Evaluating Toxicity of Chemicals using a Zebrafish Vibration Startle Response Screening System
Summary
We describe a screening system’s workflow and data analysis for evaluating chemical compound toxicity based on the zebrafish embryo vibration startle response. The system records the movements of zebrafish embryos upon exposure to a vibration stimulus and allows for an integrated evaluation of general toxicity/lethality and neuromuscular toxicity.
Abstract
We developed a simple screening system for the evaluation of neuromuscular and general toxicity in zebrafish embryos. The modular system consists of electrodynamic transducers above which tissue culture dishes with embryos can be placed. Multiple such loudspeaker-tissue culture dish pairs can be combined. Vibrational stimuli generated by the electrodynamic transducers induce a characteristic startle and escape response in the embryos. A belt-driven linear drive sequentially positions a camera above each loudspeaker to record the movement of the embryos. In this way, alterations to the startle response due to lethality or neuromuscular toxicity of chemical compounds can be visualized and quantified. We present an example of the workflow for chemical compound screening using this system, including the preparation of embryos and treatment solutions, operation of the recording system, and data analysis to calculate benchmark concentration values of compounds active in the assay. The modular assembly based on commercially available simple components makes this system both economical and flexibly adaptable to the needs of particular laboratory setups and screening purposes.
Introduction
In recent years, zebrafish have become highly popular model organisms for the evaluation of chemical compound effects, encompassing research areas from drug development to environmental toxicology1. As vertebrates, zebrafish share many aspects of their genetic makeup and overall physiology with humans2,3. Therefore, results obtained in this model often are directly relevant to human health. Several drug candidates currently in clinical trials have been identified in compound screens using zebrafish4.
Toxicity assessment is one major application where tests using zebrafish embryonic stages are of interest. Various Organisation for Economic Co-operation and Development (OECD) test guidelines exist for the use of zebrafish in environmental toxicity testing5,6. The small size and rapid development of zebrafish embryos make them highly suitable for screening approaches on a medium to high throughput scale1,3,4. Toxicological endpoints targeted by such screens include embryonic malformations and lethality7, endocrine disruption8, organ toxicity9, and behavioral assessments indicative of neural toxicity10,11. The behavioral assays are possible because zebrafish embryos show various types of locomotor responses to different stimuli depending on their stage of development. For example, 1 day post fertilization (dpf) embryos show spontaneous tail coiling12 and respond to a sequence of light pulses with a typical sequence of movements, the so-called photomotor response (PMR)10. After hatching, typically occurring around 48-72 hours post fertilization (hpf), the freely swimming eleutheroembryos13 gradually develop startle and escape responses to vibrational stimuli starting around 4 dpf14. These responses are characterized by a distinctive bend to the direction opposite the direction of the stimulus (the so-called C-bend or C-start), which is followed by a smaller counter bend and swimming behavior14,15,16,17. Notably, embryonic behaviors are governed by neural circuits using various neurotransmitter systems, allowing for probing chemical compound effects targeting these systems. For example, the PMR assay revealed the effects of compounds interfering with cholinergic, adrenergic, and dopaminergic signaling10, while the startle response involves cholinergic, glutamatergic, and glycinergic neurons16,18. Furthermore, compounds that damage the muscles or the neuro-muscular interface will also affect these behaviors, as will compounds toxic to the inner ear/lateral line hair cells19,20. Observing zebrafish locomotor behavior in response to a stimulus is thus a suitable means to assess not only neurotoxicity but equally ototoxicity and myotoxicity. Scoring locomotor behavior also serves as a proxy for general toxicity/lethality assessment since dead embryos do not move. Thereby, embryonic locomotion behaviors represent an integrative readout for a first-tier toxicity screening approach, which indicates lethal and neuromuscular compound effects in one setup. Given that the eleutheroembryos are already capable of metabolizing compounds, the approach may also detect the effects of metabolic transformation products7,21,22. Importantly, zebrafish embryos are not considered as protected life stage under some animal protection legislations until the stage of free feeding after 120 hpf13. Therefore, they are regarded as an alternative to animal toxicity testing.
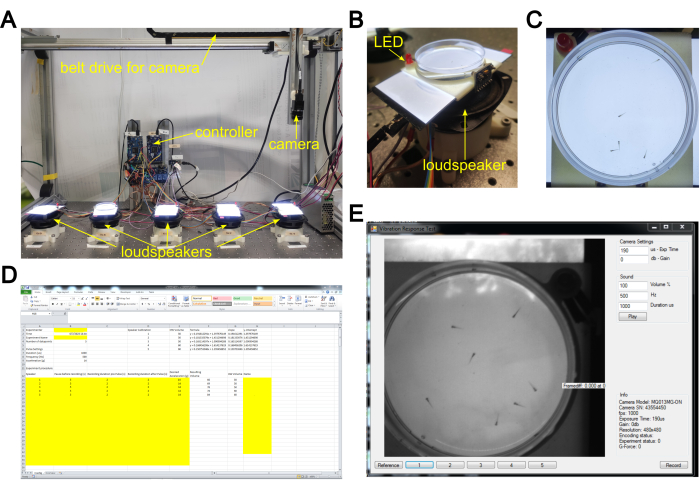
Figure 1: Vibration startle response system setup. (A) Overview of the system. Plates with embryos exposed to the test compounds are placed on the electrodynamic transducer array ("loudspeakers"). The camera is sequentially moved by the belt-driven linear drive into the recording position above the targeted transducer. (B) Detailed view of the transducer/loudspeaker with tissue culture dish inserted on top. The plates are illuminated from below by an LED light sheet at 4000-5000 lux. An LED light next to the speaker lights up while the stimulus is given. (C) Still image of video recorded by the camera upon stimulation of the embryos. (D) Screenshot of the configuration file. (E) Screenshot of the recording software interface. Please click here to view a larger version of this figure.
Here, we describe a testing protocol for the evaluation of compound effects on the vibration startle response using an in-house build simple testing device based on vibration stimuli generated by electrodynamic transducers coupled with an automated video recording of several freely moving embryos in a tissue culture dish23. The system is modular and allows for sequential recording from several tissue culture dishes in parallel. In the setup currently used, five electrodynamic transducers provide a vibrational stimulus (500 Hz, duration 1 ms) to tissue culture dishes containing 20 embryos placed on top of them (Figure 1). The plates are illuminated from below at 4000-5000 lux with LED light sheets. An LED light next to each transducer indicates periods of stimulus application, and an oscilloscope indicates waveforms and frequency of the applied stimulus (for details, see Ref. 23). The behavior of the embryos is recorded by a high-speed camera (Table of Materials) at 1000 frames per second (fps), which is moved above the targeted speaker by a belt-driven linear drive. This recording speed is required to reliably resolve the startle response. The system provides a low-cost, individually adaptable alternative to current commercial systems. The precise workflow detailed below is currently performed in the framework of the Precision Toxicology initiative24 in order to determine suitable exposure conditions for OMICS data acquisition from zebrafish embryos treated with a selected set of toxicants.
Protocol
All zebrafish husbandry and handling were performed in compliance with the German animal protection standards and approved by the Government of Baden-Württemberg, Regierungspräsidium Karlsruhe, Germany (Aktenzeichen 35-9185.64/BH KIT).
1. Preparing stock solutions of chemicals to be tested
- Label the glass vial (or chemical aliquot) with the compound name/abbreviation, stock concentration, and date of preparation. For example, CdCl2_2.5 g·L-1_210813.
- Centrifuge the chemical aliquot at 2000 x g for 1 min at room temperature (RT).
- Under a fume hood, weigh the compound (if necessary) on a scale sensitive to 0.001 g and transfer it to the labeled vial. If the compound is a liquid, add it to the vial using a pipette and plastic pipette tip.
NOTE: For example, for the tricaine methanesulfonate stock used in the result example shown in Figure 2, 400 mg was weighed into the labeled vial. - Add solvent (e.g., sterile pure water or dimethyl sulfoxide (DMSO), depending on the physicochemical properties of the compounds) using a pipette and plastic pipette tip. If possible, water is the preferred solvent.
NOTE: For example, for the tricaine stock, a 15.3 mM solution in 100 mL water was prepared. - Seal the vial, shake gently, and check for precipitation.
- Prepare dilute stock solutions (if necessary) in glass vials using pipettes and plastic pipette tips.
NOTE: For example, no further dilution was necessary for the tricaine stock. - Store the stock solution(s) at -20 °C until use.
- Store the remaining chemical aliquot in the same conditions as before.
- Record stock aliquot information in the lab notebook.
2. Collecting and raising zebrafish embryos
- Collect embryos in cleavage stages (2-8 cell stage) from natural spawning of group matings in 10 cm tissue culture dishes.
- Select a batch of appropriate quality: less than 10% unfertilized/dead eggs.
- Clean the dishes (remove unfertilized eggs, debris, scales, etc.).
- Place 60 embryos per 10 cm tissue culture dish in 15 mL of E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4)25.
- Place dishes into a humidified chamber prepared by laying out paper towels soaked with water.
- Raise embryos until 72 hpf in an incubator at 28.5 °C.
3. Preparing working dilution of chemicals to be tested
- Remove the stock solution from the -20 °C freezer and let it thaw.
- If compound solubility is high enough, prepare serial dilutions in E3 medium in glass bottles, 1 mL per replicate per concentration. Ensure that the the concentration is 10 times higher than the desired exposure concentration. This avoids having to change the entire medium of the embryos at the beginning of the exposure. In case of low solubility, prepare the serial dilutions directly at the desired exposure concentrations, 10 mL per replicate per concentration.
- Check for precipitation (if necessary). If the solution has precipitated, record this, then further dilute to achieve the next highest concentration. Check again for precipitation. Repeat until there is no precipitation.
- Check the pH of the exposure solution. If outside the range of pH 7.0-8.5, record this, and prepare the solutions in E3 containing 5 mM HEPES/pH 7.4, adjusting the pH with HCl or NaOH.
- Dispose of unused exposure media according to local regulations.
4. Exposing embryos to the chemicals to be tested
- Check the dishes with the 72 hpf old embryos for dead/unhatched embryos and remove them. If a batch of embryos contains more than 5% of unhatched eggs, remove the batch.
- Place 10 embryos per 6 cm tissue culture dish in 9 mL of E3 medium (exposure plate).
- Label exposure plates with the compound name, exposure concentration, and replicate number. For example, "CdCl2 1 mg·L-1 01". Include sufficient E3-only plates and a solvent control plate, if needed (see section 5).
- Add 1 mL of exposure solution to each plate, starting with the lowest concentration, and swirl the plate. For compounds with low solubility, replace the entire 10 mL of the plate with the exposure solution (see step 3.2.)
- Record the temporal order in which compound solutions were added to the embryos.
- Incubate the plates in the humidified chamber in an incubator at 28.5 °C for 48 h until they reach 120 hpf.
5. Performing the vibration startle assay
NOTE: Analyse the plates in the same order as recorded in step 4.5. Each run should include an E3 control plate.
- Switch on the computer and the vibration device (blue LED light should be on).
- Prepare the configuration file in a spreadsheet, as seen in Figure 1D and attached as Supplementary File 1. Record exposure information for each of the 5 plate positions (compound, concentration, replicate).
- Open the general user interface (GUI) program (available at https://git.scc.kit.edu/xk4962/vibration-startle-assay-kit , project ID 43215.)
- Check the camera movement by selecting the different positions in the GUI program and observing camera movement.
- Take out the sample plates to be measured from the incubator. Place the sample plates on the 5 positions (see Figure 1A, "loudspeakers") and let the embryos settle for several minutes.
- Click on Record; a window will open to select the configuration file.
- Select the appropriate configuration file prepared in step 5.2 for this run.
- Check that the sample description corresponds to the samples on each position (1-5).
- Measuring will be conducted automatically (10 s / position). When the sound pulse is applied by the program, an LED is turned on. Recording for 10 s/position allows acquiring enough data to estimate swimming speed and distance traveled both before and after the stimulus is applied and prevents habituation to subsequent stimuli.
- When the recording is completed, the camera goes back to position 1 and the software starts to compress the files. During this time, replace the samples with the next set that needs to be measured.
- Go to step 5.2. to record the next run.
- When all plates have been measured, collect the exposure solutions. Use a sieve to retain the embryos.
- Euthanize the embryos by rapid chill in an ice/isopropanol (5%) bath.
- Dispose of the exposure solutions and dead embryos according to the local regulations.
6. Data analysis
- Open the video data with VirtualDub (1.10.4).
- Score visually the number of embryos responding to the sound pulse (when the control LED is on).
- Enter data into a spreadsheet. Record the compound name, the replicate, the concentration of the compound, and the percentage of immotile embryos according to the template provided in Supplementary File 2, which includes the example dataset shown in Figure 2C.
NOTE: The template has a flexible structure and principally allows application for data of other organisms and endpoints. It describes the responses for each concentration and also provides endpoint descriptions as well as definitions of the parameters generated in the subsequent concentration-response modeling. - Conduct the benchmark concentration (BMC) analysis using a KNIME workflow (KNIME analytics 4.626) with embedded R scripts (R version 3.6., R-packages plotrix, drc and bmd27,28).
NOTE: In principle, the assessment could also be conducted directly in R. However, for convenience and to allow assessment as a web-based service, the analysis has been organized in a KNIME server-compliant workflow. The output of the KNIME workflow is provided in Supplementary File 3. For details on the statistical parameters generated by the KNIME workflow, refer to this template. The statistical parameters to estimate the goodness of fit and thresholds to accept determined BMC values are shown in Table 1. The KNIME workflow itself is available via GitHub (https://github.com/precisiontox/range-finding-drc). The concentration-response is modeled using a 4-parameter log-logistic model. Two of the curve fitting parameters can be fixed. Typically, one would fix the maximum to 100 in the case of percentage data. In case no background effects are observed, the minimum can be fixed to 0%.
Representative Results
Figure 2A shows the percentage of immotile embryos in 48 clutches of untreated wild-type embryos (AB2O2 strain). On average, 14.33% of untreated wild-type embryos do not react to the vibration stimulus. In 4 clutches, the percentage of immotile larvae reached 50%, but 75% of the clutches had a percentage of immotile larvae below 20%.
Figure 2B,C show an example of a typical calculation of a benchmark concentration/dose (BMC/BMD29,30) for compound effects on motility with the vibration startle assay workflow, as currently performed within the PrecisionTox consortium24. BMC10, BMC25, and BMC50 values correspond to the concentrations at which 10%, 25%, and 50% of embryos show immotility levels higher than the background, respectively. Only embryos that are completely immotile are included in this calculation, not those that still show partial responses, such as only a C-bend without subsequent escape swimming or only tail movements (Figure 2B). The embryos were exposed to 8 concentrations of the sodium channel inhibitor tricaine methanesulfonate, which is frequently used for fish anesthesia31. The data indicate a background level of around 25% immotility in response to the vibration stimulus. Starting at 1% tricaine, motility is reduced and then ceases above 2.5%. The KNIME workflow calculates the BMC50 as 164.9 µM, which corresponds to 1.07% tricaine and an immotility level of 75% (Figure 2C). The small 95% confidence intervals (indicated by the grey shades in the curve) indicate robust reproducibility of the motility values in this assay.
Figure 2D shows an example of a suboptimal assay run, the data of which should not be used for BMC calculations. Five E3 treated control groups with different embryos derived from the same clutch are shown (AB2O2 [wild-type strain] replicate 1-5). Only the first group shows a near normal response, showing around 25% immotility that is consistent with literature values32 and those obtained in the assay described here, as shown in Figure 2A, while all other groups show reduced and/or incomplete behavioral responses (e.g., showing only a C-bend not followed by swimming activity, or motility without a clear C-bend at the beginning). Such a response may occur when embryos do not develop properly and are in an immature state due to developmental delay, which impacts the robustness of the startle response14,33.
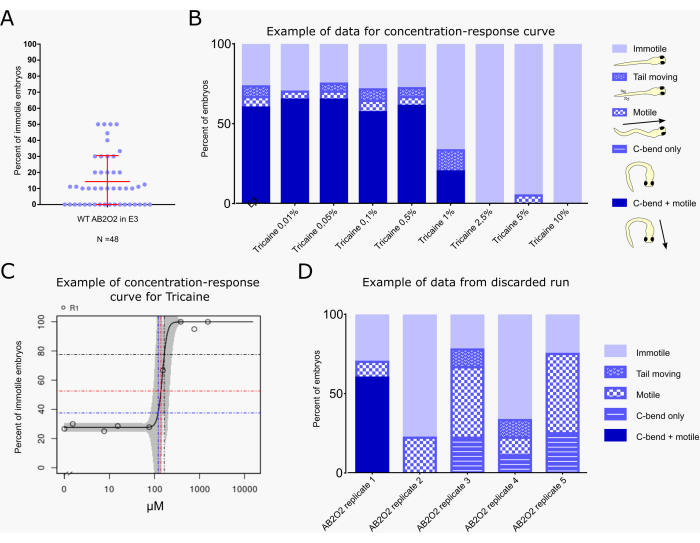
Figure 2: Example of a typical result, including benchmark dose calculation. (A) Percentage of non-responsive embryos after the sound pulse for wild-type untreated larvae for 48 clutches (n = 10 per clutch). The mean (14.33%) and standard deviation (±16.19%) are indicated in red. (B) Evaluation of startle response behavior of embryos (n=20 per condition) treated with the indicated concentration of tricaine in E3 medium or with E3 alone as a control. Behavior is classified according to the color scheme and cartoons indicated to the right of the graph, with each embryo assigned to only one of the following classes: "immotile": embryo does not show any movement; "tail moving": embryo shows tail movement, but neither C-bend nor swimming behavior; "motile": embryo shows swimming movement, but no C-bend in response to the vibrational stimulus; "C-bend only": embryo shows C-bend, but not escape swimming; "C-bend + motile": embryo shows typical C-bend behavior followed by escape swimming (the typical full startle response). The different behaviors are shown as a percentage of the total number of embryos for each treatment. (C) BMC calculation graph generated by the KNIME workflow, indicating the percentage of "immotile" embryos for each treatment concentration. Blue, red, and black lines indicate the BMC10, BMC25, and BMC50 values, i.e., the concentrations at which 10%, 25%, and 50% of embryos show immotility levels higher than the background, respectively. (D) Example of a discarded assay run. Five E3-treated control runs with different AB2O2 wild-type embryos derived from the same clutch are shown (replicate 1-5). Only replicate 1 shows a nearly normal response, while embryos of the remaining runs do not show the typical C-bend + escape swimming response. Please click here to view a larger version of this figure.
Table 1: Statistical parameters to estimate the goodness of fit and thresholds to accept determined BMC values. Please click here to download this Table.
Table 2: Properties of a selection of vibrational startle response assay systems. Please click here to download this Table.
Supplementary File 1: Excel template for configuration file. Please click here to download this File.
Supplementary File 2: KNIME input template with an example data set. Please click here to download this File.
Supplementary File 3: KNIME output file example. Please click here to download this File.
Discussion
We present the workflow and data analysis for chemical compound evaluation using a custom-built zebrafish embryo vibration startle assay setup. The workflow generates robust data that allow the calculation of typical parameters specifying compound toxicity, such as benchmark concentration/dose (BMC/BMD). The modularity of the setup allows adaptation to different needs for throughput and space requirements. As the system is made from low-cost basal components, following a relatively simple setup, it provides a cheap alternative to existing commercial systems, which are generally designed for several assay types at once, rely on proprietary software, and remain relatively costly.
Both these commercial systems and other custom-made systems allow for the assessment of single embryos or larvae in multiwell plates (e.g., 12-well34, 16-well32,35, 24-well20,33,36, 48-well37, 96-well38,39,40,41,42 and even 384-well [as 4×96 well]43), but the spatial restriction in the wells makes the analysis of some data parameters of the escape response (e.g., distance traveled) more challenging. Furthermore, in some of these setups, imaging is restricted to a subset of the wells of the plate, reducing the throughput36,39. Imaging embryos in dishes allows for better assessment of escape response parameters and enables recording the behavior of several embryos at once (up to 30 in a 6 cm dish, for example). Usually, dish-based imaging is limited to one dish per run44,45,46,47,48 (exceptions perform imaging in parallel on 6 dishes with one larva each49 or on 4 larvae in 2 split dishes50), a drawback that can be solved by parallel designs such as in our case. We have summarized some characteristics of the system used in this study and other commercial and custom-made solutions in Table 220,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,
51,52.
One advantage of the method is a readout capturing both lethality and behavioral changes, which can increase the performance of toxicity assessments. For example, while the zebrafish fish embryo acute toxicity test (FET)5 has been shown to predict toxicity in the adult fish acute toxicity test53 quite well, its prediction accuracy was improved by including behavioral readouts54. The reason for this is the weak mortality induced by neuroactive compounds seen in fish embryos, probably due to the lack of respiratory failure syndrome causing enhanced toxicity in juvenile or adult fish. Neuroactivity can, however, be identified by assessment of behavior. Furthermore, behavioral readouts can also capture myotoxic and ototoxic effects as well as other, more subtle toxic effects on physiology, which are sublethal yet influence the behavioral performance of the organism.
When conducting the assay, it is critical to ensure proper handling of compounds as well as using a homogenously developing batch of zebrafish embryos. Thus, using glass vials for compound storage should minimize the decline in concentrations of chemicals, particularly hydrophobic compounds, due to absorbance to plastic material. In the case of compounds of high absorptive potential to "plastic" polystyrene, glass plates can also be used for the incubation. Cleaning of the eggs in the tissue culture dishes used for collection and removal of dead embryos is a critical step to ensure standard development. Normal speed of development is important, as developmental delays may affect the maturity of neural networks underlying the assessed behavior14,33. Also, to enable comparison of compound effects, eggs should be derived from the same strain since different strains have been reported to present different behavioral profiles38,55,56,57. During exposure, it is important to incubate the embryos in a humidified chamber in order to avoid excessive evaporation of the E3 medium, which would alter the concentrations tested.
E3 controls should be incorporated into each run in order to determine the baseline response level of the particular batch of embryos used in the test series. Typically, we run one plate of controls along each set of 5 measurements. As illustrated in Figure 2D, this approach also allows for the detection of batches with suboptimal responses due to delayed development or for other reasons, such as genetic background effects. In case of an unexpected lack of response to the stimulus, also watch out for potential transducer failure. Typically, the startle responses show a sigmoidal concentration-response behavior that allows for curve fitting using a log-logistic model. However, in rare cases with biphasic responses, other models may have to be employed, such as Gaussian or Cedergreen models. They are available within the R packages drc and bdm27,28.
The lack of response to the vibrational stimulus may indicate simply the death of the embryos or severely impaired life functions due to general cytotoxicity, but might also reflect more specific toxicity targeting neural circuits of stimulus perception, integration, and locomotor output. Other possible compound effects are interference with the neuromuscular interface or with muscle structure and function. To distinguish between these possibilities, further assays are necessary. For example, the structural integrity of the muscles can be assessed with a birefringency assay58,59, and transgenic lines are available to assess perturbance of muscular and neural function60,61. However, the recorded video data already allow for a more detailed analysis of the morphology and the behavioral response of the embryos that can provide first additional information. Is only the C-bend impaired, or all motility? Are still remnants of neuromuscular activity present, as indicated by weak or trembling tail movements? Do such altered behaviors go along with changes in morphology, such as edema or increased body curvature? Additionally, parameters such as latency time until the C-bend or the distance travelled during the escape response can be evaluated (see, for example, Ref. 44).
The screening protocol described here allows for rapid and robust compound toxicity evaluations, with the added value of specifically detecting non-lethal neurotoxic, ototoxic, and myotoxic compounds. The provided analysis workflow is easy to implement and provides a robust readout. Modifications of the stimulus protocols used in the vibration startle assay have been used to address compound effects on more complex aspects of startle behavior as well, such as prepulse inhibition (PPI)39,44 and habituation32,33, and could be adapted to the electrodynamic transducer-based stimulus setup used in this study.
A main application of startle-response-based screening systems is the assessment of compound effects in chemical screens, which is of relevance both for human toxicity evaluation and drug development1,4,62. At the same time, by testing the early life stages of an aquatic organism, the results obtained have direct relevance for ecotoxicological risk assessment63,64. In addition, startle response systems can be used for behavioral phenotyping in genetic screens65,66,67,68,69. Our easily implementable and adaptable system provides an affordable setup to smaller laboratories intending to conduct their own specific screening projects in these various domains of application.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thankfully acknowledge the excellent technical assistance of the support staff at the IBCS-BIP fish facility and screening center. This work has received funding from the European Union's Horizon 2020 research and innovation program under Grant Agreement No 965406 (PrecisionTox). This output reflects only the authors' view, and the European Union cannot be held responsible for any use that may be made of the information contained therein.
Materials
| Fine test sieves, Brass frame, pore size 250 μm | Sigma-Aldrich | Z289744-1EA | Or comparable material |
| High-speed camera | XIMEA | MQ013MG-ON USB 3 | |
| Laboratory Bottles, Narrow Neck, with Screw Cap | VWR | 215-3261 | Reference number for 50 mL, available up to 20 L. Or comparable material |
| Pipette tip, working volume: 10 µL | SARSTEDT | 70.3010.210 | Or comparable material |
| Pipette tip, working volume: 1000 µL | SARSTEDT | 70.3050.100 | Or comparable material |
| Pipette tip, working volume: 20 µL | SARSTEDT | 70.3020.210 | Or comparable material |
| Pipette tip, working volume: 200 µL | SARSTEDT | 70.3030.100 | Or comparable material |
| Serological pipette 10 mL | SARSTEDT | 86.1254.001 | Or comparable material |
| Serological pipette 25 mL | SARSTEDT | 86.1685.001 | Or comparable material |
| Serological pipette 5 mL | SARSTEDT | 86.1253.001 | Or comparable material |
| Tissue culture dish 60,0 mm/15,0 mm vented (Polystyrene) | Greiner bio-one | 628102 | Or comparable material |
| Tissue culture dish 100, suspension (Polystyrene) | SARSTEDT | 83.3902.500 | Or comparable material |
| Transfer pipette 6 mL | SARSTEDT | 86.1175 | Or comparable material |
| Tube 15 mL 120 mm x 17 mm PP | SARSTEDT | 62.554.502 | Or comparable material |
| Tube 50 mL 114mm x 28 mm PP | SARSTEDT | 62.5472.54 | Or comparable material |
Referências
- MacRae, C. A., Peterson, R. T. Zebrafish as a mainstream model for in vivo systems pharmacology and toxicology. Annu Rev Pharmacol Toxicol. 63, 43-64 (2023).
- Howe, K., et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496 (7446), 498-503 (2013).
- Choi, T. Y., Choi, T. I., Lee, Y. R., Choe, S. K., Kim, C. H. Zebrafish as an animal model for biomedical research. Exp Mol Med. 53 (3), 310-317 (2021).
- Patton, E. E., Zon, L. I., Langenau, D. M. Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov. 20 (8), 611-628 (2021).
- OECD. Test No. 236: Fish embryo acute toxicity (FET) Test. OECD Guidelines for the Testing of Chemicals, Section 2. , (2013).
- OECD. Test No. 250: EASZY assay – Detection of endocrine active substances, acting through estrogen receptors, using transgenic tg(cyp19a1b:GFP) zebrafish embryos. OECD Guidelines for the Testing of Chemicals, Section 2. , (2021).
- Braunbeck, T., et al. The fish embryo test (FET): origin, applications, and future. Environ Sci Pollut Res Int. 22 (21), 16247-16261 (2015).
- Weger, B. D., Weger, M., Nusser, M., Brenner-Weiss, G., Dickmeis, T. A Chemical screening system for glucocorticoid stress hormone signaling in an intact vertebrate. ACS Chem Biol. 7 (7), 1178-1183 (2012).
- Pandey, G., Westhoff, J. H., Schaefer, F., Gehrig, J. A Smart imaging workflow for organ-specific screening in a cystic kidney zebrafish disease model. International Journal of Molecular Sciences. 20 (6), 1290 (2019).
- Kokel, D., et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 6 (3), 231-237 (2010).
- Zhang, K., Liang, J., Brun, N. R., Zhao, Y., Werdich, A. A. Rapid zebrafish behavioral profiling assay accelerates the identification of environmental neurodevelopmental toxicants. Environ Sci Technol. 55 (3), 1919-1929 (2021).
- Ogungbemi, A. O., Teixido, E., Massei, R., Scholz, S., Kuster, E. Optimization of the spontaneous tail coiling test for fast assessment of neurotoxic effects in the zebrafish embryo using an automated workflow in KNIME(R). Neurotoxicol Teratol. 81, 106918 (2020).
- Strahle, U., et al. Zebrafish embryos as an alternative to animal experiments–a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol. 33 (2), 128-132 (2012).
- Kimmel, C. B., Patterson, J., Kimmel, R. O. The development and behavioral characteristics of the startle response in the zebra fish. Dev Psychobiol. 7 (1), 47-60 (1974).
- Eaton, R. C., Bombardieri, R. A., Meyer, D. L. The mauthner-initiated startle response in teleost fish. Journal of Experimental Biology. 66 (1), 65-81 (1977).
- Berg, E. M., Bjornfors, E. R., Pallucchi, I., Picton, L. D., El Manira, A. Principles governing locomotion in vertebrates: Lessons from zebrafish. Front Neural Circuits. 12, 73 (2018).
- Lopez-Schier, H. Neuroplasticity in the acoustic startle reflex in larval zebrafish. Curr Opin Neurobiol. 54, 134-139 (2019).
- Hale, M. E., Katz, H. R., Peek, M. Y., Fremont, R. T. Neural circuits that drive startle behavior, with a focus on the Mauthner cells and spiral fiber neurons of fishes. J Neurogenet. 30 (2), 89-100 (2016).
- Behra, M., Etard, C., Cousin, X., Strahle, U. The use of zebrafish mutants to identify secondary target effects of acetylcholine esterase inhibitors. Toxicol Sci. 77 (2), 325-333 (2004).
- Buck, L. M., Winter, M. J., Redfern, W. S., Whitfield, T. T. Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hear Res. 284 (1-2), 67-81 (2012).
- van Wijk, R. C., Krekels, E. H. J., Hankemeier, T., Spaink, H. P., vander Graaf, P. H. Systems pharmacology of hepatic metabolism in zebrafish larvae. Drug Discovery Today: Disease Models. 22, 27-34 (2016).
- Loerracher, A. K., Braunbeck, T. Cytochrome P450-dependent biotransformation capacities in embryonic, juvenile and adult stages of zebrafish (Danio rerio)-a state-of-the-art review. Arch Toxicol. 95 (7), 2299-2334 (2021).
- Marcato, D. . Design and Development of Imaging Platforms for Phenotypic Characterization of Early Zebrafish. , (2018).
- PrecisionTox Consortium. The precision toxicology initiative. Toxicol Lett. 383, 33-42 (2023).
- Nüsslein-Volhard, C. . Zebrafish – A Practical Approach. , (2002).
- Berthold, M. R., Preisach, C. h. r. i. s. t. i. n. e., Burkhardt, H. a. n. s., Schmidt-Thieme, L. a. r. s., Decker, R. e. i. n. h. o. l. d., et al. . Data Analysis, Machine Learning and Applications. , (2008).
- Ritz, C., Baty, F., Streibig, J. C., Gerhard, D. Dose-response analysis using R. PLoS ONE. 10 (12), e0146021 (2015).
- Jensen, S. M., Kluxen, F. M., Streibig, J. C., Cedergreen, N., Ritz, C. bmd: an R package for benchmark dose estimation. Peerj. 8, e10557 (2020).
- Committee, E. S., et al. Guidance on the use of the benchmark dose approach in risk assessment. EFSA J. 20 (10), e07584 (2022).
- Haber, L. T., et al. Benchmark dose (BMD) modeling: current practice, issues, and challenges. Crit Rev Toxicol. 48 (5), 387-415 (2018).
- Carter, K. M., Woodley, C. M., Brown, R. S. A review of tricaine methanesulfonate for anesthesia of fish. Reviews in Fish Biology and Fisheries. 21 (1), 51-59 (2011).
- Wolman, M. A., Jain, R. A., Liss, L., Granato, M. Chemical modulation of memory formation in larval zebrafish. Proc Natl Acad Sci U S A. 108 (37), 15468-15473 (2011).
- Roberts, A. C., et al. Habituation of the C-start response in larval zebrafish exhibits several distinct phases and sensitivity to NMDA receptor blockade. PLoS One. 6 (12), e29132 (2011).
- Marquez-Legorreta, E., et al. Brain-wide visual habituation networks in wild type and fmr1 zebrafish. Nat Commun. 13 (1), 895 (2022).
- Panlilio, J. M., Aluru, N., Hahn, M. E. Developmental neurotoxicity of the harmful algal bloom toxin domoic acid: Cellular and molecular mechanisms underlying altered behavior in the zebrafish model. Environ Health Perspect. 128 (11), 117002 (2020).
- Zeddies, D. G., Fay, R. R. Development of the acoustically evoked behavioral response in zebrafish to pure tones. J Exp Biol. 208 (Pt 7), 1363-1372 (2005).
- Levitz, J., et al. Optical control of metabotropic glutamate receptors. Nat Neurosci. 16 (4), 507-516 (2013).
- Best, J. D., et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 33 (5), 1206-1215 (2008).
- Bhandiwad, A. A., Zeddies, D. G., Raible, D. W., Rubel, E. W., Sisneros, J. A. Auditory sensitivity of larval zebrafish (Danio rerio) measured using a behavioral prepulse inhibition assay. J Exp Biol. 216 (Pt 18), 3504-3513 (2013).
- Liu, F., et al. Solute carrier family 26 member a2 (slc26a2) regulates otic development and hair cell survival in zebrafish. PLoS One. 10 (9), e0136832 (2015).
- Singh, C., Oikonomou, G., Prober, D. A. Norepinephrine is required to promote wakefulness and for hypocretin-induced arousal in zebrafish. Elife. 4, e07000 (2015).
- Joo, W., Vivian, M. D., Graham, B. J., Soucy, E. R., Thyme, S. B. A customizable low-cost system for massively parallel zebrafish behavioral phenotyping. Front Behav Neurosci. 14, 606900 (2020).
- Tucker Edmister, S., et al. Novel use of FDA-approved drugs identified by cluster analysis of behavioral profiles. Sci Rep. 12 (1), 6120 (2022).
- Burgess, H. A., Granato, M. Sensorimotor gating in larval zebrafish. J Neurosci. 27 (18), 4984-4994 (2007).
- Marsden, K. C., Granato, M. In Vivo Ca(2+) Imaging Reveals that Decreased Dendritic Excitability Drives Startle Habituation. Cell Rep. 13 (9), 1733-1740 (2015).
- Chatterjee, P., et al. Otoferlin deficiency in zebrafish results in defects in balance and hearing: rescue of the balance and hearing phenotype with full-length and truncated forms of mouse otoferlin. Mol Cell Biol. 35 (6), 1043-1054 (2015).
- Wang, C., et al. Evaluation of the hair cell regeneration in zebrafish larvae by measuring and quantifying the startle responses. Neural Plast. 2017, 8283075 (2017).
- Xu, L., Guan, N. N., Huang, C. X., Hua, Y., Song, J. A neuronal circuit that generates the temporal motor sequence for the defensive response in zebrafish larvae. Curr Biol. 31 (15), 3343-3357.e4 (2021).
- Hecker, A., Schulze, W., Oster, J., Richter, D. O., Schuster, S. Removing a single neuron in a vertebrate brain forever abolishes an essential behavior. Proc Natl Acad Sci U S A. 117 (6), 3254-3260 (2020).
- Weber, D. N. Dose-dependent effects of developmental mercury exposure on C-start escape responses of larval zebrafish Danio rerio. Journal of Fish Biology. 69 (1), 75-94 (2006).
- Santistevan, N. J., et al. cacna2d3, a voltage-gated calcium channel subunit, functions in vertebrate habituation learning and the startle sensitivity threshold. PLoS One. 17 (7), e0270903 (2022).
- Thyme, S. B., et al. Phenotypic landscape of schizophrenia-associated genes defines candidates and their shared functions. Cell. 177 (2), 478-491.e20 (2019).
- OECD. Test No. 203: Fish, Acute Toxicity Test. OECD Guidelines for the Testing of Chemicals, Section 2. , (2019).
- Kluver, N., et al. Fish embryo toxicity test: identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ Sci Technol. 49 (11), 7002-7011 (2015).
- Monroe, J. D., et al. Hearing sensitivity differs between zebrafish lines used in auditory research. Hear Res. 341, 220-231 (2016).
- van den Bos, R., et al. Further characterisation of differences between TL and AB zebrafish (Danio rerio): Gene expression, physiology and behaviour at day 5 of the larval stage. PLoS One. 12 (4), e0175420 (2017).
- van den Bos, R., et al. Early life exposure to cortisol in zebrafish (Danio rerio): similarities and differences in behaviour and physiology between larvae of the AB and TL strains. Behavl Pharmacol. 30 (2-3), 260-271 (2019).
- Felsenfeld, A. L., Walker, C., Westerfield, M., Kimmel, C., Streisinger, G. Mutations affecting skeletal-muscle myofibril structure in the zebrafish. Development. 108 (3), 443-459 (1990).
- Berger, J., Sztal, T., Currie, P. D. Quantification of birefringence readily measures the level of muscle damage in zebrafish. Biochem Biophys Res Commun. 423 (4), 785-788 (2012).
- Shahid, M., et al. Zebrafish biosensor for toxicant induced muscle hyperactivity. Sci Rep. 6, 23768 (2016).
- Winter, M. J., et al. Functional brain imaging in larval zebrafish for characterising the effects of seizurogenic compounds acting via a range of pharmacological mechanisms. Br J Pharmacol. 178 (13), 2671-2689 (2021).
- Vorhees, C. V., Williams, M. T., Hawkey, A. B., Levin, E. D. Translating neurobehavioral toxicity across species from zebrafish to rats to humans: Implications for risk assessment. Front Toxicol. 3, 629229 (2021).
- Scholz, S., et al. The zebrafish embryo model in environmental risk assessment–applications beyond acute toxicity testing. Environ Sci Pollut Res Int. 15 (5), 394-404 (2008).
- Dutra Costa, B. P., Aquino Moura, L., Gomes Pinto, S. A., Lima-Maximino, M., Maximino, C. Zebrafish models in neural and behavioral toxicology across the life stages. Fishes. 5 (3), 23 (2020).
- Wolman, M. A., et al. A genome-wide screen identifies PAPP-AA-mediated IGFR signaling as a novel regulator of habituation learning. Neuron. 85 (6), 1200-1211 (2015).
- Marsden, K. C., et al. A Cyfip2-dependent excitatory interneuron pathway establishes the innate startle threshold. Cell Rep. 23 (3), 878-887 (2018).
- Jain, R. A., et al. A forward genetic screen in zebrafish identifies the g-protein-coupled receptor CaSR as a modulator of sensorimotor decision making. Curr Biol. 28 (9), 1357-1369.e5 (2018).
- Nelson, J. C., et al. Acute regulation of habituation learning via posttranslational palmitoylation. Curr Biol. 30 (14), 2729-2738.e4 (2020).
- Meserve, J. H., et al. A forward genetic screen identifies Dolk as a regulator of startle magnitude through the potassium channel subunit Kv1.1. PLoS Genet. 17 (6), e1008943 (2021).

