence using flow cytometry, as described in step 4.3. Select the clones to be used for further in vitro and in vivo assays based on the percentage of fluorescent parasites and the mean fluorescence intensity measured in the FL-1 channel. Aim for clones with 98%-99% of fluorescent parasites and select those with the highest mean fluorescence intensity.
Representative Results
After building the pLEXSY-eGFP construct and transforming competent E. coli cells, colonies containing the construct with the eGFP insert will generate an approximately 859 bp product after running the colony PCR described in section 1.2 (Figure 3A). Total digestion of the purified plasmid from positive colonies using SwaI should give two characteristic fragments in gel electrophoresis, a 2.9 kbp fragment that is the portion of the PLEXSY vector containing all the necessary elements for replication and selection in E. coli, and a 7-8 kpb fragment representing the linearized expression cassette that is used for transfection (Figure 3B).
Following transfection, the polyclonal selection is made mostly qualitatively. The status of the parasite cultures during selection with antibiotics is monitored microscopically, bringing special attention to parasite morphology and motility. After successful recovery of a culture of transfected parasites, those effectively expressing eGFP should be detected in the FL-1 channel of a flow cytometer (Figure 4A); transfection efficiencies may vary between different Leishmania species. In this work, after polyclonal selection, 98.44% of L. donovani parasites analyzed by flow cytometry were fluorescent in comparison with 82.00% of L. panamensis (Figure 4B). If the pLEXSY-eGFP expression cassette has been successfully integrated into the ssu locus, a 2.3 kbp product should be observed after running the diagnostic PCR described in section 5 (Figure 4C). These results ensure that recombinant parasites are obtained that constitutively express eGFP as an integrated transgene and will remain stable in subsequent passages of the parasite culture.
Cloning by limiting dilution allows the enrichment of the fluorescent parasite population with lower transfection efficiencies, such as L. panamensis, as well as the selection of clones with the highest fluorescence intensities. Four clones were obtained for each of the Leishmania species used in this work (Table 1), namely Lpan-A7, F11, F12, and H2 for L. panamensis, and Ldon-C1, G4, F2, and H1 for L. donovani. Clones with the highest percentage of fluorescent parasites (Lpan-F11 = 95.74%; Ldon-C1 = 99.16%) and mean fluorescence intensity (Lpan-F11 = 35.63 relative fluorescent units [RFU]; Ldon-C1 = 14.12 RFU) were selected for further use in drug screening assays.
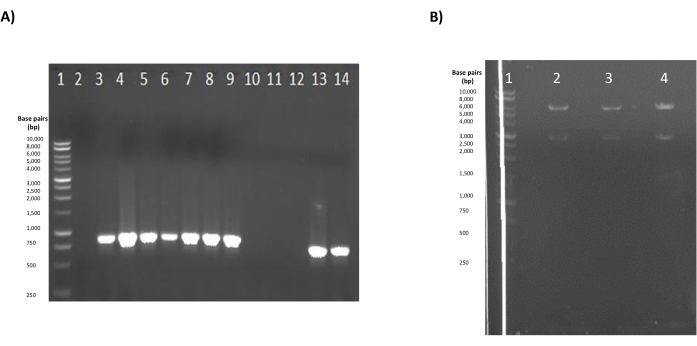
Figure 3: Representative results of preparation of the pLEXSY-eGFP construct for transfection. (A) Representative results of the colony PCR. Lane 1: 1 kb ladder (units in base pairs); lanes 2, 10, and 12: negative samples for the presence of the eGFP insert; lanes 3-9 and 13-14: positive samples for the presence of the eGFP insert. (B) Results of the linearization with SwaI. Lane 1: 1 kb ladder (units in base pairs); lanes 2-4: digestion reactions of plasmids purified from three different colonies positive for the presence of the eGFP insert. A 2.9 kbp fragment representing the elements necessary for replication and selection in E. coli, and a 7-8 kpb fragment representing the linearized expression cassette are observed. Both gels were prepared at an agarose concentration of 1%. Please click here to view a larger version of this figure.
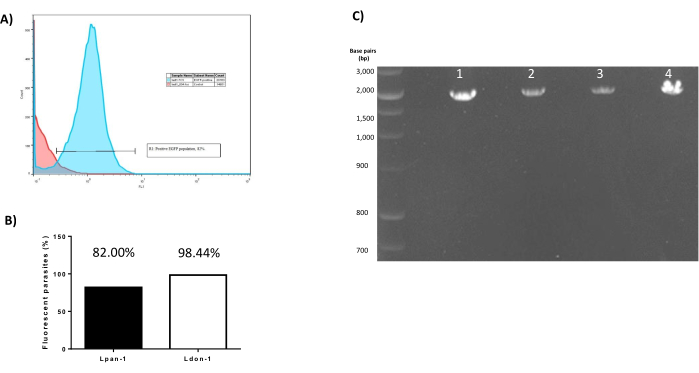
Figure 4: Representative results of confirmation of eGFP expression and chromosomal insertion of the expression cassette. (A) Flow cytometry histogram in the FL-1 channel. Red: wild-type cells without eGFP; Blue: transfected L. panamensis parasites with 82.00% of the population expressing eGFP. (B) Comparison of transfection results between L. panamensis (Lpan-1) and L. donovani (Ldon-1). A higher transfection efficiency was observed in L. donovani (98.44%) than L. panamensis (82.00%) after polyclonal selection. (C) Results of the PCR for confirmation of genomic integration. L: 100 bp ladder (units in base pairs); lanes 1-4: samples from four different clones of L. panamensis showing a characteristic 2.3 kbp product for pLEXSY-sat2.1 integration into the ssu locus. Please click here to view a larger version of this figure.
| Species | Clone code | Percentage of fluorescent parasites | Mean fluorescence intensity* |
| L. panamensis | Lpan-A7 | 90.00 | 24.70 |
| Lpan-F11 | 95.74 | 35.63 | |
| Lpan-F12 | 92.85 | 34.44 | |
| Lpan-H2 | 85.00 | 20.66 | |
| L. donovani | Ldon-C1 | 99.16 | 14.12 |
| Ldon-G4 | 99.21 | 13.96 | |
| Ldon-F2 | 97.96 | 11.67 | |
| Ldon-H1 | 98.97 | 12.90 |
Table 1: Clones obtained by limiting dilution. Four clones were obtained by limiting dilution for each of the Leishmania species used in this work. Percentage of fluorescent parasites and mean fluorescence intensity are reported for each clone. *Fluorescence is expressed in relative fluorescence units (RFU).
Discussion
The advantages and disadvantages of various reporter genes have been studied in several protozoan parasites. Among them, GFP and eGFP are intrinsically fluorescent and allow easy quantification and imaging. The fluorescent activity of these proteins can be detected with minimal manipulation using fluorescence microscopy, fluorimetry, or flow cytometry. Few studies have been carried out for generating GFP-expressing L. (Viannia) strains, despite the demonstrated robustness of GFP and their derivatives as reporter genes in Old World Leishmania species15,16,18,24. Varela et al.37 developed L. panamensis strains that express GFP as an episomal transgene. Flow cytometry analysis of both axenic promastigotes and intracellular amastigotes showed the usefulness of these parasites for drug screening assays. However, fluorescence was very heterogeneous, and the number of fluorescent parasites in the transfected population was dependent on constant selective pressure with the antibiotic tunicamycin. These facts restrict using these parasites as intracellular amastigotes for drug-screening assays, given that tunicamycin cannot be added to infected macrophages38, leaving parasites with low fluorescence present in the population of intracellular amastigotes that could bias the quantification of infected macrophages.
The use of vectors such as pLEXSY constitutes a powerful and reliable tool for the stable modification of Leishmania species parasites through the integration of transgenes by means of homologous recombination28. The pLEXSY cassette is integrated into the 18S rRNA locus (ssu), whose transcription is under the control of RNA polymerase I strong promoter, leading to higher transcription rates20. The use of this system has proven to allow stable and homogeneous expression of the target protein27. These characteristics favor the expression of eGFP and its use in intracellular assays with homogenous levels of fluorescence in infected cells, as well as its use for experimental infections in murine models. The pLEXSY-2.1 vector family used in this protocol has been designed with an additional Leishmania ribosomal promoter in front of the expression cassette, enhancing protein production29,30.
In this protocol, there are several critical steps for obtaining optimal results. Firstly, cloning of the target gene should be performed using a high-fidelity polymerase33, and digestion of the target DNA and expression vector should be done using a sequential digestion protocol when using the BglII/KpnI combination for cloning. The latter is because there is no buffer in which both BglII and KpnI exhibit optimal activity, although both enzymes are optimally active at the same temperature (37 °C). Secondly, the selection of recombinant clones after transformation in E. coli should be carried out at 30 °C, as pLEXSY plasmids are more stable at this temperature in E. coli28,29. Additionally, the growth rates of L. panamensis and L. donavani could be quite different. During the performance of the experiments described in this article, L. panamensis usually took longer to reach the stationary phase. Growth rates can also vary from strain to strain in different laboratories; therefore, it is necessary to characterize the growth rates of the laboratory strains for estimation of the time required to reach the parasite concentration required for electroporation. Using the buffer reported by Bolhassani et al.27 for electroporation is an important modification that significantly increases the survival of the electroporated parasites in comparison with direct electroporation in a culture medium, as suggested by the original Jena Bioscience protocol. During polyclonal selection, it is extremely important not to let the electroporated cultures overgrow before adding the selective antibiotic; otherwise, non-recombinant parasites will outgrow the recombinant ones27. It will take one or two consecutive passages (1:10 inoculum) into fresh medium with the appropriate antibiotic to get a turbid culture of antibiotic-resistant recombinant parasites and a clear negative control. Passages should be done within 5 days of the first antibiotic addition. Finally, there are notably differences in the efficiency of transfection and integration of the pLEXSY expression cassette between L. panamensis and L. donovani24,37. While in L. donovani almost 98% of the total population analyzed through flow cytometry after polyclonal selection shows fluorescence, in L. panamesis, the percentage of fluorescent parasites is around 80%. For this reason, the optional step of cloning by limiting dilution is a modification mostly required to obtain an L. panamensis population where more than 95% of the parasites analyzed through flow cytometry are fluorescent. The use of pLEXSY plasmids is limited to genomic modification of Leishmania species, as they were originally designed for using L. tarentolae as protein production platforms28. Although pLEXSY has proven to work in several Leishmania species different from L. tarentolae21,24,27, it is important to verify the conservation of the ssu sequence when working with a new Leishmania species to make sure that recombination sites in the pLEXSY plasmid will work.
The method described in this article allows the production of recombinant Leishmania parasites with a constitutive and stable expression of eGFP or any other reporter of interest. In these parasites, fluorescence is homogeneous, and it is maintained in intracellular forms. Therefore, strains generated through this process could be used for standardizing high-throughput drug-screening assays to evaluate the potential anti-leishmania activity of molecules of natural and synthetic origin.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was funded by the Secretaría Nacional de Ciencia, Tecnología e Innovación (SENACYT), Panamá, grant number NI-177-2016, and Sistema Nacional de Investigación (SNI), Panamá, grant numbers SNI-169-2018, SNI-008-2022, and SNI-060-2022.
Materials
| 96 Well Microplates | Corning | CLS3340 | Flat bottom clear, black polystyrene, sterile, lid |
| Agarose | Sigma-Aldrich | A4718 | |
| Ampicillin sodium salt | Sigma-Aldrich | A8351 | BioXtra, suitable for cell culture |
| BglII restriction enzyme | New England BioLabs | R0144S | 2,000 units. 10,000 units/mL |
| Cell Culture Flasks | Corning | CLS430168 | Surface area 25 cm2, canted neck, cap (plug seal) |
| ChemiDoc Imaging System | Bio-Rad | 17001401 | |
| CyFlow Space | Sysmex | Not available | |
| D-(+)-Glucose | Sigma-Aldrich | G7021 | powder, BioReagent, suitable for cell culture, suitable for insect cell culture, suitable for plant cell culture, ≥99.5% |
| Fetal Bovine Serum | Sigma-Aldrich | F7524 | |
| Gel Loading Buffer | Sigma-Aldrich | G2526 | The rate of migration varies with gel composition. Dilute 1:3 to 1:6 with sample before loading. |
| Gene Pulser Xcell Electroporation System | Bio-Rad | 1652660 | The system is composed of a main unit, two accessory modules, the capacitance extender (CE module) and the pulse controller (PC module), and a ShockPod cuvette chamber. |
| Gene Pulser/MicroPulser Electroporation Cuvettes | Bio-Rad | 1652086 | Pkg of 50, 0.2 cm–gap sterile electroporation cuvette, for use with the Gene Pulser and MicroPulser Systems, for mammalian and other eukaryotic cells |
| Gentamicin solution | Sigma-Aldrich | G1397 | 50 mg/mL in deionized water, liquid, 0.1 μm filtered, BioReagent, suitable for cell culture |
| GoTaq Long PCR Master Mix | Promega | M4021 | |
| HEPES solution | Sigma-Aldrich | H0887 | 1 M, pH 7.0-7.6, sterile-filtered, BioReagent, suitable for cell culture |
| Inverted microscope | Olympus | IXplore Standard | |
| KpnI-HF restriction enzyme | New England BioLabs | R3142S | 4,000 units. 20,000 units/mL |
| LB Broth with agar | Sigma-Aldrich | L3147 | Highly-referenced nutrient-rich microbial growth powder medium with Agar, suitable for regular E.coli culture. |
| LB Broth | Sigma-Aldrich | L2542 | Liquid microbial growth medium |
| Mini-Sub Cell GT Horizontal Electrophoresis System | Bio-Rad | 1640300 | Mini horizontal electrophoresis system, includes 8- and 15-well combs, 7 cm x 10 cm UV-transparent tray |
| pEGFP-N1-1x | Addgene | 172281 | Expressing eGFP mRNA fused with 1 tandem repeat of a 50-base sequence |
| pLEXSYcon2.1 expression kit | Jena Bioscience | EGE-1310sat | Contains integrative constitutive expression vector pLEXSY-sat2.1. Antibiotic selection of transfectants with Nourseothricin (NTC, clonNAT). Contains all primers for diagnostic PCRs and sequencing. |
| Potassium Chloride | Millipore | 529552 | Molecular Biology Grade – CAS 7447-40-7 – Calbiochem |
| PureYield Plasmid Miniprep System | Promega | A1222 | Up to 15 μg of Transfection-Ready Plasmid from 3 mL cultures. |
| Schneider′s Insect Medium | Sigma-Aldrich | S0146 | Medium used in our laboratory for culturing Leishmania. |
| SOC Medium | Sigma-Aldrich | S1797 | |
| Sodium chloride | Sigma-Aldrich | S3014 | for molecular biology, DNase, RNase, and protease, none detected, ≥99% (titration) |
| Sodium phosphate dibasic | Sigma-Aldrich | RDD038 | BioReagent, suitable for cell culture, suitable for insect cell culture, ≥99.0%, free-flowing, Redi-Dri |
| SwaI restriction enzyme | New England BioLabs | R0604S | 2,000 units. 10,000 units/mL |
| Syringe filters | Corning | CLS431212 | regenerated cellulose membrane, diam. 4 mm, pore size 0.2 μm |
| T100 Thermal Cycler | Bio-Rad | 1861096 | Thermal cycler system, includes 96-well thermal cycler, power cord, tube support ring |
| T4 DNA Ligase | Promega | M1801 | Joins two DNA strands with cohesive or blunt ends |
| Tris-Borate-EDTA buffer | Sigma-Aldrich | T4415 | BioReagent, suitable for electrophoresis, 10× concentrate |
| Wizard Genomic DNA Purification Kit | Promega | A1120 | |
| Wizard SV Gel and PCR Clean-Up System | Promega | A9285 | |
| XL10-Gold Ultracompetent Cells | Agilent | 200317 | XL10-Gold Kanr Ultracompetent Cells, 10 x 0.1 mL. Features the kanamycin-resistance gene on the F' episome, for extremely demanding cloning in chloramphenicol-resistant vectors. Efficiency: > 5 x 10 9 transformants/µg pUC18 DNA. |
Referências
- Alvar, J., et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 7 (5), e35671 (2012).
- Franssen, S. U., et al. Global genome diversity of the Leishmania donovani complex. eLife. 9, e51243 (2020).
- Saldaña, A., et al. Clinical cutaneous leishmaniasis rates are associated with household Lutzomyia gomezi, Lu. Panamensis, and Lu. trapidoi abundance in Trinidad de Las Minas, western Panama. The American Journal of Tropical Medicine and Hygiene. 88 (3), 572-574 (2013).
- Ramírez, J. D., et al. Taxonomy, diversity, temporal and geographical distribution of Cutaneous Leishmaniasis in Colombia: A retrospective study. Scientific Reports. 6, 28266 (2016).
- Ponte-Sucre, A., et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Neglected Tropical Diseases. 11 (12), e0006052 (2017).
- Croft, S. L., Seifert, K., Yardley, V. Current scenario of drug development for leishmaniasis. Indian Journal of Medical Research. 123 (3), 399-410 (2006).
- Croft, S. L., Yardley, V., Kendrick, H. Drug sensitivity of Leishmania species: some unresolved problems. Transactions of the Royal Society of Tropical Medicine and Hygiene. 96, S127-S129 (2002).
- Sereno, D., Cordeiro da Silva, A., Mathieu-Daude, F., Ouaissi, A. Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitology International. 56 (1), 3-7 (2007).
- Don, R., Ioset, J. R. Screening strategies to identify new chemical diversity for drug development to treat kinetoplastid infections. Parasitology. 141 (1), 140-146 (2014).
- Sereno, D., Roy, G., Lemesre, J. L., Papadopoulou, B., Ouellette, M. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrobial Agents and Chemotherapy. 45 (4), 1168-1173 (2001).
- Roy, G., et al. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Molecular and Biochemical Parasitology. 110 (2), 195-206 (2000).
- Lang, T., Goyard, S., Lebastard, M., Milon, G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cellular Microbiology. 7 (3), 383-392 (2005).
- Ashutosh, G. S., Ramesh, S. S., Goyal, N. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrobial Agents and Chemotherapy. 49 (9), 3776-3783 (2005).
- Buckner, F. S., Wilson, A. J. Colorimetric assay for screening compounds against Leishmania amastigotes grown in macrophages. The American Journal of Tropical Medicine and Hygiene. 72 (5), 600-605 (2005).
- Okuno, T., Goto, Y., Matsumoto, Y., Otsuka, H., Matsumoto, Y. Applications of recombinant Leishmania amazonensis expressing egfp or the beta-galactosidase gene for drug screening and histopathological analysis. Experimental Animals. 52 (2), 109-118 (2003).
- Kamau, S. W., Grimm, F., Hehl, A. B. Expression of green fluorescent protein as a marker for effects of antileishmanial compounds in vitro. Antimicrobial Agents and Chemotherapy. 45 (12), 3654-3656 (2001).
- Singh, N., Dube, A. Short report: fluorescent Leishmania: application to anti-leishmanial drug testing. The American Journal of Tropical Medicine and Hygiene. 71 (4), 400-402 (2004).
- Dube, A., Singh, N., Sundar, S., Singh, N. Refractoriness to the treatment of sodium stibogluconate in Indian kala-azar field isolates persist in in vitro and in vivo experimental models. Parasitology Research. 96 (4), 216-223 (2005).
- Chan, M. M. Y., Bulinski, J. C., Chang, K. P., Fong, D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitology Research. 89 (4), 266-271 (2003).
- Boucher, N., McNicoll, F., Dumas, C., Papadopoulou, B. RNA polymerase I-mediated transcription of a reporter gene integrated into different loci of Leishmania. Molecular and Biochemical Parasitology. 119 (1), 153-158 (2002).
- da Silva Santos, A. C., Moura, D. M. N., dos Santos, T. A. R., de Melo Neto, O. P., Pereira, V. R. A. Assessment of Leishmania cell lines expressing high levels of beta-galactosidase as alternative tools for the evaluation of anti-leishmanial drug activity. Journal of Microbiological Methods. 166, 105732 (2019).
- Calvo-Álvarez, E., et al. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Neglected Tropical Diseases. 9 (3), 0003666 (2015).
- García-Bustos, M. F., et al. Development of a fluorescent assay to search new drugs using stable tdtomato-leishmania, and the selection of galangin as a candidate with anti-leishmanial activity. Frontiers in Cellular and Infection Microbiology. 11, 666746 (2021).
- Singh, N., Gupta, R., Jaiswal, A. K., Sundar, S., Dube, A. Transgenic Leishmania donovani clinical isolates expressing green fluorescent protein constitutively for rapid and reliable ex vivo drug screening. Journal of Antimicrobial Chemotherapy. 64 (2), 370-374 (2009).
- De Rycker, M., et al. Comparison of a high-throughput high-content intracellular Leishmania donovani assay with an axenic amastigote assay. Antimicrobial Agents and Chemotherapy. 57 (7), 2913-2922 (2013).
- Peña, I., et al. New compound sets identified from high throughput phenotypic screening against three kinetoplastid parasites: an open resource. Scientific Reports. 5, 8771 (2015).
- Bolhassani, A., et al. Fluorescent Leishmania species: development of stable GFP expression and its application for in vitro and in vivo studies. Experimental Parasitology. 127 (3), 637-645 (2011).
- Breitling, R., et al. Non-pathogenic trypanosomatid protozoa as a platform for protein research and production. Protein Expression and Purification. 25 (2), 209-218 (2002).
- Pulido, S. A., et al. Improvement of the green fluorescent protein reporter system in Leishmania spp. for the in vitro and in vivo screening of antileishmanial drugs. Acta Tropica. 122 (1), 36-45 (2012).
- Bastos, M. S. E., et al. Achievement of constitutive fluorescent pLEXSY-egfp Leishmania braziliensis and its application as an alternative method for drug screening in vitro. Memórias do Instituto Oswaldo Cruz. 112 (2), 155-159 (2017).
- Vincze, T., Posfai, J., Roberts, R. J. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Research. 31 (13), 3688-3691 (2003).
- Chen, M., et al. A molecular beacon-based approach for live-cell imaging of RNA transcripts with minimal target engineering at the single-molecule level. Scientific Reports. 7 (1), 1550 (2017).
- Green, M., Sambrook, J. . Molecular Cloning: A Laboratory Manual. Fourth Edition. , (2012).
- Santarém, N., et al. The impact of distinct culture media in Leishmania infantum biology and infectivity. Parasitology. 141 (2), 192-205 (2014).
- Medina-Acosta, E., Cross, G. A. Rapid isolation of DNA from trypanosomatid protozoa using a simple "mini-prep" procedure. Molecular and Biochemical Parasitology. 59 (2), 327-329 (1993).
- Ye, M., Wilhelm, M., Gentschev, I., Szalay, A. A modified limiting dilution method for monoclonal stable cell line selection using a real-time fluorescence imaging system: a practical workflow and advanced applications. Methods and Protocols. 4 (1), 16 (2021).
- Varela, M. R. E., et al. Leishmania (Viannia) panamensis: an in vitro assay using the expression of GFP for screening of antileishmanial drug. Experimental Parasitology. 122 (2), 134-139 (2009).
- Komura, T., et al. ER stress induced impaired TLR signaling and macrophage differentiation of human monocytes. Cellular Immunology. 282 (1), 44-52 (2013).

