Non-Nutritive Suck Parameters Measurements Using a Custom Pressure Transducer System
Summary
The non-nutritive suck (NNS) device can easily collect and quantify NNS features using a pacifier connected to a pressure transducer and recorded through a data acquisition system and laptop. Quantification of NNS parameters can provide valuable insight into a child’s current and future neurodevelopment.
Abstract
The non-nutritive suck (NNS) device is a transportable, user-friendly pressure transducer system that quantifies infants’ NNS behavior on a pacifier. Recording and analysis of the NNS signal using our system can provide measures of an infant’s NNS burst duration (s), amplitude (cmH2O), and frequency (Hz). Accurate, reliable, and quantitative assessment of NNS has immense value in serving as a biomarker for future feeding, speech-language, cognitive, and motor development. The NNS device has been used in numerous research lines, some of which have included measuring NNS features to investigate the effects of feeding-related interventions, characterizing NNS development across populations, and correlating sucking behaviors with subsequent neurodevelopment. The device has also been used in environmental health research to examine how exposures in utero can influence infant NNS development. Thus, the overarching goal in research and clinical utilization of the NNS device is to correlate NNS parameters with neurodevelopmental outcomes to identify children at risk for developmental delays and provide rapid early intervention.
Introduction
Non-nutritive suck (NNS) is one of the first occurring behaviors that an infant can perform with their mouth soon after birth and therefore has the potential to provide meaningful insights into brain development1. NNS refers to sucking movements without nutritional intake (e.g., sucking on a pacifier) and is characterized by a series of rhythmic expressions and suction movements of the jaw and tongue with pause breaks for breathing. Common parameters of NNS have been noted to include an average NNS burst (series of suck cycles) of 6-12 suck cycles with an intra-burst frequency of two sucks per second2; however, NNS features vary among clinical populations3,4 and dynamically change during the first year of life5. These changes are attributed to the growth of the oral cavity and associated anatomy, maturation of feeding skills and neurodevelopment, and experiences. The neural bases of NNS mainly include the suck central pattern generator in the central gray of the brainstem, comprising an intricate network of interneurons and the facial and trigeminal motor neuron nuclei6. A coordinated NNS also relies on intact neural pathways among cortical and brainstem regions to modulate its performance to sensory stimuli7,8, which makes NNS a viable indicator of early neural function and development.
NNS measures are linked to feeding success in premature infants9,10, and both sucking and feeding outcomes have been linked to subsequent motor, communication, and cognitive development11,12,13. In a retrospective study that characterized 23 preschool-aged children with language and motor impairments, 87% had a history of early feeding issues, which included difficulties in sucking11. Nutritive sucking performance immediately following birth and caregiver reports of feeding difficulties were significantly associated with multiple domains of neurodevelopment in children 18 months of age12,14. Interestingly, the sensitivity and specificity of feeding performance were higher than ultrasound assessment of the brain on neurodevelopmental outcome measures12. In another study, sucking/oral motor performance scores assessed via the neonatal oral-motor assessment scale15 in early infancy were associated with motor skills, language, and measures of intelligence at 2 and 5 years of age in a cohort of children born prematurely13,16.
Given that sucking and feeding can be sensitive indicators of neurodevelopmental outcomes throughout childhood, there is a critical need for accessible, accurate, and quantitative assessment of NNS to help identify children at risk for delayed and disordered development to provide early intervention. This need led to the design and research utilization of the Speech & Neurodevelopment Lab's (SNL) NNS device. This portable device includes a pacifier attached to the end of an easy-to-hold handle, connected to a customized pressure transducer designed in-house, and connected to a data acquisition center (DAC). The DAC connects to a laptop, and the data is recorded via data acquisition and analysis software. The pressure transducer measures pressure changes inside the pacifier and converts it into a voltage signal. The DAC contains converters that change the analog voltage signal to digital values in cmH2O that are visualized and recorded via the data acquisition and analysis software. NNS outcome measures that can be analyzed from the suck signal waveform include NNS duration (how long a suck burst lasts measured in s), amplitude (measured as peak height subtracted by peak-trough in cmH2O), cycles/burst (number of suck cycles within a burst), frequency (intra-burst frequency measured in Hz), cycles (number of suck cycles that occur in a min), and bursts (number of suck bursts that occur in a min).
Protocol
Northeastern University's institutional review board has approved studies using the NNS device with human subjects (15-06-29; 16-04-06; 17-08-19). Informed consent was obtained from the children's caregivers. All research personnel have completed human subject training prior to collecting any data with the NNS device. The SNL team has generated several training resources and protocols for new research personnel to complete prior to data collection using the NNS device. These training sessions include reviewing the following protocol.
1. NNS device setup
- Open the transportable case (Figure 1) and remove the following device components: DAC and its power cord, customized pressure transducer box (NNS box) with pacifier receiver handle and gray cable attached, laptop computer and the USB cord that connects it to the DAC, and pacifier.
- Plug in the following components: the power cord into the DAC and a three-pronged power outlet, a grey cable connected to the NNS box into the first front round port of the DAC, and a USB cord into the laptop computer and DAC (Figure 2).
- Turn on the DAC using the power switch in its back and log onto the laptop/computer.
2. NNS device calibration
- Remove the pressure calibrator and 1 mL syringe from the case.
- Unscrew the black pacifier receiver from the handle. Screw the handle onto the pressure calibrator so that the handle is horizontal with the pressure calibrator (Figure 3A-C).
- Draw the syringe plunger fully out and then screw it into the upper position on the pressure calibrator. The syringe should be perpendicular to the pressure calibrator (Figure 3D).
- On the laptop computer, open the spreadsheet labeled SNL Suck Analyzer Calibration File.
NOTE: This file contains formulas that assess pressure variability between the data acquisition and analysis application and pressure calibrator device measured in psi. There is a box in the upper left corner for data entry, which is used to enter data readings from the pressure calibrator and the LabChart Calibration File (described below).- Right-click the tab that reads Duplicate and Rename as Date and select Move or Copy.
- In the Move or Copy pop-up window, click the box Create a Copy within the SNL Suck Analyzer Calibration File and then click Ok.
- Double-click the tab that was just copied and rename it as the current date.
- Open the data acquisition and analysis file on the laptop computer's desktop labeled Calibration File.
NOTE: Ensure the spreadsheet is still viewable on the laptop computer screen, which may require minimizing and re-arranging the spreadsheet and data acquisition and analysis application windows. - Press the Power button on the pressure calibrator device to turn it on.
- On the laptop computer, select Start on the Calibration File while the NNS box gear is at Zero. Check the waveform sampling across time on the file.
NOTE: The NNS box has two setting options: Zero and Sample. Ensure that it is set to Zero before starting calibration. The file's Start button will only activate when the file is opened after the DAC has been powered on. If the file is opened and the Start button cannot be clicked, close the file, power on the DAC, and reopen the file. - In their respective cells in the spreadsheet (i.e., under the DAC Program and Red Calibrator columns), record the value at the top right corner of the Calibration File and the value on the pressure calibrator device while psi is at 0.00 (Figure 4A).
- Turn the gear from Zero para Sample on the NNS box. Wait approximately 15 s to allow adequate time for the pressure transducer to change recording functions.
- Slowly depress the syringe plunger until the pressure calibrator reaches a value as close to 0.2 psi as possible, and then fill in the Calibration File with the pressure calibrator values in their respective cells in the spreadsheet.
- Repeat step 2.10. for the following psi values: 0.4, 0.6, and 0.8 (Figure 4A).
- Once all values are inputted into the spreadsheet, click Stop in the Calibration File. In the spreadsheet, check the Slope and Goodness of Fit cells located to the right of the table that was used to plug in the psi values from the data acquisition and analysis application and the calibration device (Figure 4B). If both cells are highlighted in green, the calibration was successful; proceed to step 2.13.
NOTE: If one or both cells are red, clear the values in the psi measurement cells in the spreadsheet, turn the NNS box from Sample para Zero, close out the Calibration File, turn off the pressure calibrator by pressing the Power button, fully unscrew the syringe off the pressure calibrator, and pull the syringe plunger fully out before screwing it back on. Repeat steps 2.5. – 2.12. - Close the Calibration File without saving, turn the gear on the NNS box to Zero, and turn the pressure calibrator off by pressing the Power button.
- Unscrew the syringe off the pressure calibrator. Pull the syringe plunger fully out again and then screw it back on the pressure calibrator.
- On the computer's desktop, select and open the file labeled Master Settings File. On the top channel in the file, click the arrow for drop-down options on Suck Pressure and select Arithmetic.
NOTE: Ensure the spreadsheet is still viewable on the laptop/computer screen, which may require minimizing and re-arranging the spreadsheet and data acquisition and analysis application windows. - Within the parentheses of the Formula text box in the data acquisition and analysis file, type in the values from the spreadsheet that are located in the blue cells above the Slope and Goodness of Fit cells (Figure 4C). Click OK on the file.
- Turn the pressure calibrator back on using the Power button. Press Start on the Master Settings File. Turn the NNS box back to Sample and wait for 15 s.
- Depress the syringe plunger as close to 0.5 psi as read on the pressure calibrator.
- Scroll to the right within the spreadsheet and record the Master Settings File value under the cell labeled DAC and the pressure calibrator's value under the cell labeled Calibrator (Figure 4D). If the percentage error cell is highlighted in green, the calibration is successfully completed. If it is red, clear the data entered in this step and restart the calibration process from step 2.13.
- Click Stop on the Master Settings File. Turn the NNS box to Zero. Save the Master Settings File by selecting File, then Save as Settings. Name the file as the date of successful calibration.
- In the spreadsheet, select File > Save and then File > Close.
- Turn off the pressure calibrator by pressing the Power button. Unscrew the handle and syringe from the pressure calibrator and screw the black receiver back on the handle. Turn off, unplug, and pack up device components back in the case.
3. Collecting non-nutritive suck data
- Complete steps 1.1. – 1.3. for NNS device setup.
- Wash hands, put on latex gloves, and attach a newly opened pacifier to the pacifier receiver (Figure 5).
- Open the data acquisition and analysis file on the laptop computer's desktop with the latest calibration date. Once the file is opened, select Start.
- Turn the NNS box gear from Zero para Sample. Wait approximately 15 s to allow adequate time for the pressure transducer to change recording functions.
- Offer the pacifier to the child in a comfortable position and hold it for them to suck on for 2-5 min (or however long is tolerable for the child and comfortable with their caregiver).
NOTE: Preferable positions to measure NNS in children would be optimal feeding positions for their age. The researcher or a caregiver can offer the child the pacifier (Figure 6). - When the child is finished or 5 min has passed, retrieve the pacifier handle from whoever was holding it for the child and press Stop on the file. Change the NNS box gear from Sample para Zero.
- Remove the pacifier from the receiver and safely dispose of it following any institutional sanitary protocols. Safely remove and dispose of gloves and wash hands.
- Save the file by selecting Save as and name the file with the participant's ID number and the date of data collection. Save the file to the desktop of the laptop computer.
- Turn off, unplug, and pack up device components back in the case.
4. Analyzing non-nutritive sucks amples
- Using a desktop or laptop that has the data acquisition and analysis software, open up the participant's NNS data file on the desktop by double-clicking on it.
- Manually identify suck bursts using the following criteria: NNS bursts having more than one suck cycle, each suck cycle having an amplitude of at least 1 cmH2O, and waveforms within 1000 ms of each other being considered as part of the same suck burst (Figure 7).
NOTE: It is helpful to modify the view of the waveform (click the Set horizontal scaling box on the bottom right of the screen to have zoom-in and out options) to better identify NNS cycles from noise. The analysis is completed in a 50:1 view. It is important to note as we explore NNS across populations, these criteria may change as various populations exhibit altered NNS patterns. - To set peak analysis settings, select Peak Analysis, then Settings, then Table Options. Check the T Start, T End, Height, Peak Area, and Period boxes. All other boxes should be unchecked.
- Use the cursor to click and drag a box around the first NNS burst identified with the criteria described in step 4.2.
- Click Analyze (as part of peak analysis options in the top toolbar), which will identify peaks with parameters specified in step 4.3.
- Click the Burst Analysis Macro button, which will generate a pop-up data pad menu.
- In the data pad, insert a row in the column above the data by right clicking that column, selecting Insert Row for the first NNS burst, and typing Min 0-1 (or whichever minute the first burst occurs in).
- Continue steps 4.4. – 4.6. until all NNS bursts have been selected. Continue to keep track of the minute in which bursts occur by characterizing the specific minute (e.g., Min 1-2, Min 2-3) in the data pad.
- Once the analysis is complete, select File > Save As, and save the analyzed NNS file as the participant ID, date, and researcher initials. Additionally, select File > Export > Data Pad Only as Text File > Save to save the data pad file separately.
NOTE: It is important to save the raw NNS file, analyzed NNS file, and text file. - Process the text file through a custom NNS burst macro. This produces an analyzed text file that contains the following burst variables: duration, frequency, height (amplitude), burst count, cycles/burst, and cycles/minute for each NNS burst. It also contains an average for the two consecutive minutes of NNS with the highest cycle count, which is often used for final analyses. Adjust depending on what analysis window needs to be analyzed.
Representative Results
The NNS device has been used in numerous published studies that incorporate NNS outcome measures17,18,19. In the example data shown in Figure 7, bursts have been manually identified with the following criteria: more than one suck cycle per burst, cycles having at least an amplitude of 1 cmH2O, and suck waveforms within 1000 ms of each other. Once bursts are identified, the custom Macro outputs the NNS outcomes.
The SNL has used the device to assess NNS parameters in 25 infants immediately before and after frenotomy (a surgical procedure to alleviate a tight lingual frenulum)17. Following frenotomy, infants demonstrated a decrease in NNS amplitude (M = 13.52 cmH2O, SD = 5.39 pre-frenotomy; M = 10.25 cmH2O, SD = 4.93 post-frenotomy) and burst duration (M = 5.21 s, SD = 2.62 pre-frenotomy; M = 4.04 s, SD = 1.72 post-frenotomy); however, these results, which indicated reduced NNS behavior, could have been related to pain following the surgery17. This study highlights that the NNS device system could be used as a pre-/post-outcome measure following feeding-related interventions and/or surgeries to inform practitioners of their efficacy. An investigation of birth order effects on a variety of caregiver and infant feeding outcomes in 56 pairs of mothers and infants reported no difference in NNS features measured via the NNS device between infants with (duration M = 4.41 s, SD = 2.39; frequency M = 2.03 Hz, SD = 0.41; amplitude M = 12.74 cmH2O, SD = 6.99; bursts M = 4.33, SD = 0.41) and without (duration M = 5.70 s, SD = 4.15; frequency M = 2.11 Hz, SD = 0.21; amplitude M = 16.28 cmH2O, SD = 8.13; bursts M = 4.85, SD = 2.30) siblings18. These non-statistically significant results on NNS outcomes matched the result of no difference in feeding performance assessed via the oral feeding skills scores among these infants18. The NNS device has been used in a research line evaluating the relationship between the early oromotor behaviors of NNS and babbling. In a group of 26 full-term infants, Murray et al.19 reported NNS burst duration (M = 4.93 s, range = 0.94 – 11.97), frequency (M = 2.06 Hz, range = 1.36 – 2.75), and amplitude (M = 12.32 cmH2O, range = 1.19 – 28.03) were significant predictors of the coefficient of variation babbling vocalization measure of voice onset time (VOT) in a multiple regression model (F[4,18] = 3.613, p = 0.02, R2 = 0.45). Specifically, longer NNS burst duration and higher NNS intraburst frequency drove increased variation in VOT. Further research on the relationship between early NNS behavior and subsequent oromotor skills is ongoing in the SNL.
Several studies using the NNS device have contributed to furthering our understanding of NNS development, features, and how additional sensory experiences may modulate its performance5,20,21. Martens et al.5 captured differences in NNS outcomes throughout the first year of life in a longitudinal, repeated measures study in 26 full-term infants at 3 and 12 months of age. Specifically, NNS outcome measures of suck bursts/min (3-month Mdn = 4.50; 12-month Mdn = 2.50), cycles/burst (3-month Mdn = 9.60; 12-month Mdn = 2.50), and burst duration (3-month Mdn = 4.74 s; 12-month Mdn = 1.67 s) decreased, NNS amplitude (3-month Mdn = 14.05 cmH2O; 12-month median = 19.75 cmH2O) increased, and NNS frequency (3-month Mdn = 2.09 Hz; 12-month Mdn = 2.11 Hz) remained constant with age5. Zimmerman et al.21 used the NNS device to standardize NNS measurement and investigate NNS characteristics within a single suck sample. In 54 full-term infants at 3 months of age, infants averaged 14.50 suck bursts (cycles/burst range = 2 – 69; amplitude range = 0.55 – 34.60 cmH2O; frequency range 0.69 – 7.81 Hz) during a 5 min sample. Breakpoint analyses revealed physiologic differences in NNS cycles/burst and amplitude throughout the 5 min of sampling NNS behavior, emphasizing the importance of collecting more than one NNS suck burst to assess suck function21. Establishing norms of NNS outcomes and standardized measurement protocols are paramount for valid and reliable NNS assessment to more accurately identify children who may have delayed or disordered oromotor behaviors. Zimmerman and DeSousa20 have used the NNS device to examine how visual stimuli affect the NNS response in a group of 15 full-term infants under 6 months of age. A repeated measures ANOVA showed a significant main effect for NNS bursts and visual stimuli (F[2, 26] = 8.975, p = 0.001), and post-hoc pairwise comparisons revealed infants increased the number of NNS bursts when visually presented with a woman's face compared to a visual stimulus of a car while exposed to maternal scent. These results highlight the saliency of social and maternal effects on feeding-related behavior.
Another line of research in which the NNS device has been utilized is examining the effects of exposures in utero, like environmental and maternal factors, on infant NNS development22,23,24,25. Prenatal exposure to certain metalloids, fine air pollution, and phthalates, as measured in urinary samples from mothers during pregnancy in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort, have been significantly associated with differences in NNS parameters22,23,24. Specifically, in near or over 200 groups of PROTECT mother-infant participants, NNS amplitude (M = 17.1 cmH2O, SD = 6.9) and burst duration (M = 6.1 s, SD = 3.6) were associated with prenatal exposure to concentrations of fine particulate matter23 and NNS amplitude (M = 16.7 cmH2O, SD = 6.59) and frequency (M = 1.92 Hz, SD = 0.25) were related with levels of gestational phthalate exposure24. Prenatal maternal stress has also been reported to have effects on NNS outcomes, as higher reported maternal stress levels were associated with longer suck bursts (Mdn = 5.29, IQR = 3.95, 95% CI = 0.01 – 0.17) and fewer suck bursts/min (Mdn = 5.00, IQR = 3.00, 95% CI = -0.13 – -0.02) in a large cohort of 237 mother-infant dyads from the Environmental influences on Child Health Outcomes (ECHO) Program25. NNS measures using the NNS device have been sensitive to detect relationships among these environmental and maternal exposures, which can inform and facilitate positive changes in environmental and public health.
Collectively, results from projects that have used the NNS device have demonstrated its effectiveness in quantifying NNS performance and reliable patterns of results that have greatly contributed to the NNS literature. In the PROTECT cohort, higher prenatal metal exposure was associated with decreased NNS amplitude and increased NNS burst duration, cycles/burst, and cycles/min in the first 2 months of life in full-term infants22,23. Additionally, longer NNS burst durations and more NNS cycles/burst and cycles/min at 3 months were associated with lower scores in cognitive development assessments at 12 months26. Thus, larger amplitudes and shorter burst cycles and durations may indicate more organized NNS behavior in the 1st year of life. This hypothesis matches the changes in NNS development previously described by Martens et al.5, which supports the notion that these NNS parameters represent organized performance and healthy development.
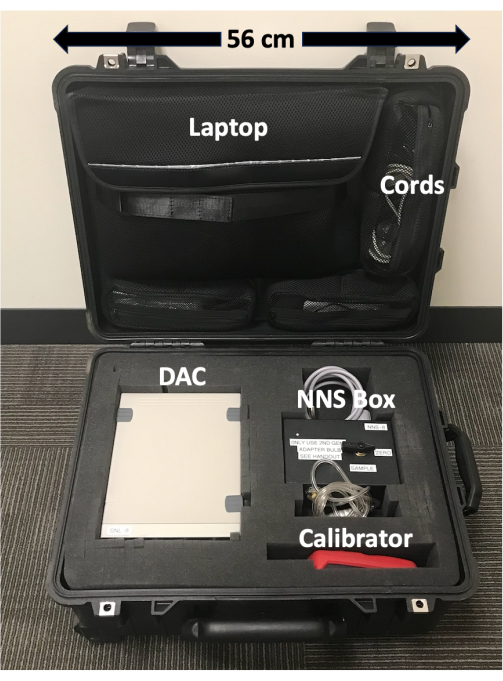
Figure 1: Portable NNS device case. Device components are labeled and scaled. The case keeps device components safe and contains wheels and an extendable handle which makes transportation of the device easy. Please click here to view a larger version of this figure.
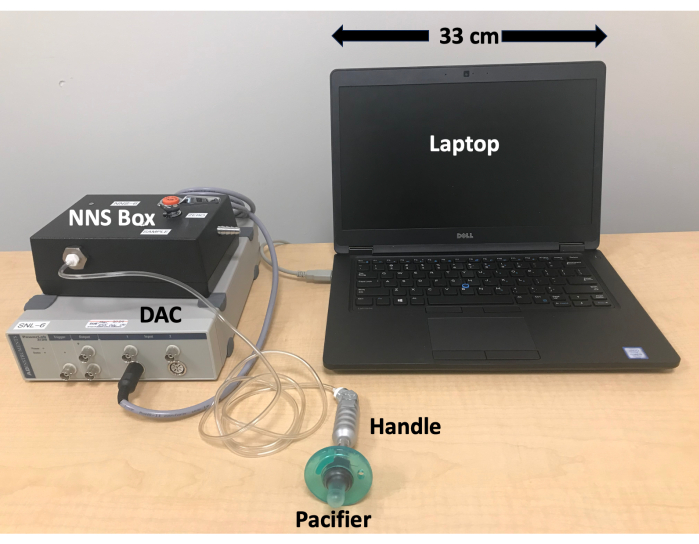
Figure 2: NNS device set-up. Device components are labeled and scaled. The NNS device does not require much space to set up and all cords and plug-ins for the system are long, which gives flexibility for a variety of different data collection rooms and researcher/caregiver positions. Please click here to view a larger version of this figure.

Figure 3: Pressure calibration set-up. (A) Pressure calibrator and pacifier receiver and handle. (B) Pressure calibrator with pacifier receiver screwed off the handle. (C) Pressure calibrator with handle attached. (D) Pressure calibrator with handle and 1 mL syringe attached. The syringe plunger needs to be fully pulled out prior to it being screwed onto the pressure calibrator. Please click here to view a larger version of this figure.
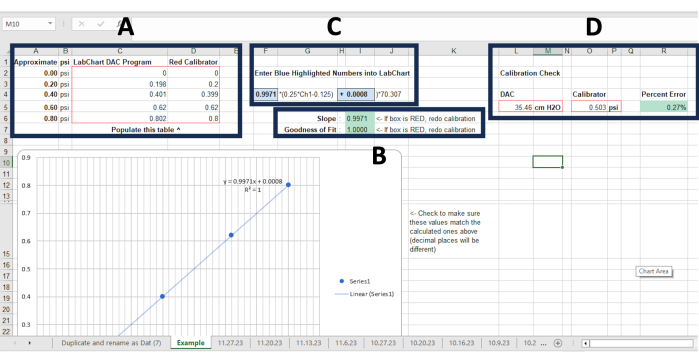
Figure 4: Suck analyzer calibration file. (A) Cells where pressure recordings are denoted as measured by the Calibration file and calibration device using the 1 mL syringe at 0.0, 0.2, 0.4, 0.6, and 0.8 psi. (B) The Slope and Goodness of fit cells will populate automatically once all pressure recordings are inputted in (A). Green cells indicate calibration was successful, red cells require the calibration process to be re-done. (C) These blue cells will also automatically populate once pressure recordings are completed. These values need to be input into the Master Settings File in the Formula text box. (D) Cells where pressure recordings are denoted measured via the Master Settings File and calibration device using the 1 mL syringe at 0.5 psi. The Percent Error cell will automatically populate once measures are plugged in. Green means calibration is successful, red requires the calibration process to be re-done starting at step 2.13. in the protocol. Please click here to view a larger version of this figure.
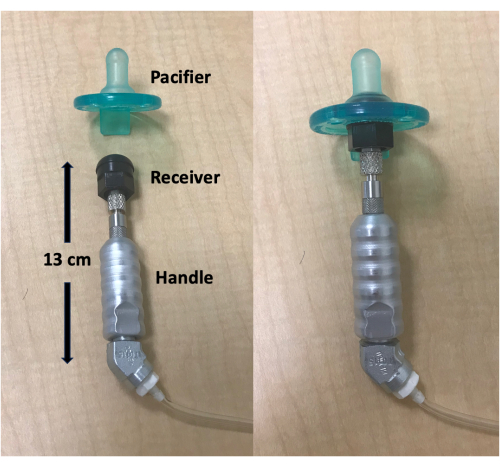
Figure 5: Pacifier, receiver, and handle. Device components are labeled and scaled. A newly opened pacifier easily attaches to the receiver. Please click here to view a larger version of this figure.

Figure 6: NNS data collection example. A researcher or caregiver can offer the pacifier to the participant and then hold the handle like a bottle during data collection. Please click here to view a larger version of this figure.
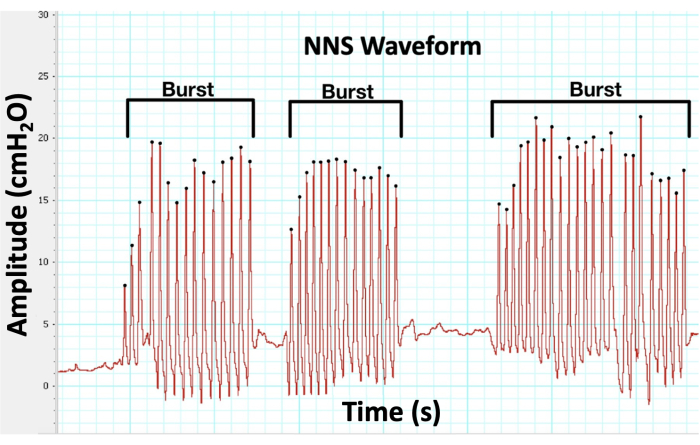
Figure 7: NNS waveform. The custom pressure transducer measures NNS compression in cmH2O over time, and the software provides live biofeedback of NNS performance and records its data for analysis. Please click here to view a larger version of this figure.
Discussion
The NNS device has several limitations that are important to acknowledge. Although NNS provides critical insight into feeding9, there is a considerable amount of extrapolation from NNS to feeding performance. Solutions to this limitation have included research teams pairing NNS results with actual feeding observations and comprehensive feeding-related questionnaires for caregivers to more fully capture how NNS relates to feeding18. In addition, an infant can have a well-patterned NNS but still have challenges with feeding due to the additional demands of coordinating the swallowing of nutrients. Furthermore, due to standardization and for equipment purposes, the NNS device has only used one specific pacifier in investigations of NNS. Sucking performance may not be fully representative of the child's NNS characteristics if babies use different pacifiers at home, as pacifiers can significantly differ in physical properties, which can affect NNS performance27,28. Another challenge with the NNS device is that it only captures the expression aspect of non-nutritive sucking and does not measure any suction parameters. The expression refers to the positive pressure an infant exerts on a nipple or pacifier by closing on it using the tongue and mandible. The suction component is when an infant lowers the tongue and mandible to enlarge the oral cavity while sealing the nasopharynx to create negative intra-oral pressure. Although suction is an important characteristic of a suck response, its accurate measurement would require participants to achieve an adequate seal around the device and the ability to create intra-oral negative pressure. These requirements would be challenging in clinical populations, particularly in infants with cleft lip and/or palate29. The NNS device is currently being employed in clinical populations that would have difficulty sealing around a nipple and creating intra-oral negative pressure, and evaluation of their expression characteristics still provides valuable information on their NNS behavior.
The NNS device has advantages in describing NNS function compared to non-instrumental options, particularly with its accuracy. Assessment of NNS in clinical settings often occurs without biomedical equipment, which makes its evaluation subjective and limited to an evaluator's experience. Significant differences have been reported in the evaluation of oropharyngeal task performance between clinical subjective ratings and biomedically recorded objective measurement30, the effect of which has been specifically observed in NNS assessment31. Wahyuni et al.31 reported significant differences between subjective scoring and a biomedical suction pressure transducer device in NNS outcomes of the number of sucks per burst, the time between bursts, and sucking pressure in a large cohort of premature babies. Common ways NNS can be clinically assessed is through observations of infants sucking on a pacifier or their hands/fingers and potentially with a clinician's gloved finger as a surrogate to a pacifier. Neiva et al.32 developed and validated an assessment for NNS performance in premature infants using a gloved finger and a comprehensive scoring system. Although clinical assessment is limited in its evaluation and accuracy, it can offer some insight into NNS behavior and is commonly employed due to the challenges of accessing, using, and interpreting objective methods to measure NNS function in clinical settings.
Besides the NNS device, there are several quantitative options to measure NNS performance. Pereira et al.33 described a system that measures NNS parameters and can provide NNS stimulation via a pneumatic pump on a pacifier with a complex system of device components, microcontrollers, and multiple software programs. Grassi et al.34 developed a device using a pacifier with an unconventional shape that can quantitatively measure NNS compression and suction pressures simultaneously. They reported preliminary results in a small group of infants in the neonatal intensive care unit (NICU). Cunha et al.35 designed a device that measures NNS pressures on a pacifier using a pressure sensor and amplifier circuit and investigated differences between NNS and nutritive sucking on their device between a small cohort of pre- and full-term infants. Nascimento et al.36 developed the S-FLEX device, which is a portable system that includes a pacifier attached to a pressure sensor that can measure the maximum and mean pressure of NNS behavior. Truong et al.37 described an NNS system comprised of a single-use pacifier, pressure sensor, data acquisition unit, and customized software that assessed intraoral vacuum measures during NNS performance in a cohort of healthy full-term infants. Ebrahimi et al.38 presented preliminary results on a broad range of NNS outcomes using a wireless and portable pacifier comprised of pressure measurement and power supply units on four infants from a NICU. Akbarzadeh et al.39 developed a sensitized non-nutritive sucking system that can evaluate expression and suction pressures with a research pacifier that has an implemented analog-to-digital converter and microcontroller, which can wirelessly transmit data. This sensitized system has been employed in studies with large sample sizes of premature infants investigating NNS performance with Apgar scores39 and full attainment of oral feeds40. Another quantitative option for NNS assessment is the NTrainer system, which includes a pneumatic amplifier attached to a silicone pacifier that can assess NNS parameters and deliver pulsated orosensory stimulation that mimics typical NNS motor patterns41. The NTrainer system has been used to characterize NNS behavior in clinical populations and as an intervention strategy for infants in the NICU to improve NNS parameters, facilitate oral feeding success, and decrease hospital stay41,42,43. Although it requires considerable resources and training to clinically implement, NICU team members and parents of infants in the NICU report positive effects of its use44.
In addition to the other quantitative methods that describe NNS behavior, the NNS device is an ideal option to assess NNS function. The device system is transportable and easy to set up, and has been used to collect data in hospital, clinical, and home settings. The NNS device is highly safe and hygienic, as the only material in contact with the infant is a common commercial pacifier that can be easily attached and removed from the pacifier handle (Figure 5). NNS data collection is simple with the device, as the easy-to-hold handle allows caregivers or clinical staff to offer the pacifier like a bottle (Figure 6) while the laptop provides real-time biofeedback of the NNS waveform (Figure 7). The customized and streamlined calibration and analysis pipelines facilitate its accessibility to other research teams17,25 and clinicians. Additionally, the NNS device measures NNS behavior with high accuracy. The calibration process of the NNS device verifies that the pressure transducer sensor is recording precision pressure measurements to a ground truth signal. This process ensures that data is valid, and the sensor is accurate.
Research and clinical implications of the NNS device are vast. Quantitative, valid, and reliable measures of NNS function have immense value in the early assessment of infants' neuromotor status. NNS is one of the first observable motor functions in utero and during infancy, and it can serve as an accessible and reliable behavior to identify children at risk for potential delays or impairment in feeding, cognitive, speech-language, and motor developmental domains9,11,12,14. Numerous studies have demonstrated the research possibilities using the NNS device, as it's been used to assess the effects of feeding-related experiences and interventions/surgical procedures17,20, characterize NNS development throughout the first year of life5, correlate sucking behavior with the development of other oromotor behaviors19, and identify environmental and maternal exposures that can negatively impact children's neurodevelopment22,23,24,25. Future directions for the NNS device include the characterization of NNS profiles in clinical populations to improve its diagnostic capabilities in identifying children at risk for disrupted development.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge the following NIH funding sources: DC016030 and DC019902. We would also like to thank the members of the Speech & Neurodevelopment Lab and the families who participated in our numerous studies.
Materials
| Case | Pelican | 1560 | |
| Data Acquisition and Analysis Software/LabChart | ADInstruments | 8.1.25 | |
| Data Acquisition Center (PowerLab 2/26) | ADInstruments | ML826 | |
| Laptop | Dell | Latitude 5480 | |
| Pressure Calibrator | Meriam Process Technologies | M101 | |
| Soothie Pacifier | Phillips Avent | SCF190/01 | |
| Syringe | CareTouch | CTSLL1 |
Referências
- Poore, M. A., Barlow, S. M. Suck predicts neuromotor integrity and developmental outcomes. Pers Speech Sci Orofacial Disorders. 19 (1), 44-51 (2009).
- Wolff, P. H. The serial organization of sucking in the young infant. Pediatrics. 42 (6), 943-956 (1968).
- Estep, E., Barlow, S. M., Vantipalli, R., Finan, D., Lee, J. Non-nutritive suck parameters in preterm infants with RDS. J Neonatal Nur. 14 (1), 28-34 (2008).
- Lau, C., Alagugurusamy, R., Schanler, R. J., Smith, E. O., Shulman, R. J. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 89 (7), 846-852 (2000).
- Martens, A., Hines, M., Zimmerman, E. Changes in non-nutritive suck between 3 and 12 months. Early Human Dev. 149, 105141 (2020).
- Barlow, S. M., Estep, M. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J Comm Disorders. 39 (5), 366-380 (2006).
- Poore, M., Zimmerman, E., Barlow, S. M., Wang, J., Gu, F. Patterned orocutaneous therapy improves sucking and oral feeding in preterm infants. Acta Paediatr. 97 (7), 920-927 (2008).
- Zimmerman, E., Foran, M. Patterned auditory stimulation and suck dynamics in full-term infants. Acta Paediatr. 106 (5), 727-732 (2017).
- Bingham, P. M., Ashikaga, T., Abbasi, S. Prospective study of non-nutritive sucking and feeding skills in premature infants. Arch Dis Childhood. 95 (3), F194-F200 (2010).
- Pineda, R., Dewey, K., Jacobsen, A., Smith, J. Non-nutritive sucking in the preterm infant. Am J of Perinatol. 36 (3), 268-277 (2019).
- Malas, K., Trudeau, N., Chagnon, M., McFarland, D. H. Feeding-swallowing difficulties in children later diagnosed with language impairment. Dev Med Child Neurol. 57 (9), 872-879 (2015).
- Mizuno, K., Ueda, A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 47 (5), 299-304 (2005).
- Wolthuis-Stigter, M. I., et al. Sucking behaviour in infants born preterm and developmental outcomes at primary school age. Dev Med Child Neurol. 59 (8), 871-877 (2017).
- Adams-Chapman, I., Bann, C. M., Vaucher, Y. E., Stoll, B. J. Association between feeding difficulties and language delay in preterm infants using Bayley scales of infant development – Third edition. J Pediatr. 163 (3), 680-685 (2013).
- Palmer, M. M., Crawley, K., Blanco, I. A. Neonatal oral-motor assessment scale: A reliability study. J Perinatol. 13 (1), 28-35 (1993).
- Wolthuis-Stigter, M. I., et al. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J Pediatr. 166 (1), 26-30 (2015).
- Hill, R. R., Hines, M., Martens, A., Pados, B. F., Zimmerman, E. A pilot study of non-nutritive suck measures immediately pre- and post-frenotomy in full term infants with problematic feeding. J Neonatal Nurs. 28 (6), 413-419 (2022).
- Hines, M., Hardy, N., Martens, A., Zimmerman, E. Birth order effects on breastfeeding self-efficacy, parent report of problematic feeding and infant feeding abilities. J Neonatal Nurs. 28 (1), 16-20 (2022).
- Murray, E. H., Lewis, J., Zimmerman, E. Non-nutritive suck and voice onset time: Examining infant oromotor coordination. PLoS One. 16 (4), 30250529 (2021).
- Zimmerman, E., DeSousa, C. Social visual stimuli increase infants suck response: A preliminary study. PLoS One. 13 (11), e0207230 (2018).
- Zimmerman, E., Carpenito, T., Martens, A. Changes in infant non-nutritive sucking throughout a suck sample at 3-months of age. PLoS One. 15 (7), e0235741 (2020).
- Kim, C., et al. Associations between biomarkers of prenatal metals exposure and non-nutritive suck among infants from the PROTECT birth cohort in Puerto Rico. Front Epidemiol. 2, 1057515 (2022).
- Morton, S., et al. Non-nutritive suck and airborne metal exposures among Puerto Rican infants. Sci Total Environ. 789, 148008 (2021).
- Zimmerman, E., et al. Associations of gestational phthalate exposure and non-nutritive suck among infants from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) birth cohort study. Environ Int. 152, 106480 (2021).
- Zimmerman, E., et al. Examining the association between prenatal maternal stress and infant non-nutritive suck. Pediatr Res. 93, 1285-1293 (2023).
- Martens, A., Phillips, H., Hines, M., Zimmerman, E. An examination of the association between infant non-nutritive suck and developmental outcomes at 12 months. PLoS One. 19 (2), e0298016 (2024).
- Zimmerman, E., Barlow, S. M. Pacifier stiffness alters the dynamics of the suck central pattern generator. J Neonatal Nurs. 14 (3), 79-86 (2008).
- Zimmerman, E., Forlano, J., Gouldstone, A. Not all pacifiers are created equal: A mechanical examination of pacifiers and their influence on suck patterning. Am J Speech-Lang Pathol. 26 (4), 1202-1212 (2017).
- Choi, B. H., Kleinheinz, J., Joos, U., Komposch, G. Sucking efficiency of early orthopaedic plate and teats in infants with cleft lip and palate. Int J Oral Maxillofacial Surg. 20 (3), 167-169 (1991).
- Clark, H. M., Henson, P. A., Barber, W. D., Stierwalt, J. A. G., Sherrill, M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. Am J Speech-Lang Pathol. 12 (1), 40-50 (2003).
- Wahyuni, L. K., et al. A comparison of objective and subjective measurements of non-nutritive sucking in preterm infants. Paediatr Indonesia. 62 (4), 274-281 (2022).
- Neiva, F. C. B., Leone, C., Leon, C. R. Non-nutritive sucking scoring system for preterm newborns. Acta Paediatr. 97 (10), 1370-1375 (2008).
- Pereira, M., Postolache, O., Girão, P. A smart measurement and stimulation system to analyze and promote non-nutritive sucking of premature babies. Measurement Sci Rev. 11 (6), 173-180 (2011).
- Grassi, A., et al. Sensorized pacifier to evaluate non-nutritive sucking in newborns. Med Eng Phys. 38 (4), 398-402 (2016).
- Cunha, M., et al. A promising and low-cost prototype to evaluate the motor pattern of nutritive and non-nutritive suction in newborns. J Pediatr Neonatal Individualized Med. 8 (2), 1-11 (2019).
- Nascimento, M. D., et al. Reliability of the S-FLEX device to measure non-nutritive sucking pressure in newborns. Audiol Comm Res. 24, e2191 (2019).
- Truong, P., et al. Non-nutritive suckling system for real-time characterization of intraoral vacuum profile in full term neonates. IEEE J Translat Eng Health Med. 11, 107-115 (2023).
- Ebrahimi, Z., Moradi, H., Ashtiani, S. J. A compact pediatric portable pacifier to assess non-nutritive sucking of premature infants. IEEE Sensors J. 20 (2), 1028-1034 (2020).
- Akbarzadeh, S., et al. Evaluation of Apgar scores and non-nutritive sucking skills in infants using a novel sensitized non-nutritive sucking system. 42nd Ann Int Conf IEEE Eng Med Biol Soc. , 4282-4285 (2020).
- Akbarzadeh, S., et al. Predicting feeding conditions of premature infants through non-nutritive sucking skills using a sensitized pacifier. IEEE Trans Biomed Eng. 69 (7), 2370-2378 (2022).
- Barlow, S. M., Finan, D. S., Lee, J., Chu, S. Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. J Perinatol. 28, 541-548 (2008).
- Barlow, S. M., et al. Frequency-modulated orocutaneous stimulation promotes non-nutritive suck development in preterm infants with respiratory distress syndrome or chronic lung disease. J Perinatol. 34, 136-142 (2014).
- Song, D., et al. Patterned frequency-modulated oral stimulation in preterm infants: A multicenter randomized controlled trial. PLoS One. 14 (2), e0212675 (2019).
- Soos, A., Hamman, A. Implementation of the NTrainer system into clinical practice targeting neurodevelopment of pre-oral skills and parental involvement. Newborn Infant Nurs Rev. 15 (2), 46-48 (2015).

