Echocardiography Recording in Awake Miniature Pigs
Summary
A simple cart construct, built to perform research echocardiography in standing awake minipigs, is described, along with building considerations, training techniques, and representative ultrasound images.
Abstract
Echocardiography uses ultrasonic waves to non-invasively assess cardiac structure and function and is the standard of care for cardiac assessment and monitoring. The miniature pig, or minipig, is increasingly being used as a model of cardiac disease in medical research. Pigs are notoriously difficult to restrain and handle safely, and, therefore, research echocardiography in this species is almost always performed under anesthesia or heavy sedation. Anesthetics and sedatives universally affect cardiovascular function and may cause the depression of cardiac output and blood pressure, increases or decreases in heart rate and systemic vascular resistance, changes in the electrical rhythm, and altered coronary blood flow. Therefore, sedated or anesthetized echocardiography may not accurately depict the progression of cardiac disease in large animal models, thereby limiting the translational value of these important studies. This paper describes a novel device that allows for standing awake echocardiography in minipigs. In addition, training techniques used to teach pigs to tolerate this painless and non-invasive procedure without the need for hemodynamic-altering anesthetics are described. Standing awake echocardiography represents a safe and feasible way to perform the most common cardiac monitoring test in minipigs for cardiovascular research.
Introduction
Heart failure is an increasing burden to medical institutions in the United States and abroad, with a worldwide prevalence of 38 million patients1. Approximately 19 million deaths globally were attributed to cardiovascular disease in 2020, demonstrating an 18.7% increase from 20102. New therapy development is slow to catch up with this alarming trend. Heart failure is, therefore, a critical area of research, and the importance of high-fidelity tools for capturing disease development and progression cannot be overstated.
Echocardiography is currently the most clinically important tool for non-invasively measuring the progression of cardiac disease, but in large animal research models, it can be challenging to implement3. Echocardiography uses ultrasonic waves to assess cardiac structure and function and is the standard of care in the clinical setting for cardiac assessment and monitoring4. Preclinical large animal models of cardiac diseases, such as pigs, play a critical role in translating basic science to the development of cardiovascular therapeutics5. It follows, then, that translating echocardiography to large animal models in developing these therapeutics is an important part of this critical endeavor.
Pigs are one of several species commonly used as large animal models of ischemic, pressure overload, and rapid-pacing simulations of heart failure5,6. Pigs are especially important in preclinical studies, as neurohormonal compensatory mechanisms and cardiac remodeling closely mirror human pathophysiology6,7. More recently, miniature pigs, or minipigs, have demonstrated promise as a multiple comorbidities model of cardiac disease, with obesity, hypertension, hypercholesterolemia, and diabetes reliably resulting in cardiac dysfunction and remodeling8,9.
Safely performing echocardiography in most large animals requires heavy sedation or general anesthesia. However, all anesthetic and sedative drugs depress cardiac function in a dose-dependent manner10,11. Anesthetics and sedatives may cause the depression of cardiac output and blood pressure, increases or decreases in heart rate and systemic vascular resistance, changes in the electrical rhythm, and altered coronary blood flow12. In most cases, anesthetics reduce sympathetic tone, decreasing venous return and lowering blood pressure13. Importantly, anesthetics also affect echocardiographic parameters, complicating the interpretation of this examination in monitoring cardiac disease in animal models14. Awake echocardiography is the closest representation of the native cardiac function.
A porcine restraint device, readily accepted by awake minipigs, is described here that can be used for basic echocardiographic monitoring without requiring the administration of hemodynamic-altering anesthetics.
Protocol
The building and use of the echocardiography cart were conducted in compliance with the animal handling and training standards of the University of Utah's Institutional Animal Care and Use Committee.
1. Considerations for building the echocardiography cart
- Build a contraption that allows access to lateral and ventral standing echocardiography imaging windows.
- Use a cart with tall sides, front, and back to prevent the pigs from climbing or jumping out during echocardiography.
- Use a length- and width-adjustable cart to accommodate pigs of various sizes and ages; however, in research studies in which all pigs are of the same size, breed, and age, this may not be necessary.
- Procure a non-slip ramp for mounting and dismounting the cart. Pigs prefer to walk forward, so a backward dismount is undesirable. In this example, a detachable ramp was utilized, such that it could be disconnected from one end after mounting the cart and then moved to the other end of the cart for dismounting.
- Use a wheeled cart with locking wheels as this allows the contraption to be moved from storage to the animal room.
- To comply with vivarium cleaning and disinfection standards, use plastic, metal, and rubber material.
- Finally, include a detachable feeding trough to the front of the cart to provide the pigs with a distraction during the echocardiography.
2. Cart-building specifications
NOTE: The pigs utilized in our study were Yucatan and Gottingen miniature pigs aged 5-10 months old, and, therefore, our cart was constructed with this size in mind.
- While a similar structure could be built from scratch, to decrease some of the building work, start from a premade heavy-duty utility cart (Figure 1). Locking wheels are recommended.
- Saw off and remove the front and back of the utility cart, and replace with gates constructed of PVC pipe and chain link. Use the chain link material for hanging a food trough using hooks or carabiners (Figure 2). With the removal of the front and back, the integrity and holding strength of the plastic are reduced, so for larger and heavier pigs, reinforce with metal bars on the undersides of the cart shelves.
- Create an aperture on the top floor of the cart that is large enough to pass a hand holding an ultrasound probe. Retain a plastic cover piece fitted to the top of the aperture, which can be removed for subxiphoid echocardiography access once the pigs are safely standing in the cart (Figure 3).
- As a custom fit, use aluminum stock (e.g., square tube, bar, and sheet metal) to build a metal mount/dismount ramp with reinforcements to attach to the modified cart at certain attachment points. Add removable rubber padding for grip using bolts and grommets (Figure 4).
- Create a hinge mechanism for the side gates, with simple pins used to narrow the pig standing area for improved restraint (Figure 5). This provides a snug fit for the pig within the cart and ensures the pigs are restrained in a forward-facing direction without the ability to turn around.
3. Training the minipigs to stand in the cart
- Pigs must be trained to eat from the trough for a prolonged period of time, walk up the ramp, and walk down the ramp.
- Filling the trough with a frozen treat prolongs the pigs' standing time. Use combinations such as juice, meal replacer drink, or yogurt with cereal and standard chow, biscuits, and/or fruit bars. Freeze these combinations together to create long-lasting frozen treat troughs (Figure 6). Another option to consider is to withhold the animal's normal meal and instead feed it in the trough in the cart during the echocardiography period.
- Prior to teaching the pigs to eat the frozen treats in the contraption, introduce the frozen treat troughs on the ground, encouraging the recognition of the trough as providing a high-value treat.
- Train the pigs to accept standing echocardiograms for 5-7 days, with one training session per day. Perform the following steps to do this.
- Introduce the pigs to the cart by surrounding the cart with high-value treats (biscuits or cereal) for 1-2 days.
- Introduce the pigs to the ramp for 1-2 days by placing high-value treats along the ramp and providing additional rewards when the pigs walk up the ramp.
- Allow the pigs to stand on the cart without engaging the side restraints or gates (which can induce fear), and provide frozen treat troughs at the front gate for 2-3 days. During the last 1-2 days, while the pigs eat from the troughs, place the ultrasound probe with ultrasound gel on the pigs to accustom them to the sensation of contact with the probe.
- After this training regimen, the pigs will easily allow the restraints and gates to be closed and ultrasound to be performed for the acquisition of echocardiography imaging.
4. Image acquisition
- Obtain images in the echocardiography cart, which allows probe positioning in the following positions, as described below.
- Take images through the sides of the cart to the right and left axillae. These positions are used to obtain the right and left parasternal imaging planes.
- Take images through the floor of the cart to the subxiphoid region to obtain apical views.
- Take images for both B-mode and M-mode imaging from these standing positions.
- For a mock awake echo recording procedure, see Video 1. In the video, the animal subject is represented by a real-sized minipig doll, constrained like a real minipig with limited moving room in the cart. The best access window and positioning of the recording echocardiography probe are also shown.
Representative Results
The representative images acquired in a Yucatan minipig at approximately 8 months of age are presented here. The animal was never sedated and was enjoying feed or frozen treat troughs during the image acquisition.
The echocardiography cart is primarily useful for obtaining simple images for calculating left ventricular chamber volumes and ejection fraction (EF) from B-mode or M-mode images and videos. More sensitive imaging, such as vascular imaging or tissue Doppler, may prove too challenging with this technique, as the awake pigs retain limited mobility, and the imaging time frame is limited by the feeding duration.
The laboratory uses a bedside ultrasound machine without post-hoc image analysis capabilities. Therefore, the videos and still images require processing using editing software and measurement using a scientific image analysis software.
Short-axis transverse-view M-mode images (Figure 7) were obtained through the sides of the cart, which were used for the analysis of the left ventricular internal diameter in systole and diastole (LVIDs and LVIDd, respectively) and the subsequent calculation of the ejection fraction (EF), where EF = (EDV – ESV)/EDV × 100% (EDV: end diastolic volume; ESV: end systolic volume) based on volumes calculated with the Teichholz formula (volume = 7D3/[2.4 + D]) (D: linear LV diameter)15. Representative data generated from four M-mode scans obtained from two minipigs are included in Table 1, as well as data generated from M-mode scans recorded from the same animals during sedated echo sessions. As expected, the EF generated from sedated echo sessions tended to be lower than the EF from the conscious echo session.
Parasternal long-axis view B-mode images were also obtained from the sides of the cart (Figure 8). The area-length method for calculating the EF was utilized with these B-mode images16. First, the left ventricular EDV was calculated from the end-diastolic left ventricular major axis length and chamber area using the formula EDV = (0.85 × area2)/length. The left ventricular ESV was calculated in the same manner using the systolic measurements. The ejection fraction was then calculated as EF = (EDV – ESV)/EDV × 100%. Representative data generated from eight B-mode scans obtained from two minipigs are included in Table 2. For comparison, data generated from B-mode scans recorded from the same animals during sedated echo sessions are also included. Using the B-mode images, the EF generated from sedated and conscious echo sessions were closely matched with each other (Table 2).

Figure 1: Side view of the echocardiography cart. The echocardiography cart is built using a pre-made heavy-duty utility cart. Please click here to view a larger version of this figure.

Figure 2: Head view of the echocardiography cart. The front and back of the pre-made cart are replaced with hinged gates made of PVC pipe and chain link (A), which also accommodates a hanging food trough (B). Please click here to view a larger version of this figure.
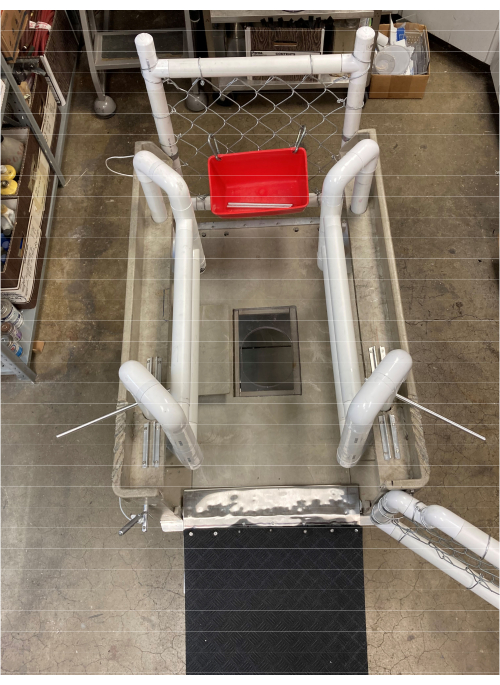
Figure 3: Top view of the echocardiography cart. An aperture is created on the top floor of the cart for passing a hand holding an ultrasound probe. A plastic over-piece is fitted for safe cart mounting and dismounting. Please click here to view a larger version of this figure.

Figure 4: Aluminum ramp. An aluminum ramp is attached to the front or back of the cart, and removable rubber padding is added for grip using bolts and grommets. Please click here to view a larger version of this figure.

Figure 5: Side gate. Hinges are created for the side gates, with pins to allow for more accurate sizing and restraint. Please click here to view a larger version of this figure.

Figure 6: Treats for conscious echo sessions. Combinations of juice, meal replacer drink, or yogurt combined with cereal and standard chow, biscuits, and/or fruit bars are frozen to create long-lasting frozen treat troughs. Please click here to view a larger version of this figure.
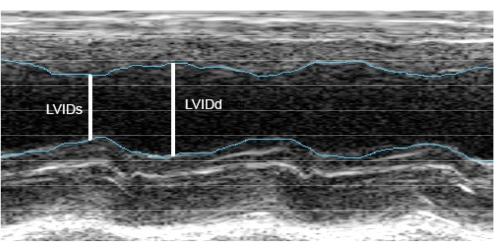
Figure 7: A representative M-mode scan obtained from a conscious animal. Sample image analysis for calculating the left ventricular ejection fraction from M-mode imaging. Please click here to view a larger version of this figure.
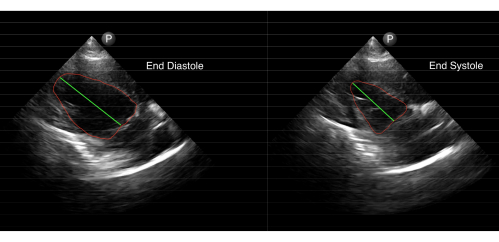
Figure 8: A representative B-mode scan obtained from a conscious animal. Sample image analysis for calculating the left ventricular ejection fraction from B-mode imaging. Please click here to view a larger version of this figure.
| Mean ± SD | LVIDd (cm) | LVIDs (cm) | EF (%) |
| Conscious Echo (N=4/2) | 3.8 ± 0.5 | 2.5 ± 0.5 | 64.3 ± 5.4 |
| Sedated Echo (N=4/2) | 3.9 ± 0.2 | 3.0 ± 0.0 | 48.5 ± 7.9 |
| EF, ejection fraction; SD, standard deviation. | |||
| N = 4 echo scans obtained from two animals | |||
Table 1: Comparison of the parameters generated from M-mode images recorded in sedated minipigs versus conscious minipigs constrained in the cart.
| LV-MALd (cm) | LV-MALs (cm) | LV-CAd (cm2) | LV-CAs (cm2) | EF (%) | |
| Conscious Echo (N=8/2) | 5.8 ± 0.8 | 4.5 ± 0.6 | 18.6 ± 5.0 | 10.7 ± 2.8 | 57.3 ± 5.2 |
| Sedated Echo (N=8/2) | 5.9 ± 0.5 | 4.8 ± 0.4 | 21.8 ± 2.7 | 13.1 ± 2.4 | 55.3 ± 9.0 |
| LV-MAL, left ventricular major axis length; LV-CA, left ventricular chamber area; | |||||
| EF, ejection fraction; SD, standard deviation. N=8 echo scans obtained from 2 animals | |||||
Table 2: Comparison of the parameters generated from B-mode images recorded in sedated minipigs versus conscious minipigs constrained in the cart.
Video 1: A mock awake echocardiogram recording procedure performed on a real-sized minipig doll using the echocardiography cart. Please click here to download this Video.
Discussion
The echocardiography cart represents an easily replicable method for monitoring the heart structure and function in an important cardiac research model, the minipig. The cart’s novelty lies in the ability to capture echocardiographic images without its biggest caveat: the necessity of using anesthetics or sedatives that change the animals’ cardiac function and alter the very measurements used to assess the effects of cardiac therapeutics. Moreover, the cart is safe, inexpensive, and an easy training target for pigs.
The authors first identified the desired features of the cart and then worked closely with a carpenter to design the product. Standard positive reinforcement training techniques were easy and rapid for teaching the pigs to fearlessly accept the cart and utilize it. With ultrasonography practice, the authors were able to rapidly find and record standard two-dimensional echocardiography imaging planes for later processing. During these standing echocardiograms, sedatives or anesthetics were never administered, and, therefore, the videos and images were representative of awake cardiac function.
The building of the echocardiography cart is relatively simple for an experienced carpenter or handyman after identifying the key features important to the research group (for example, size adjustability, height, or ultrasound probe access points). During the building process, the features of the cart can be altered to suit individual laboratories’ needs. The materials are largely inexpensive, and building the cart may save on the cost of performing echocardiograms with the sedatives and anesthetics typically used.
The limitations of the technique included the motion and the limited timeframe to obtain the images. While the cart could be adjusted to varying sizes to restrain the pigs, and while the animals could not turn around and could only move a few inches in each direction, the animals were still capable of motion within the confines of the cart. A headgate, squeeze chute, or stanchion, such as those used with farm animals, could potentially provide better restraint with additional training. Similarly, successful imaging relied on the animals being distracted by their feed or frozen treats during the echocardiograms. Typically, this allowed approximately 15 min of imaging, which was not always sufficient for obtaining all the desired images. The ability to easily replace the food trough or add feed while the animal remained restrained may have prolonged imaging duration. Finally, due to both of the above limitations, more sensitive imaging techniques, such as tissue Doppler, proved difficult to perform in the standing echocardiography cart.
Other porcine experimental models often utilize non-anesthetic handling techniques, such as the commercially available Panepinto sling17. However, the authors found the sling technique more cumbersome for training pigs, and the sling did not provide the ultrasonographer access to the imaging planes required for echocardiography. Other potential applications for the echocardiography cart could include other non-painful procedures such as abdominal ultrasonography, skin lesion observation, or obtaining blood samples from a vascular access port. The authors often utilize the cart to easily restrain pigs to perform electrocardiograms and program pacemakers, for example.
In conclusion, the described awake echocardiography technique is easy to perform and valuable for obtaining basic ultrasound imaging of the heart without the cardiovascular depression typical of anesthetic or sedative use. This technique can be utilized to compare anesthetized images to awake images in large animals or for the everyday monitoring of cardiac disease progression in the valuable porcine preclinical translational model of heart disease and failure.
Declarações
The authors have nothing to disclose.
Acknowledgements
Funding for this research includes NIH-T32 (T.H.), R01HL133286 (TT.H.), R01HL094414 (R.M.S.), R01HL138577 (R.M.S.), R01HL159983 and R21AG074593 (R.M.S. and TT.H.). We extend our gratitude to all members of the research group, adjunct investigators, and staff in the Nora Eccles Harrison Cardiovascular Research and Training Institute and Comparative Medicine at the University of Utah. We would also like to extend our appreciation to Dr. Joseph Palatinus MD PhD for his valuable echocardiography training and assistance.
Materials
| Access Ramp | N/A – shop built | 58" L x 18" W. Rise of 19" not to exceed 22.5 degree angle. | Any removable aluminum ramp with capacity to hold weight of pigs |
| Fence Feeder with Clips | DuraFlex | E011772 | Feed trough with clips for hanging on chain link, used for frozen treats or feed to distract pigs during echocardiography |
| Heavy Duty Utility Cart | Baxter Medical Equipment & Supplies | Cart # unk / 45x25x33"; Pipes, sch 40 PVC | Made of heavy plastic, with three shelves |
| Image Analysis Software | Image J FIJI | https://imagej.net/software/fiji/ | Free scientific image analysis software |
| Lumify Ultrasound with S4-1 Phased Array Transducer | Philips | FUS6884 | Handheld bedside ultrasound with cardiac probe, used with a tablet device and proprietary software |
| Video Editing Software | Adobe Premiere Pro 2022 | https://www.adobe.com/products/premiere.html | Commen software part of Adobe Creative Cloud. |
Referências
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet. 385, 812-824 (2015).
- Tsao, C. W., Aday, A. W., Almarzooq, Z. I., et al. Heart disease and stroke statistics – 2022 Update: A report from the American Heart Association. Circulation. 145 (5), e153-e639 (2022).
- Billig, S., et al. Transesophageal echocardiography in swine: evaluation of left and right ventricular structure, function, and myocardial work. International Journal of Cardiovascular Imaging. 37 (3), 835-846 (2021).
- Boon, J. A. . Veterinary Echocardiography., 2nd edition. , (2011).
- Silva, K. A. S., Emter, C. A. Large animal models of heart failure: A translational bridge to clinical success. Journal of the American College of Cardiology: Basic to Translational Science. 5 (8), 840-856 (2020).
- Pilz, P. M., et al. Large and small animal models of heart failure with reduced ejection fraction. Circulation Research. 130 (12), 1888-1905 (2022).
- Paslawska, U., et al. Normal electrocardiographic and echocardiographic (M-mode and two-dimensional) values in Polish Landrace pigs. Acta Veterinaria Scandinavica. 56 (1), 54 (2014).
- Sharp, T. E., et al. Novel Gottingen miniswine model of heart failure with preserved ejection fraction integrating multiple comorbidities. Journal of the American College of Cardiology: Basic to Translational Science. 6 (2), 154-170 (2021).
- Olver, T. D., et al. Western diet-fed, aortic-banded Ossabaw swine: A preclinical model of cardio-metabolic heart failure. Journal of the American College of Cardiology: Basic to Translational Science. 4 (3), 404-421 (2019).
- Merin, R. G. Effect of anesthetic drugs on myocardial performance in man. Annual Review of Medicine. 28, 75-83 (1977).
- El Mourad, M. B., Shaaban, A. E., El Sharkawy, S. I., Afandy, M. E. Effects of propofol, dexmedetomidine, or ketofol on respiratory and hemodynamic profiles in cardiac patients undergoing transesophageal echocardiography: A prospective randomized study. Journal of Cardiothoracic and Vascular Anesthesia. 35 (9), 2743-2750 (2021).
- Stoelting, R. K., Hillier, S. C. . Handbook of Pharmacology & Physiology in Anesthetic Practice., 2nd edition. , (2006).
- Kristensen, S. D., et al. ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). European Heart Journal. 35 (35), 2383-2431 (2014).
- Roth, D. M., Swaney, J. S., Dalton, N. D., Gilpin, E. A., Ross Jr, J. Impact of anesthesia on cardiac function during echocardiography in mice. American Journal of Physiology – Heart and Circulatory Physiology. 282 (6), H2134-H2140 (2002).
- Chengode, S. Left ventricular global systolic function assessment by echocardiography. Annals of Cardiac Anaesthesia. 19 (Suppl 1), S26-S34 (2016).
- Cacciapuoti, F. Echocardiographic evaluation of ejection fraction: 3DE versus 2DE and M-Mode. Heart Views. 9 (2), 71-79 (2008).
- Yang, H., Galang, K. G., Gallegos, A., Ma, B. W., Isseroff, R. R. Sling training with positive reinforcement to facilitate porcine wound studies. JID Innovations. 1 (2), 100016 (2021).

