Balloon Tag Manufacturing Technique for Sensor Fish and Live Fish Recovery
Summary
A protocol is presented for designing and manufacturing balloon tags to recover Sensor Fish and live fish, allowing assessment of their physical condition and biological performance in hydraulic structures. The method optimizes balloon tag performance by considering factors such as balloon volume, inflation/deflation times, component selection, and the characteristics of the injected water.
Abstract
Fish may experience injuries and mortality when they pass through hydraulic conveyances at hydropower dams, even if these conveyances are designed to be fish-friendly, such as downstream bypass systems, modified spillways and turbines. The main methods used to study fish passage conditions in hydraulic structures involve direct, in situ testing using Sensor Fish technology and live fish. Sensor Fish data helps identify physical stressors and their locations in the fish passage environment, while live fish are assessed for injuries and mortality. Balloon tags, which are self-inflating balloons attached externally to Sensor Fish and live fish, aid in their recovery after passing through hydraulic structures.
This article focuses on the development of balloon tags with varying numbers of dissolvable, vegetable-based capsules containing a mixture of oxalic acid, sodium bicarbonate powders, and water at two different temperatures. Our research determined that balloon tags with three capsules, injected with 5 mL of water at 18.3 °C, consistently achieved the desired balloon volume. These tags had a mean inflation volume of 114 cm3 with a standard deviation of 1.2 cm3. Among the balloon tags injected with water at 18.3 °C, it was observed that the two-capsule balloon tags took the longest time to reach full inflation. In addition, the four-capsule balloon tags demonstrated a faster inflation start time, while the three-capsule balloon tags demonstrated a faster deflation start time. Overall, this approach proves to be effective for validating the performance of new technologies, improving turbine design, and making operational decisions to enhance fish passage conditions. It serves as a valuable tool for research and field evaluations, aiding in the refinement of both the design and operation of hydraulic structures.
Introduction
Hydropower is a significant renewable energy resource worldwide. In the United States, hydropower contributes an estimated 38% or 274 TWh of electricity generated from renewable sources1 and has the potential to add approximately 460 TWh per year2. However, as hydropower development increases, concerns about fish injury and mortality during hydraulic passage have become paramount3. Various mechanisms contribute to fish injuries during passage, including rapid decompression (barotrauma), shear stress, turbulence, strikes, cavitation, and grinding4. Although these injury mechanisms may not have an immediate impact on the overall condition of the fish, they can render them more vulnerable to diseases, fungal infections, parasites, and predation5. Additionally, direct physical injuries resulting from collisions with turbines or other hydraulic structures can lead to significant mortality, emphasizing the importance of mitigating these risks in hydropower development.
One of the most common methods for evaluating fish passage conditions is releasing Sensor Fish and live fish through hydraulic structures6,7. The Sensor Fish is an autonomous device designed to study the physical conditions that fish experience during passage through hydraulic structures, including turbines, spillways, and dam bypass alternatives8,9. Equipped with a 3D accelerometer, 3D gyroscope, temperature sensor, and pressure sensor9, the Sensor Fish provides valuable data on fish passage conditions.
Balloon tags, which are self-inflating balloons attached externally to Sensor Fish and live fish, assist in their recovery after passing through hydraulic structures. The balloon tags consist of dissolvable capsules filled with gas-generating chemicals (e.g., oxalic acid and sodium bicarbonate), a silicone stopper, and a fishing line. Prior to deployment, water is injected through the silicone stopper into the balloon. The water dissolves the vegetable-based capsules, triggering a chemical reaction that produces gas inflating the balloon. In this neutralization reaction, sodium bicarbonate, a weak base, and oxalic acid, a weak acid, react to form carbon dioxide, water, and sodium oxalate10. The chemical reaction is provided below:
2NaHCO3+ H2C2O4 → 2CO2 + 2H2O + Na2C2O4
The inflated balloon increases the buoyancy of the Sensor Fish and live fish, enabling them to float on the water surface for easier recovery.
The number of balloon tags required to achieve flotation and facilitate the retrieval of a sample (e.g., Sensor Fish or live fish) may vary based on the volume and mass characteristics of the sample. The duration of balloon tag inflation can be adjusted by injecting water at different temperatures. Colder water will increase the inflation time, while warmer water will decrease it. Balloon tags have been successfully employed in various locations, including the Farmers Screen, a unique horizontal, flat-plate fish and debris screen structure in Hood River, Oregon11, and a Francis turbine at Nam Ngum Dam in the Lao People's Democratic Republic12. Another commercially available balloon tag example is the Hi-Z Turb'N Tag13,14. The Hi-Z Turb'N Tag allows inflation time to be adjusted between 2 min and 60 min, depending on the injected water temperature13. This technology has been used in fish studies at many field sites, including studies involving Chinook salmon smolts released at Rocky Reach Dam on the Columbia River and juvenile American shad at Hadley Falls Dam on the Connecticut River15,16. Both technologies utilize acid-base chemical reactions to inflate the balloon tags for recovery.
This method offers cost-effectiveness and simplicity in manufacturing, with an estimated material cost of only $0.50 per balloon. As described here, the manufacturing process is easy to follow, making balloon tag production accessible to anyone.
Protocol
1. Acid/base encapsulation
- Mix a 1:2 ratio by the weight of H2C2O4 (oxalic acid) and NaHCO3 (sodium bicarbonate) in a mixing cup (see Table of Materials). If the acid-base powder mix is crystallized, grind it down using a mortar and pestle (Figure 1A).
- Retrieve the size 3 vegetable-based capsules and the semi-automatic capsule filling machine to begin the process (see Table of Materials).
- Place the cap sheet flat on a clean, dry surface. Align the encapsulation sheet on top of the cap sheet using the black pegs to fix it correctly in place (Figure 1B).
- Separate the capsule tops and bottoms, unless using pre-separated capsules. The size #3 vegetable capsules, when closed, have overall dimensions of 15.9 mm in length, 5.57 mm in outer diameter (OD), 0.30 mL in volume, and weigh 47 mg.
- Pour capsule tops into the encapsulation sheet (Figure 1C). Gently shake the tops into the holes with a circular motion. While doing this, cover the gap in the wall of the encapsulation sheet with one hand or a powder spreader to avoid spilling the tops (Figure 1D).
- Once the holes are filled, pour the excess capsule tops into a clean cup (Figure 1E). Identify any upside-down capsule tops and turn them over (Figure 1F). Ensure that all capsule tops are facing the correct direction in the cap sheet. It is important to ensure proper orientation, as incorrect alignment may result in capsule tops not properly joining with the capsule bottoms.
- Remove the encapsulation sheet and set aside the filled cap sheet.
- Take out the body or "bottom" sheet. Place it on a clean, dry, flat surface. Fix the encapsulation sheet to the bottom sheet, ensuring proper alignment by utilizing the black pegs to position it correctly in place.
- Pour capsule bottoms into the encapsulation sheet and shake as before in a circular motion to fill the holes. Pour off excess capsule bottoms. Identify any upside-down capsule bottoms and turn them over.
- Remove the encapsulation sheet from the bottom sheet and set it aside.
- Pour the acid/base powder mixture onto the filled bottom sheet (Figure 1G). Use a plastic spreader to fill the capsule bottoms with the powder (Figure 1H). Check that all the capsule bottoms are filled (Figure 1I). Remove any unused acid/base powder.
- Place the cap sheet on a flat surface and position the middle sheet on top, aligning it with the black pegs to ensure a correct fit. Ensure to line up all the capsule tops with the corresponding holes in the middle sheet.
- Invert the cap sheet with the affixed middle sheet and align it with the filled bottom sheet (Figure 1J).
- Gently press down on the cap sheet equally on all sides to join the tops and bottoms, fitting both sides of the capsule together (Figure 1K).
- Remove the cap sheet and middle sheet from the bottom sheet. At this point, the capsule bottoms and tops should be properly joined together.
- Verify that each capsule top and bottom are fitted tightly; if not, manually press the capsule top and bottom together to create a tight fit. Remove the filled capsules and place them in an airtight, sealable container (Figure 1L).
NOTE: For safe handling, it is essential for users to wear personal protective equipment (PPE) and face protection. Adequate ventilation should be ensured, and precautions should be taken to avoid ingestion, inhalation, and contact with the substance on the skin, eyes, or clothing. Additionally, it is important to prevent the generation of dust. For detailed information regarding safety, please refer to the safety data sheet (SDS) for oxalic acid and sodium bicarbonate. To maintain the integrity of the acid/base capsules, it is advised to store them away from direct sunlight and high humidity. Store the unused capsules in a sealed, airtight container. As long as the capsules are kept dry and free from moisture, they can be used effectively to ensure optimal functionality.
- Verify that each capsule top and bottom are fitted tightly; if not, manually press the capsule top and bottom together to create a tight fit. Remove the filled capsules and place them in an airtight, sealable container (Figure 1L).
2. Silicone stopper manufacture
- Using a fused deposition modeling (FDM) 3D printer (see Table of Materials), print a mold plate using the STL file provided in Supplementary File 1.
- Place a clear packing tape on the bottom side of the mold plate so that each opening is sealed (Figure 2A).
- Mix a 1:1 ratio by weight (e.g., 50 g each of Part A and Part B) of the commercially available silicone mold material into a mixing cup (see Table of Materials). Using a disposable spoon, thoroughly mix the chemical compound for approximately 5 min, or until it has become uniform.
- Place the mold plate with the packing tape over a piece of paper. The paper will catch any potential silicone spill from the mold plate.
- Begin pouring the silicone mixture into each stopper hole, ensuring they are all filled (Figure 2B). Use a rubber squeegee to spread the silicone into each stopper hole (Figure 2C). Remove the leftover silicone mixture from the surface of the mold plate.
- Let the rubber stoppers dry for 4 h. After making sure the stoppers have fully cured (e.g., the silicone mixture has completely dried and hardened), remove the tape from the back of the mold plate (Figure 2D), and then begin pulling the stoppers out of the mold (Figure 2E).
- Remove any excess silicone attached to the stoppers (Figure 2F).
3. Balloon tag assembly
- Carefully insert the piercing tool (e.g., straight dental pick) into the silicone stopper (Figure 3A) (see Table of Materials). Insert the piercing tool into a 15 G syringe needle and then remove the piercing tool from the silicone stopper, leaving only the 15 G needle inside (Figure 3B). The piercing tool will create a slit inside the silicone stopper without cutting or removing any material.
- Cut a piece of 50 lb. fishing line (see Table of Materials) to a length of 150 mm. Insert the fishing line through the 15 G syringe needle and into the silicone stopper (Figure 3C).
- While carefully holding the stopper and the fishing line together, remove the 15 G syringe needle from the stopper's body, leaving the fishing line inside the stopper (Figure 3D). Ensure the fishing line lengths are even on both sides of the stopper.
- Insert two acid/base-powder-filled capsules into a latex balloon (Figure 3E) (see Table of Materials). Expand the balloon opening using the rubber-band expansion tool (i.e., castration band pliers) and then carefully insert one silicone stopper into the balloon opening (Figure 3F), leaving the fishing line's two ends outside the balloon.
- Place two O-rings (1.6 mm wide, 8.1 mm ID, see Table of Materials) onto the rubber-band expansion tool and expand them. Insert the neck of the latex balloon through the two expanded O-rings (Figure 3G). Carefully pull the two O-rings away from the rubber-band expansion tool, leaving them tightly wrapped around the balloon's neck, centered on the stopper (Figure 3H).
4. Balloon tag attachment to Sensor Fish caps
- Put one end of the fishing line through one of the small holes in the Sensor Fish cap (see Table of Materials) and bring it through the large hole in the center of the cap (Figure 4A).
- Tie the two ends of the fishing line together, leaving about 13 to 26 mm between the top of the cap and the base of the balloon. Use four overhand knots on top of each other when tying the fishing line.
- Leave the extra fishing line attached, as cutting it too close to a knot could potentially cause the knot to come undone (Figure 4B).
- Test the knot by gripping the fishing line on each side of the knot with the fingers and pulling as hard as possible. Be cautious not to pull too close to the balloon, as it could unintentionally tear the fishing line through the rubber stopper.
Representative Results
A study was conducted to determine the optimal methods for manufacturing balloon tags, focusing on the volume and temperature of water injected into the balloon. The study examined various input parameters, including the inflation start time, full inflation time, deflation start time, and the volume of the balloon at full inflation. The study was conducted at a desk with an ambient temperature of 21 °C.
A total of 360 balloon tags were prepared for the study. The tags were divided into 36 sets, with each set containing 10 balloon tags. The sets were categorized based on the number of capsules, including two, three, or four capsules. The tags in each set were injected with 5, 6, 7, 8, 9, or 10 mL of water at temperatures of either 18.3 or 12.7 °C. The temperature of 12.7 °C was chosen as the lowest temperature that still allowed for capsule dissolution, while 18.3 °C represented room temperature for practicality.
The results showed that full inflation occurred faster when using water at 18.3 °C compared to 12.7 °C (Figure 5). The slower dissolution of the vegetable-based capsules at lower temperatures caused a delay in inflation. Among the tested conditions, the three-capsule balloon tags injected with 5 mL of water at 18.3 °C exhibited consistent size, with a mean volume of 114 cm3 and a standard deviation of 1.28 cm3 (Table 1). At 18.3 °C, the four-capsule balloon tags demonstrated a faster inflation start time, while the three-capsule balloon tags demonstrated a faster deflation start time (Figure 6). However, the full inflation times for the two-capsule and four-capsule balloon tags were nearly identical. The three-capsule begins deflating first, followed by the four-capsule, and lastly the two-capsule.
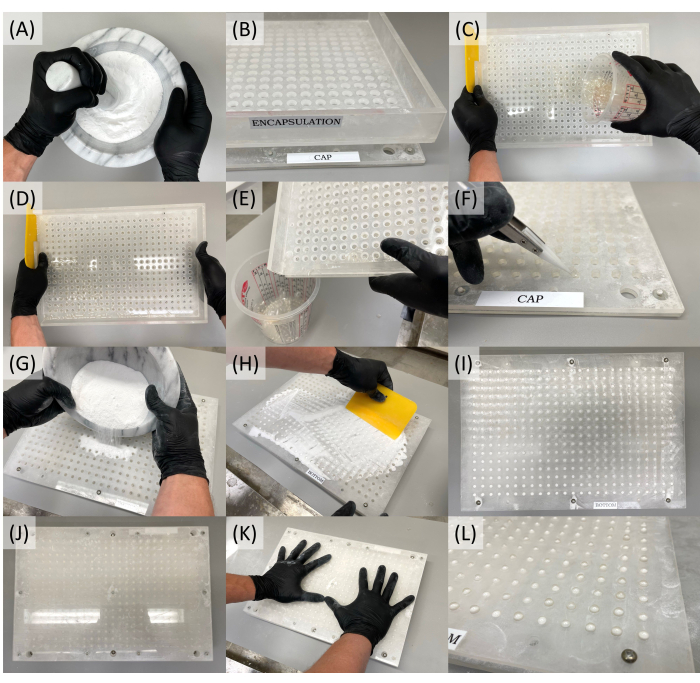
Figure 1: Step-by-step images illustrating the process of filling balloon tag inflation reagent capsules. (A) Mixing and grinding oxalic acid and sodium bicarbonate. (B) Aligning the encapsulation sheet on top of the cap sheet. (C) Pouring capsule tops into the encapsulation sheet. (D) Shaking tops into the holes of the encapsulation sheet. (E) Pouring excess tops into a clean cup. (F) Identifying upside-down capsule tops and flipping them over. (G) Pouring the acid/base powder mixture onto the bottom sheet. (H) Spreading the powder to fill the capsule bottoms. (I) Verifying that all capsule bottoms are filled. (J) Inverting the cap sheet with the affixed middle sheet and aligning it with the filled bottom sheet. (K) Pressing down on the cap sheet to join the top and bottom capsules. (L) Ensuring a tight fit of each capsule top and bottom. Please click here to view a larger version of this figure.
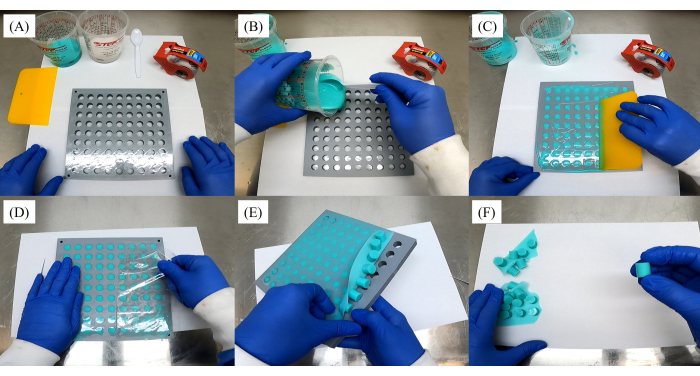
Figure 2: Step-by-step images demonstrating the process of making balloon tag silicone stoppers. (A) Sealing each opening with clear packing tape on the bottom side of the mold plate. (B) Pouring the silicone mixture into each stopper hole. (C) Spreading the silicone into each stopper hole using a rubber squeegee. (D) Removing the tape from the back of the mold plate after the stoppers have cured. (E) Removing the stoppers from the mold. (F) Removing any excess silicone attached to the stoppers. Please click here to view a larger version of this figure.
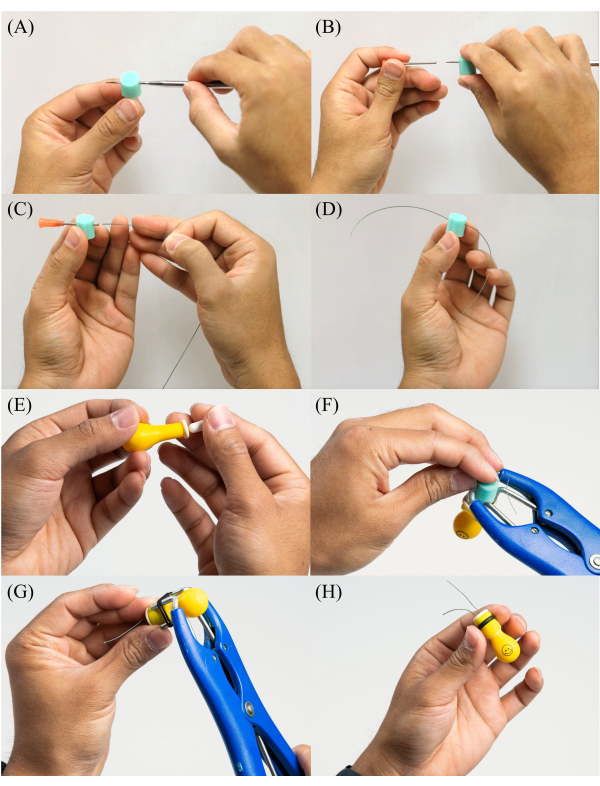
Figure 3: Step-by-step photos illustrating the assembly of a balloon tag. (A) Inserting a piercing tool into the silicone stopper. (B) Inserting a piercing tool into a 15 G syringe needle. (C) Cutting a 6-inch piece of 50 lb. fishing line and threading it through the 15 G syringe needle and into the silicone stopper. (D) Removing the 15 G syringe needle from the stopper, leaving the fishing line inside. (E) Inserting two acid/base-filled capsules into the latex balloon. (F) Expanding the balloon opening with a rubber-band expansion tool and inserting one silicone stopper. (G) Placing two O-rings onto the rubber-band expansion tool, expanding them, and inserting the latex balloon neck through the expanded O-rings. (H) Carefully pulling two O-rings away from the rubber-band expansion tool, tightly wrapping them around the balloon neck, centered on the stopper. Please click here to view a larger version of this figure.
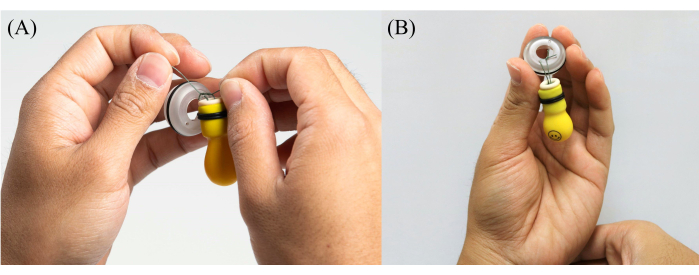
Figure 4: Step-by-step photos demonstrating the process of tying a balloon tag to a Sensor Fish cap. (A) Threading one end of the fishing line through a small hole in the Sensor Fish cap, bringing it through the large center hole, and tying both ends together, leaving a 13 to 26 mm gap between the cap's top and the balloon's base. (B) Balloon tag attached to a Sensor Fish cap. Please click here to view a larger version of this figure.
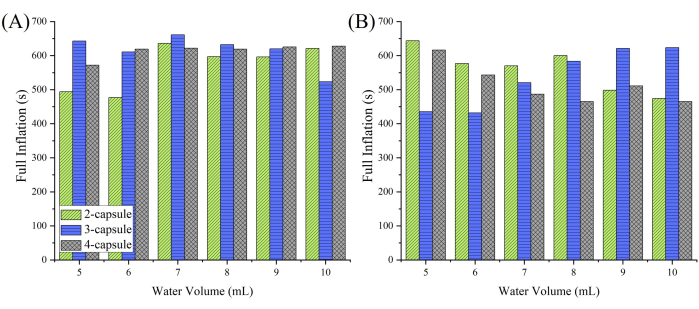
Figure 5: Inflation of balloon tags. Average inflation time for balloon tags with water at (A) 12.7 °C and (B) 18.3 °C using 5 to 10 mL of water for two-capsule (green), three-capsule (blue), and four-capsule (gray) balloon tags. Please click here to view a larger version of this figure.
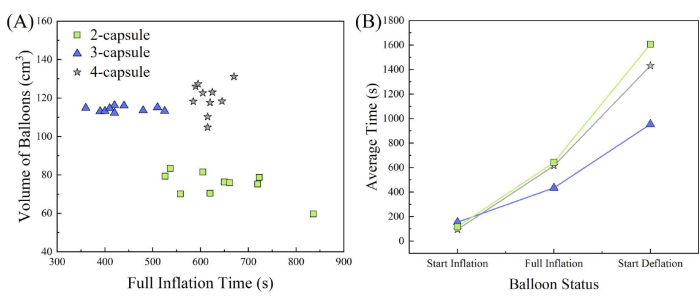
Figure 6: Volume and inflation time of balloon tags. (A) Volumes of balloons at full inflation time, and (B) average times to start of inflation, full inflation, and start of deflation for two-capsule (squares), three-capsule (triangles), and four-capsule (stars) balloon tags with 5 mL of water at 18.3 °C. Please click here to view a larger version of this figure.
| Water Temperature | 18.3 °C | 12.7 °C | ||||
| Quantity of Capsules | 2 | 3 | 4 | 2 | 3 | 4 |
| Average Volume | 76.1 | 114 | 120 | 72.1 | 103 | 117 |
| Standard Deviation | 6.53 | 1.28 | 7.53 | 6.82 | 5.07 | 6.14 |
Table 1: Average volume and standard deviation (cm3) of two-capsule, three-capsule, and four-capsule balloon tags after injecting 5 mL of water at 18.3 °C and 12.7 °C.
Supplementary File 1: STL file for printing the mold plate. Please click here to download this File.
Supplementary File 2: Citric Acid. Please click here to download this File.
Discussion
This study concluded that three-capsule balloon tags injected with 5 mL of water at 18.3 °C had a slower start inflation time and consistently larger volume compared to two-capsule and four-capsule balloon tags. When the balloon tags were injected with water at 12.7 °C, the average volume was smaller, and the inflation time was longer. The three-capsule begins deflating first, followed by the four-capsule, and lastly the two-capsule. The inflation and deflation periods associated with each water temperature can be helpful in the field. For studies requiring a longer inflation time, colder water may result in a slower inflation of the balloon tags, allowing for testing at large facilities where fish or Sensor Fish may be more widely distributed and require a longer retrieval time, similar to the field studies conducted by Martinez et al.7,12. Warmer water could be utilized to increase the inflation rate for testing reduced-scale models and small hydraulic structures, such as farmer screens and scaled hydroturbines11,17.
The most critical steps in manufacturing the balloon tags include ensuring that the sodium bicarbonate and oxalic acid powders are thoroughly mixed using a mortar and pestle before encapsulation. This will produce a finely ground chemical compound without clumps that could otherwise alter the chemical ratio. After manufacturing, the capsules must be kept away from direct sunlight and sealed in an airtight container to prevent moisture absorption from the air, which can degrade the vegetable-based capsules18.
The main advantage of this method is its cost-effectiveness and simple manufacturing process. The estimated material cost to produce one balloon is only $0.50. This is advantageous for studies with limited budgets that require a large sample size. The balloon tags will support Sensor Fish deployments and fish survival and injury assessments at hydroelectric dams and other hydraulic structures. This method addresses the growing need for sustainable energy and continued turbine replacements in the United States19. After the deployment of new technology, field evaluation is necessary to validate the design improvements of the technology20. The evaluation results can also provide insights for improved turbine design and inform management decisions regarding the operation of turbines to enhance fish passage conditions21.
The manufacturing and use of balloon tags have certain limitations that need to be considered. The manual mixing process using a mortar and pestle to ensure thorough mixing of sodium bicarbonate and oxalic acid powders before encapsulation can be time-consuming and labor-intensive, limiting scalability. Furthermore, the vegetable-based capsules used in the tags require careful storage away from direct sunlight in an airtight container to prevent degradation, adding complexity to handling and transportation, especially in field settings. Additionally, the performance of the balloon tags is temperature-dependent, with colder water resulting in smaller average volume and longer inflation time, limiting their suitability for studies requiring shorter inflation periods or testing at smaller hydraulic structures. Conversely, warmer water can increase the inflation rate but may limit applicability in colder environments or larger facilities that require longer retrieval times. These limitations should be carefully considered and addressed for the optimal use of balloon tags in various research scenarios.
To ensure your safety when working with hazardous chemicals, such as those detailed in this manuscript, it is imperative to consult the SDS for comprehensive guidance on their proper handling and storage. Specifically, oxalic acid poses risk to human health if it comes into contact with the skin or is ingested. Furthermore, it exhibits sensitivity to heat and can react violently with oxidizing agents, such as nitrates, potentially resulting in fires and explosions22. Therefore, when handling oxalic acid, it is essential to work in a well-ventilated fume hood and wear PPE, such as eye protection, a mask, and gloves, to prevent injury or irritation.
Citric acid can serve as an alternative chemical for the balloon tags instead of oxalic acid, primarily due to its Food and Drug Administration recognition as a safe substance for use in both food and skin products23. In contrast to oxalic acid, citric acid exhibits reduced sensitivity to heat and is incompatible with oxidizing agents, strong bases, or acids. Just like with oxalic acid, handling citric acid necessitates the use of a well-ventilated fume hood and appropriate PPE.
The reaction involving citric acid (C6H8O7) and sodium bicarbonate (NaHCO3) in water also generates carbon dioxide (CO2) for inflating the balloon tags. This chemical process results in the formation of sodium citrate (Na3C6H5O7), water, and carbon dioxide, as illustrated in the following equation:
C6H8O7 + 3NaHCO3 → Na3C6H5O7 + 3H2O +3CO2
The limitation of using citric acid is that, for the same mass of material (acid + sodium bicarbonate) stored inside the balloon tag, the amount of CO2 generated is approximately 81% of what is produced by oxalic acid. This is a crucial consideration because it reduces the size of the balloon tag, and the balloon tag's full inflation duration is longer. If citric acid is used in place of oxalic acid, it is recommended to use a mass ratio of 1:2 (sodium bicarbonate to citric acid) to achieve a balloon volume of 46 cm3 and a full inflation time of 15 minutes. For more information, please refer to Supplementary File 2: Citric Acid.
This research focuses on developing and utilizing balloon tag technology, a tool designed to locate and help recover Sensor Fish and live fish after they navigate through hydraulic structures. The primary objective is to improve the understanding of how these structures impact aquatic animals, ultimately facilitating the creation of more fish-friendly turbines. This approach not only offers cost-effectiveness but also encompasses a straightforward manufacturing process, which, when optimized, could enable large-scale production of these tags. Moreover, these tags can be customized to accommodate various species and aquatic environments. Future research will delve into optimizing balloon tag performance under different conditions, exploring their effects on fish behavior, and addressing environmental concerns. While our preliminary results show promise, extensive field tests are necessary for real-world validation and long-term durability assessment. Overall, this research aims to promote sustainable and responsible hydropower development by providing a tool that assists in assessing and mitigating the impacts of hydraulic structures on fish.
Declarações
The authors have nothing to disclose.
Acknowledgements
This study was funded by the U.S. Department of Energy (DOE) Water Power Technologies Office. The laboratory studies were conducted at Pacific Northwest National Laboratory, which is operated by Battelle for the DOE under Contract DE-AC05-76RL01830.
Materials
| 3D Printed Silicone Stopper Plate | NA | NA | |
| ARC800 Sensor Fish | ATS | NA | |
| FDM 3D printer | NA | NA | |
| Manual Capsule Filler Machine CN-400CL (Size #3) | Capsulcn | NA | |
| Mold Star 15 SLOW | Smooth-On | NA | |
| Oil-Resistant Buna-N O-Ring | McMaster-Carr | SN: 9262K141 | |
| Oxalic Acid, 98%, Anhydrous Powder (C2H2O4) | Thermo Scientific | CAS: 144-62-7 | |
| Rubber Band Expansion Tool | iplusmile | NA | |
| Separated Vegetable Cellulose Capsules (Size #3) | Capsule Connection | NA | |
| Smiley Face YoYo Latex balloon | YoYo Balloons, Etc. | NA | |
| Sodium Bicarbonate Powder (CHNaO3) | Sigma | CAS: 144-55-8 | |
| Spectra Fiber Braided Fishing Line (50 lbs.) | Power Pro | NA |
Referências
- Uria-Martinez, R., et al. U.S. Hydropower Market Report. Oak Ridge National Laboratory. , (2021).
- Kao, S., et al. New stream-reach development: a comprehensive assessment of hydropower energy potential in the United States. Oak Ridge National Laboratory. , (2014).
- Martinez, J. J., Deng, Z. D., Mueller, R., Titzler, S. In situ characterization of the biological performance of a Francis turbine retrofitted with a modular guide vane. Applied Energy. 276, 115492 (2020).
- Čada, G. l. e. n. n. . F. The development of advanced hydroelectric turbines to improve fish passage survival. Fisheries. 26, 14-23 (2001).
- Tuononen, E. I., Cooke, S. J., Timusk, E. R., Smokorowski, K. E. Extent of injury and mortality arising from entrainment of fish through a Very Low Head hydropower turbine in central Ontario, Canada. Hydrobiologia. 849, 407-420 (2020).
- Deng, Z., Carlson, T. J., Duncan, J. P., Richmond, M. C., Dauble, D. D. Use of an autonomous sensor to evaluate the biological performance of the advanced turbine at Wanapum Dam. Journal of Renewable and Sustainable Energy. 2, 053104 (2010).
- Martinez, J. J., et al. Hydraulic and biological characterization of a large Kaplan turbine. Renewable energy. 131, 240-249 (2019).
- Zhiqun Deng, , et al. Six-degree-of-freedom sensor fish design and instrumentation. 7, 3399-3415 (2007).
- Deng, Z. D., et al. Design and implementation of a new autonomous sensor fish to support advanced hydropower development. Review of Scientific Instruments. 85, 115001 (2014).
- Deng, Y., Jia, Y., Haoran, L. Effects of ionicity and chain structure on the physicochemical properties of protic ionic liquids. AIChE Journal. 66 (10), e16982 (2020).
- Salalila, A., Deng, Z. D., Martinez, J. J., Lu, J., Baumgartner, L. J. Evaluation of a fish-friendly self-cleaning horizontal irrigation screen using autonomous sensors. Marine and Freshwater Research. 70, 1274-1283 (2019).
- Martinez, J., et al. In situ characterization of turbine hydraulic environment to support development of fish-friendly hydropower guidelines in the lower Mekong River region. Ecological engineering. 133, 88-97 (2019).
- Heisey, P. G., Mathur, D., D’Allesandro, L. A new technique for assessing fish passage survival at hydro power stations. International Atomic Energy Agency. , (1993).
- Heisey, P. G., Mathur, D., Rineer, T. A reliable tag-recapture technique for estimating turbine passage survival: application to young-of-the-year American shad (Alosa sapidissima). Canadian Journal of Fisheries and Aquatic Sciences. 49 (9), 1826-1834 (1992).
- Mathur, D., Heisey, P. G., Euston, E. T., Skalski, J. R., Hays, S. Turbine passage survival estimation for chinook salmon smolts (Oncorhynchus tshawytscha) at a large dam on the Columbia River. Canadian Journal of Fisheries and Aquatic Sciences. 53 (3), 542-549 (1996).
- Mathur, D., Heisey, P. G., Robinson, D. A. Turbine-passage mortality of juvenile American shad at a low-head hydroelectric dam. Transactions of the American Fisheries Society. 123 (1), 108-111 (1994).
- Watson, S., et al. Safe passage of American Eels through a novel hydropower turbine. Transactions of the American Fisheries Society. 151, 711-724 (2022).
- Al-Tabakha, M. o. a. w. i. a. . M., et al. Influence of capsule shell composition on the performance indicators of hypromellose capsule in comparison to hard gelatin capsules. Drug Development and Industrial Pharmacy. 41 (10), 1726-1737 (2015).
- . Hydropower Vision. U.S. Department of Energy. , (2016).
- Duncan, J. o. a. n. n. e. . P., et al. Physical and ecological evaluation of a fish-friendly surface spillway. Ecological Engineering. 110, 107-116 (2018).
- Trumbo, B. r. a. d. l. y. . A., et al. Improving hydroturbine pressures to enhance salmon passage survival and recovery. Reviews in fish biology and fisheries. 24, 955-965 (2014).
- Pohanish, R. P. . Sittig’s handbook of toxic and hazardous chemicals and carcinogens. , (2017).
- U.S. Food and Drug Administration. . CFR – Code of Federal Regulations Title 21. , (1994).

