In vivo Characterization of Endocrine Disrupting Chemical Effects via Thyroid Hormone Action Indicator Mouse
Summary
The Thyroid Hormone Action Indicator mouse model was developed to enable tissue-specific quantification of local thyroid hormone action using its endogenous regulatory machinery. Recently, it has been shown that the model is suitable for characterizing endocrine-disrupting chemicals interacting with thyroid hormone economy, both by ex vivo and in vivo methodologies.
Abstract
Thyroid hormones (TH) play a critical role in cell metabolism and tissue function. TH economy is susceptible to endocrine disrupting chemicals (EDCs) that can disturb hormone production or action. Many environmental pollutants are EDCs, representing an emerging threat to both human health and agricultural production. This has led to an increased demand for proper test systems to examine the effects of potential EDCs. However, current methodologies face challenges. Most test systems use endogenous markers regulated by multiple, often complex regulatory processes, making it difficult to distinguish direct and indirect effects. Moreover, in vitro test systems lack the physiological complexity of EDC metabolism and pharmacokinetics in mammals. Additionally, exposure to environmental EDCs usually involves a mixture of multiple compounds, including in vivo generated metabolites, so the possibility of interactions cannot be ignored. This complexity makes EDC characterization difficult. The Thyroid Hormone Action Indicator (THAI) mouse is a transgenic model that carries a TH-responsive luciferase reporter system, enabling the assessment of tissue-specific TH action. One can evaluate the tissue-specific effects of chemicals on local TH action by quantifying luciferase reporter expression in tissue samples. Furthermore, with in vivo imaging, the THAI mouse model allows for longitudinal studies on the effects of potential EDCs in live animals. This approach provides a powerful tool for testing long-term exposure, complex treatment structures, or withdrawal, as it enables the assessment of changes in local TH action over time in the same animal. This report describes the process of in vivo imaging measurements on THAI mice. The protocol discussed here focuses on developing and imaging hyper- and hypothyroid mice, which can serve as controls. Researchers can adapt or expand the treatments presented to meet their specific needs, offering a foundational approach for further investigation.
Introduction
Thyroid hormone (TH) signaling is a fundamental regulator of cellular metabolism, essential for normal development and optimal tissue function in adulthood1. Within tissues, TH action is finely controlled by a complex molecular machinery, allowing for tissue-specific maintenance of local TH levels. This autonomy of different tissues from circulating TH levels is of great importance2,3,4.
Numerous chemicals have the potential to disrupt endocrine functions and are found in the environment as pollutants. It is a growing concern that these molecules can enter the food chain through wastewater and agricultural production, thereby impacting the health of livestock and humans5,6,7.
One of the significant challenges in addressing this issue is the sheer number of compounds involved, including both authorized and already banned, but still persistently present, molecules. In recent years, substantial efforts have been made to develop test systems for screening and identifying the disruptive potential of various chemicals8,9,10,11. While these methods excel in high-throughput screening of thousands of compounds and identifying potential threats, a detailed analysis of specific in vivo effects of these molecules is essential to establish the hazards of human exposure. Thus, a multifaceted approach is necessary when studying and characterizing endocrine-disrupting chemicals (EDCs).
In the context of TH regulation, understanding the tissue-specific consequences of EDC exposure requires quantifying local TH action. Although several in vivo models have been developed for this purpose, most rely on endogenous markers as their output measure. Despite being physiological, these markers are subject to numerous regulatory mechanisms, both direct and indirect, making their interpretation more challenging. Therefore, characterizing EDC effects on TH regulation at the tissue level remains a significant challenge12,13.
To address the challenges of measuring tissue-specific TH signaling, the Thyroid Hormone Action Indicator (THAI) mouse model was recently developed. This model allows for specific quantification of changes in local TH action under endogenous conditions. A luciferase transgene was introduced into the mouse genome, which is highly sensitive to regulation by TH action14. This model has demonstrated effectiveness in answering various research questions that require quantifying changes in local tissue TH signaling14,15,16,17,18.
Recognition of one potential use of the THAI model is characterizing tissue-specific effects of EDCs on TH signaling. The model has recently been employed successfully to investigate the tissue-specific effects of tetrabromobisphenol A and diclazuril on TH signaling15. Here, baseline protocols are presented for utilizing in vivo imaging techniques on the THAI model as a test system for characterizing EDCs that disrupt TH function. This method leverages the bioluminescent nature of the luciferin-luciferase reaction. Essentially, the transgenically expressed luciferase enzyme catalyzes the oxidation of administered luciferin, generating luminescent light proportional to the amount of luciferase in the tissue (Figure 1). Consequently, the biological response measured is luciferase activity, which has been validated as a suitable measure of local TH action14. While the THAI model is applicable for quantifying TH action in virtually all tissues, in vivo imaging primarily focuses on TH action in the small intestine (ventral imaging) and the interscapular brown adipose tissue (BAT, dorsal imaging)14.
A significant advantage of the in vivo imaging technique is that it eliminates the need to sacrifice animals for measurements. This allows investigators to design longitudinal and follow-up experiments as self-controlled studies, reducing between-subjects bias and the number of animals used. This aspect is particularly crucial in EDC characterization, and the method's strength and versatility for this purpose have been previously demonstrated14,15.
Protocol
The present protocol was reviewed and approved by the Animal Welfare Committee at the Institute of Experimental Medicine (PE/EA/1490-7/2017, PE/EA/106-2/2021). The presented data is from FVB/Ant background14, 3-month-old male THAI mice (n = 3-6/group). FVB/Ant background THAI animals tend to have highly pigmented spots on their skin that may distort measurements. Hence, search for pigmented spots on the skin of the imaged area after fur removal. Animals do not require special housing conditions unless the experiment specifically requires so (e.g., a special diet).
1. Hyperthyroid treatment
NOTE: A general protocol for inducing hyperthyroidism in mice is provided here. The ATA guide19 offers detailed explanations on the background of the methods with alternatives mentioned.
- Dissolve T3 (3,5,3'-triiodothyronine, see Table of Materials) in 40 mM NaOH to create a stock solution with a concentration between 5-10 mg/mL.
- Dilute the stock solution with saline to a final concentration of 0.1 µg/µL.
- Inject the diluted T3 solution intraperitoneally (i.p.) into awake animals at a volume of 10 µL per gram of body weight (bwg). After 24 h, the animals will be considered hyperthyroid.
NOTE: T3 treatment can be replaced by any other kind of treatment. The treatment does not affect the protocol of in vivo imaging.
2. Hypothyroid treatment
NOTE: Here, only a general protocol for inducing hypothyroidism in mice is provided. ATA guide19 describes detailed explanations on the background of the methods with alternatives mentioned.
- Switch the diet to an iodine-free chow diet and add KClO4 and methimazole to the drinking water (0.01 % methimazole, 0.05 % KClO4) (see Table of Materials).
- Replace the drinking solution regularly (every 2-3 days) with a fresh drinking solution because methimazole is light-sensitive and degrades quickly.
- Keep up the treatment regime for at least 2 weeks, no more than 4 weeks. Animals will lose weight and will show discomfort. If animals are hardly moving around, have lost much of their hair, or are barely conscious, use a humane endpoint and terminate them by any method (following institutionally approved protocols).
- Continue hypothyroid treatment when combined with other treatments to prevent potential recovery from hypothyroidism.
3. In vivo imaging
- Start the software compatible with the in vivo imaging system (see Table of Materials).
- Log in and wait for the "Imaging Wizard" panel to load. It is a smaller panel on the bottom left of the window.
- On the "Imaging Wizard" initiate camera cooling by clicking on Initialize in the "Image Wizard panel". This makes the instrument run a setup protocol, wait for it to complete. The "Imaging Wizard panel" turns blue, and a green light will turn on in the panel when the camera temperature is low enough and the instrument is ready.
- Set the temperature of the heating pad to 30-37 °C to keep the measured animals warm.
NOTE: Continue with the protocol while waiting for the camera temperature to be optimal. - Do not keep or treat animals near the instrument. Avoid excess amounts of hair circulating in the air around the instrument.
- Anesthetize 1-3 animals with ketamine-xylazine i.p. injection (ketamine 50 mg/kg body weight, xylazine 10 mg/kg body weight, see Table of Materials). Alternatively, if an isoflurane anesthetic system is installed, follow institutionally approved protocols to use isoflurane anesthesia, replacing the ketamine-xylazine mixture.
NOTE: Hypothyroid mice are more sensitive to ketamine-xylazine; use half dose. - Use eye protection gel during anesthesia.
- Check pedal reflex by pinching the footpads. No pedal reflex confirms the state of surgical plane anesthesia.
- After the anesthesia takes effect, remove fur from the imaged body parts using the most suitable fur removal method (epilator, shaving, cream, etc.). Ensure no fur is left on the imaged body parts to prevent the scattering of luminescent light.
- Dissolve Na-luciferin (see Table of Materials) in 1x phosphate-buffered saline (PBS) at a concentration of 15 mg/mL. Luciferin is light-sensitive; avoid direct light exposure. Store the solution in amber tubes or wrap it in aluminum foil.
- Treat shaven animals with luciferin solution 10 µL/bwg i.p.
- Place the animals into the instrument with the camera's center point marked as a '+' on the pad. Ensure proper placement by checking the gridlines and confirming with a single 'Photo' capture if unsure.
- Wait for 15 min after substrate administration before taking the first measurement. During this time, set the imaging time in the "Image Wizard" panel to 3 min for luminescence and check the boxes for Photo and Luminescence. The 'Photo' is necessary to coincide with luminescence to identify the source of the measured signal.
NOTE: 15 min is required for optimal substrate uptake and tissue distribution. The luminescent signal plateaus 15-20 min after luciferin administration. The signal starts to slowly decrease after the plateau. - Take the first measurement by clicking on Measure in the "Imaging Wizard" panel.
- If both ventral and dorsal imaging are performed, rearrange animals for the second body part to be imaged immediately after concluding the first.
- After imaging is done, return the animals to their cages and continue the experiment with the next set of animals.
- Allow the animals to recover, which typically takes 1-2 h at most. Place a tube filled with warm water near the animals to facilitate recovery, and monitor vital signs such as breathing and perfusion.
- Decide the fate of measured animals. In the data presented in this article, the measured animals were euthanized following institutionally approved protocols for ex vivo measurements. However, this is not necessary. Consider whether euthanasia or follow-up experiments are ethical.
4. Data analysis
- Open the "ClickInfo" file in the software. A panel named "Tool Palette" for image analysis and editing will open on the right side of the window.
- Convert scale to radiance in the top left corner of the image.
- Click on "Image Adjust".
- Decide on the optimal binning and color scale of the images. Make all images utilize the same settings.
- Click on "ROI tools" on the "Tool palette".
- Select areas of interest by clicking on "Place ROI" in the "ROI tools". Using the same sized ROIs or different sized ROIs can also be meaningful depending on the experimental design.
- Click on "Measure ROIs". A new window will open with the data of placed ROIs. Export the data with ctr + c-ctrl + v Windows command into an organizing or statistical software of choice.
- Data can be exported as total flux or average radiance. Choose which variable is the most relevant in the current experimental setting.
- Continue data analysis in accordance with the experimental design. Individual calculation of (treated-background) values as "measured effect in one animal" is recommended.
Representative Results
Generally, the measured radiance ranges from magnitudes of 105 to 1010 p/s/cm2/sr. However, exact values can vary among animals within the same image and across different images. Therefore, comparing raw data might be misleading. It's crucial to establish control and background signals in all experiments, making self-controlled designs highly recommended.
Figure 2 presents representative images and data from ventral and dorsal views in an experimental setup involving hypo-, eu-, and hyperthyroid mice. The lowest signals are expected in hypothyroid mice, often falling below the lower limit of the color scale.
Areas lacking fur, such as footpads, tail, and nose, exhibit relatively high basal signals. Importantly, the luciferase signal is influenced by the thyroid hormone (TH) status, as observed in Figure 2. Intriguingly, Figure 3 demonstrates that TH action is significantly heightened in the brown adipose tissue (BAT) of cold-stressed THAI mice14. However, this treatment does not impact the luciferase signal in the footpads and tail. This disparity underscores the potential for markedly distinct TH actions in tissues of the same organism. Cold exposure triggers BAT activation, necessitating localized upregulation of type 2 deiodinase-mediated TH activation20,21. Following 24 h of cold exposure, circulating TH levels remain unaltered, resulting in no TH-dependent signal change in the footpads and tail. In contrast, in the scenario presented in Figure 2, where elevated blood TH levels correspondingly increase TH action in the footpad, tail, and BAT.
Both Figure 2 and Figure 3 reveal robust signals in the testicular region. This is attributed to the TH-independent high basal expression of the luciferase transgene in the testicles, a characteristic of the THAI model. In this organ, the luciferase signal remains unaffected by changes in circulating TH levels.
As previously mentioned, hypo- and hyperthyroid treatments can be substituted with other interventions, such as testing endocrine-disrupting compounds (EDCs). Figure 4 displays BAT imaging in a three-week-long follow-up experiment involving diclazuril, a veterinary drug with EDC potential16. Signals between time points are easily distinguishable, and the method effectively captures the accumulation and clearance of diclazuril.
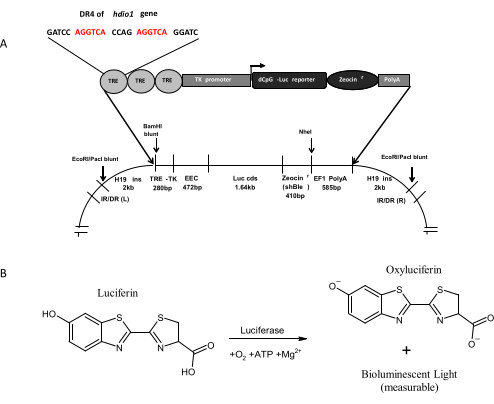
Figure 1: Concept and working principles of the THAI Construct. (A) The Recombinant THAI construct. This figure is adapted from Mohácsik et al.14. (B) Schematic illustration of luciferase-catalyzed luciferin oxidation. Please click here to view a larger version of this figure.
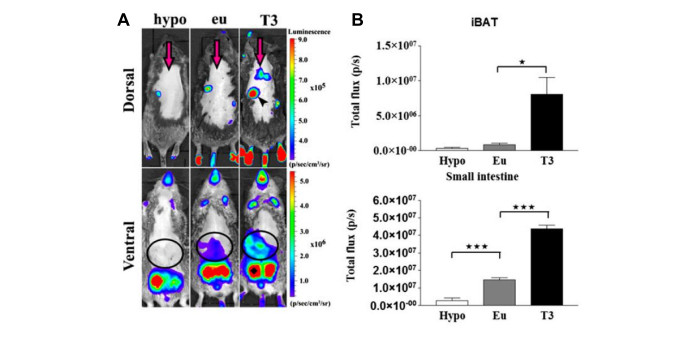
Figure 2: Representative dorsal and ventral images of hypo-, eu-, and hyperthyroid THAI Mice, along with an intensity diagram of luciferase activity. (A) Representative Images of hypo-, eu-, and hyperthyroid THAI mice. (B) Quantification of signals in (A). Mean photon/s ± SEM (n = 3). *P < 0.05; ***P < 0.001, determined by one-way ANOVA, followed by Newman-Keuls post hoc test. This figure is adapted from Mohácsik et al.14. Please click here to view a larger version of this figure.
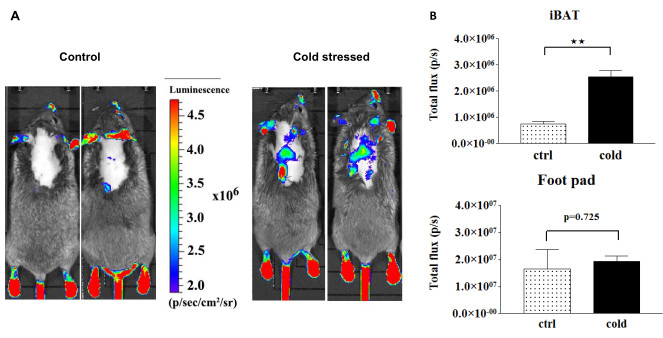
Figure 3: Paw, tail, and BAT signals before and after cold stress, along with light intensity diagram of luciferase activity. (A) Representative dorsal images of control and cold-stressed THAI mice. (B) Quantification of signals in (A). Mean photon/s ± SEM (n = 4). **P < 0.001, determined by Student's t-test. This figure is adapted from Mohácsik et al.14. Please click here to view a larger version of this figure.
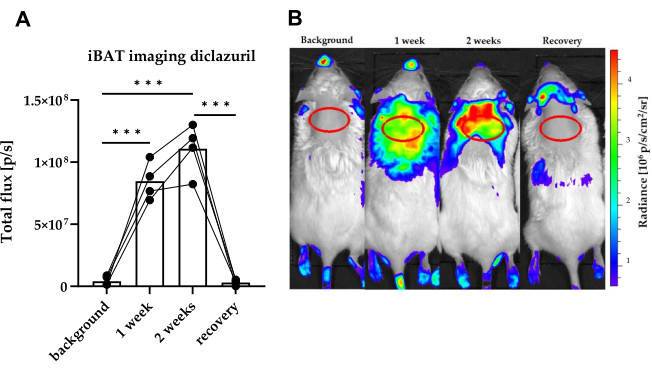
Figure 4: Representative BAT images of a three-week long diclazuril follow-up study, accompanied by light intensity diagram of luciferase activity. THAI mice were orally treated for 2 weeks with 10 mg/bwkg/day of diclazuril as a saline suspension, followed by one week of recovery. (A) Quantification of BAT bioluminescent signals in (B) before, during, and after diclazuril treatment. (B) Representative dorsal images of THAI mice before, during and after diclazuril treatment. n = 4-6 mice/group; the figure displays a Tukey Box Plot of photon/s, α = 0.05; ***: p < 0.001. This figure is adapted from Sinko et al.15. Please click here to view a larger version of this figure.
Discussion
The threats posed by Endocrine-Disrupting Chemicals (EDCs) to human health are well recognized; however, research on EDCs faces formidable challenges. These challenges are partially a consequence of the complexity of the endocrine system. Many EDCs have been identified to simultaneously disrupt multiple endocrine systems22. Additionally, in the context of Thyroid Hormone (TH) economy, there exists an additional layer of complexity due to tissue-specific differences in regulating TH action. This complexity provides a novel perspective on expanding the assessment of TH signaling by characterizing TH action in various tissues. The challenge is further exacerbated by the metabolism of compounds, which can either enhance or attenuate their effects on the endocrine system. It's important to note that current screening methods for identifying compounds are well established and function with high performance8,11. However, there is still a lack of test systems to characterize tissue-specific effects and consequences of identified compounds.
The THAI mouse model was developed to tackle the challenges of characterizing tissue-specific thyroid hormone economy. Its potential has been demonstrated under various circumstances14,15,16,18. The THAI model offers an advantage in characterizing chemicals that disrupt thyroid hormones. It's important to note that the model isn't intended for rapid compound screening, but to provide insights into disruption mechanisms in an in vivo mammalian model.
In this article, a protocol is presented outlining how the THAI mouse can be used for in vivo imaging studies. This method allows testing of treatments affecting the Hypothalamus-Pituitary-Thyroid (HPT) axis and/or thyroid hormone action in animals. The protocol supports self-controlled studies and follow-up designs. Additionally, the protocol can be used to generate control animals with various thyroid hormone states, serving as references in experimental settings. The presented hypo- and hyperthyroid treatments can be replaced, extended, and combined with other treatments as needed. This versatility is valuable for assessing tissue thyroid hormone economy, especially for endocrine-disrupting chemical (EDC) characterization.
In vivo imaging signals on the dorsal side come from the Brown Adipose Tissue (BAT), while ventral signals originate from the small intestines14. Thus, the method primarily characterizes these tissues, and measuring other body parts might be challenging. Overcoming these technical limitations through organ exposure requires careful consideration of ethical and technical implications.
Combining in vivo imaging with ex vivo studies on the THAI model allows for assessing thyroid hormone signaling in various tissues and brain regions14. For instance, qPCR can extend and specify the effects seen in in vivo imaging. However, this sacrifices follow-up and self-controlled designs, so costs and benefits should be evaluated. Combining in vivo imaging with ex vivo measurements is recommended for a comprehensive investigation.
Declarações
The authors have nothing to disclose.
Acknowledgements
This work was supported by Project no. RRF-2.3.1-21-2022-00011, titled National Laboratory of Translational Neuroscience has been implemented with the support provided by the Recovery and Resilience Facility of the European Union within the framework of Programme Széchenyi Plan Plus.
Materials
| 3,5,3'-triiodothyronine (T3) | Merck | T2877 | |
| Animals, mice | THAI mouse | ||
| Eye protection gel | Oculotect | 1000 IU/g | |
| Falcon tube | Thermo Fisher Scientific | 50 mL volume | |
| Iodine-free chow diet | Research Diets | custom | |
| IVIS Lumina II in vivo imaging system | Perkin Elmer | – | |
| Ketamine | Vetcentre | E1857 | |
| Living Image software 4.5 | Perkin Elmer | – | provided with the instrument |
| Measuring cylinder | 250 mL | ||
| methimazole | Merck | M8506 | |
| Microfuge tubes | Eppendorf | For diluting treatment materials | |
| NaClO4 | Merck | 71852 | |
| Na-luciferin, substrate | Goldbio | 103404-75-7 | |
| NaOH | Merck | 101052833 | |
| Phoshphate buffer saline | Chem Cruz | sc-362302 | |
| Pipette | Gilson | For diluting treatment materials | |
| Pipette tips | Axygen | For diluting treatment materials | |
| Shaving cream/epilator/shaver | Personal preference | ||
| Syringe | B Braun | 1 mL volume | |
| Syringe needle | B Braun | 0.3 x 12 mm | |
| Xylazine | Vetcentre | E1852 |
Referências
- Larsen, P. R., Davies, T. F., Hay, I. D., Wilson, J. D., Foster, D. W., Kronenberg, H. M., Larsen, P. R. . Williams Textbook of Endocrinology. , 389-515 (1998).
- Gereben, B., et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 29 (7), 898-938 (2008).
- Fekete, C., Lechan, R. M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 35 (2), 159-194 (2014).
- Bianco, A. C., et al. Paradigms of Dynamic Control of Thyroid Hormone Signaling. Endocr Rev. 40 (4), 1000-1047 (2019).
- Zoeller, R. T. Endocrine disrupting chemicals and thyroid hormone action. Adv Pharmacol. 92, 401-417 (2021).
- Guarnotta, V., Amodei, R., Frasca, F., Aversa, A., Giordano, C. Impact of chemical endocrine disruptors and hormone modulators on the endocrine system. Int J Mol Sci. 23 (10), 5710 (2022).
- La Merrill, M. A., et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 16 (1), 45-57 (2020).
- Fini, J. B., et al. An in vivo multiwell-based fluorescent screen for monitoring vertebrate thyroid hormone disruption. Environ Sci Technol. 41 (16), 5908-5914 (2007).
- Mughal, B. B., Fini, J. B., Demeneix, B. A. Thyroid-disrupting chemicals and brain development: an update. Endocr Connect. 7 (4), 160-186 (2018).
- Dong, M., Li, Y., Zhu, M., Li, J., Qin, Z. Tetrabromobisphenol a disturbs brain development in both thyroid hormone-dependent and -independent manners in xenopus laevis. Molecules. 27 (1), 249 (2021).
- Beck, K. R., Sommer, T. J., Schuster, D., Odermatt, A. Evaluation of tetrabromobisphenol A effects on human glucocorticoid and androgen receptors: A comparison of results from human- with yeast-based in vitro assays. Toxicology. 370, 70-77 (2016).
- Li, J., Li, Y., Zhu, M., Song, S., Qin, Z. A multiwell-based assay for screening thyroid hormone signaling disruptors using thibz expression as a sensitive endpoint in xenopus laevis. Molecules. 27 (3), 798 (2022).
- Myosho, T., et al. Preself-feeding medaka fry provides a suitable screening system for in vivo assessment of thyroid hormone-disrupting potential. Environ Sci Technol. 56 (10), 6479-6490 (2022).
- Mohacsik, P., et al. A Transgenic mouse model for detection of tissue-specific thyroid hormone action. Endocrinology. 159 (2), 1159-1171 (2018).
- Sinko, R., et al. Tetrabromobisphenol A and diclazuril evoke tissue-specific changes of thyroid hormone signaling in male thyroid hormone action indicator Mice. Int J Mol Sci. 23 (23), 14782 (2022).
- Sinko, R., et al. Different hypothalamic mechanisms control decreased circulating thyroid hormone levels in infection and fasting-induced non-thyroidal illness syndrome in male thyroid hormone action indicator mice. Thyroid. 33 (1), 109-118 (2023).
- Salas-Lucia, F., et al. Axonal T3 uptake and transport can trigger thyroid hormone signaling in the brain. Elife. 12, 82683 (2023).
- Liu, S., et al. Triiodothyronine (T3) promotes brown fat hyperplasia via thyroid hormone receptor alpha mediated adipocyte progenitor cell proliferation. Nat Commun. 13 (1), 3394 (2022).
- Bianco, A. C., et al. American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 24 (1), 88-168 (2014).
- Silva, J. E., Larsen, P. R. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature. 305 (5936), 712-713 (1983).
- Bianco, A. C., Silva, J. E. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest. 79 (1), 295-300 (1987).
- Caporale, N., et al. From cohorts to molecules: Adverse impacts of endocrine disrupting mixtures. Science. 375 (6582), 8244 (2022).

