Free-Hand Intracerebroventricular Injections in Mice
Summary
Here, a simple and rapid approach for performing intracerebroventricular injections in mice using a free-hand approach (that is, without a stereotaxic device) is described.
Abstract
The investigation of neuroendocrine systems often requires the delivery of drugs, viruses, or other experimental agents directly into the brains of mice. An intracerebroventricular (ICV) injection allows the widespread delivery of the experimental agent throughout the brain (particularly in the structures near the ventricles). Here, methods for making free-hand ICV injections in adult mice are described. By using visual and tactile landmarks on the heads of mice, injections into the lateral ventricles can be made rapidly and reliably. The injections are made with a glass syringe held in the experimenter's hand and placed at approximate distances from the landmarks. Thus, this technique does not require a stereotaxic frame. Furthermore, this technique requires only brief isoflurane anesthesia, which permits the subsequent assessment of mouse behavior and/or physiology in awake, freely behaving mice. Free-hand ICV injection is a powerful tool for the efficient delivery of experimental agents into the brains of living mice and can be combined with other techniques such as frequent blood sampling, neural circuit manipulation, or in vivo recording to investigate neuroendocrine processes.
Introduction
The delivery of experimental agents, such as drugs1, viruses2, or cells3, to the brain is often necessary for neuroendocrine research. If the agent does not readily cross the blood-brain barrier or the experimental objective is to specifically test the central effects of the agent, it is important to have a reliable method for delivering injections into the brain. Moreover, injection into the intracerebroventricular (ICV) space provides the opportunity to distribute the agent widely in the brain and provides a large target area, thus increasing the likelihood of successful injection2.
A common method for making ICV injections involves the placement of a permanent indwelling cannula. In this approach, a stereotaxic frame is necessary to position the commercially available or custom-made cannula, as the cannula is glued or cemented in place. Often, upon recovery, a supraphysiological dose of angiotensin II is administered through the cannula, and if drinking behavior is immediately observed, then the cannula is considered correctly placed4. This approach has many advantages, including the ability to perform long-term infusion and the ability to inject the same animal multiple times; additionally, if angiotensin II is employed, correct placement can be confirmed prior to the administration of experimental compounds. However, there are some limitations to placing a permanent cannula, including the requirement for expensive equipment (stereotaxic frame), the possibility of damage to the cannula after placement (e.g., mice can chew on the cannula of a cage mate), and the possibility of infections around the permanent cannula. Single ICV injections can be made with the use of a stereotaxic frame3, which, although effective, requires substantial exposure to anesthesia and, thus, may obscure some acute physiological and behavioral effects of the treatment. Additionally, the placement of mice in a stereotaxic frame requires substantial training to achieve stable placement and prevent the rupturing of the ear canals.
Here, an established method for making free-hand injections in mice is described. This method is based on previous reports5,6. The advantages of this technique are that it is simple, rapid, and does not require specialized equipment such as a stereotaxic frame. As described below, this procedure involves manipulating a glass syringe relative to landmarks on the mouse head to make the injections, which can be done rapidly and, thus, requires only a few minutes of gas anesthesia on the experimental day.
Protocol
All the procedures were approved by the Colorado State University (#3960) and University of California San Diego Institutional Animal Care and Use Committees, where the representative data were collected (S13235, PI Kellie Breen Church). Data from five adult female and two adult male C57/BL6 mice (9-16 weeks old) are depicted in the representative data section. Female mice were ovariectomized 3-4 weeks prior to ICV injection and blood collection as described previously7. Prior to experimentation, these mice were housed with a 12 h light/12 h dark light cycle and had free access to feed and water in accordance with the Guide for the Care and Use of Laboratory Animals.
1. Performing craniotomy
NOTE: Craniotomy can be performed one or more days prior to the actual injection, which makes the injection process quicker on the day of the experiment.
- Prepare the materials for the craniotomy as described below.
- Place a clean bench pad or drape material on the work surface. Tape the nose cone of an isoflurane vaporizer to the work surface (close to, but facing away from, the experimenter; see Figure 1A).
- Place silastic tubing on the end of a sterile sharp 18 G needle so that ~1 mm of the needle tip protrudes. Place silastic tubing on the end of a sterile blunt needle so that ~1 mm of the needle tip protrudes.
NOTE: Separate and sterile pre-packaged needles (sharp and blunt) should be used for each mouse. - Gather an electric shaver, iodine scrub, sterile gauze, and alcohol pads for preparing the injection site.
- Practice moving a needle 2 mm lateral and 1 mm caudal as described below.
- Use a ruler and a pen to mark a sheet of paper with a starting point (this will be bregma), a mark 2 mm to either the left or right, and another mark 1 mm above the last one (this will be the injection site; see Figure 1B).
- Practice moving the needle from the starting point to mark point 1 to mark point 2 (bregma to injection site) until confident that the movement is repeatable without guides.
NOTE: The coordinates described here are effective in adult C57/BL6 mice, but other strains or age groups may require adjustment.
- Prepare the mouse for craniotomy as described below.
- Place the mouse in an induction chamber and anesthetize the mouse with 3%-4% isoflurane in medical-grade oxygen. With practice and familiarity with the procedure, reduce the total duration of isoflurane exposure to less than 10 min for the craniotomy procedure.
CAUTION: Isoflurane exposure is hazardous to human health; use an approved and inspected isoflurane vaporizer in a well-ventilated area with means of scavenging waste gases. - Once the toe reflex is absent, shave the head of the animal. Apply eye lubricant.
- Place the mouse on the work surface with the head in the nose cone to maintain anesthesia.
- Administer an analgesic agent, such as buprenorphine (0.6-0.8 mg/kg mouse body weight, subcutaneously), as directed by the supplier.
- Clean the injection site by wiping the head with sterile gauze dipped in an iodine solution (perform three scrubs), and then wipe with an alcohol scrub pad (perform three times).
NOTE: Remove the fur and clean the skin around the injection site and use sterile instruments and needles, as recommended by the American Veterinary Medical Association to prevent infections.
- Place the mouse in an induction chamber and anesthetize the mouse with 3%-4% isoflurane in medical-grade oxygen. With practice and familiarity with the procedure, reduce the total duration of isoflurane exposure to less than 10 min for the craniotomy procedure.
- Firmly hold the head of the mouse with the non-dominant hand. Position the head as flat on the work surface as possible.
- Locate the site of the injection.
- Use the prepared sharp 18 G needle to first identify bregma; for this, drag the needle across the skin of the head along the midline, moving in the rostral-caudal plane. Imagine an equilateral triangle in which the two vertices are the eyes and the third vertex is the approximate location of bregma (see Figure 1C).
- From bregma, move the needle 2 mm lateral and 1 mm caudal to the site of injection.
- While holding the needle vertically, firmly push the needle through the skin and bone until the tubing is flush with the skin.
- Retract the needle, rotate the needle, and press through the skin and bone again. Repeat the process until a small hole is created in the bone.
- Use the blunt needle to check that a sufficient hole has been produced in the bone. Aim to make an opening in the bone large enough for the blunt needle to pass through.
- Remove the mouse from the nose cone, clean off any blood from the injection site with sterile gauze, and place the mouse in a cage on a cage warmer until awake. Since the opening made by the craniotomy is small (18 G needle), the site does not need to be closed with a suture or wound clips.
NOTE: The opening produced here is small (18 G), so many institutions consider this procedure an injection as opposed to a surgery. Moreover, although an opening through skin and bone to the brain is made, holding the skin taut results in the holes not aligning, which likely aids in preventing skin flora or cage bedding from contacting the brain.
2. Making the injection
- Prepare the materials for the injection as described below.
- Place a clean bench pad or drape material on the work surface. Tape the nose cone of an isoflurane vaporizer to the work surface (close to, but facing away from, the experimenter; see Figure 1A).
- Attach a 10 mm long 27 G needle with a 45° bevel onto a 5 µL glass syringe. Place silastic tubing on the needle so that 3.5 mm of the needle tip protrudes; use laboratory tape to hold the tubing to the syringe body (see Figure 1D).
- Prepare the injection media (drug, virus, or other liquid for injection) in a tube. For the representative results in this study, sterile isotonic saline was injected.
NOTE: Select a tube that permits access by the glass syringe and needle, such as a 2 mL microcentrifuge tube. - Draw 3 µL of injection media into the glass syringe. First, draw more than the desired volume, and eject until 3 µL remains in the syringe.
- Gather iodine scrub, sterile gauze, and alcohol pads for preparing the injection site.
- Start a laboratory timer in count-up mode and place the timer so that it is visible to the experimenter while making injections.
- Practice moving a needle 2 mm lateral and 1 mm caudal as performed on the previous day.
- Prepare the mouse for injection as described below.
- Place the mouse in the induction chamber and anesthetize the mouse with isoflurane. Once the toe reflex is absent, apply eye lubricant, and place the mouse on the work surface with the head in the nose cone.
- Clean the injection site with three wipes each of iodine and alcohol. Identify the injection site with an 18 G needle (or blunted needle) as described in step 1.8. The hole created during craniotomy should be detectable.
NOTE: If the craniotomy has not been performed in advance, or if the hole in the skull is not detectable, the craniotomy can be performed here.
- Perform the injection with a glass syringe as described below.
- Firmly hold the head of the mouse with the non-dominant hand. Position the head as flat on the work surface as possible.
- Place the needle of the glass syringe through the hole in the skull until the tubing placed over the needle prepared in step 2.1.2 rests on the skin of the mouse.
- Hold the syringe as vertically as possible, paying attention to both the coronal and sagittal planes. Slowly inject the media over a period of 1 min.
- Hold the syringe and needle in place for another minute after the completion of the injection to minimize backflow. Slowly retract the needle from the head of the mouse.
- Remove the mouse from the nose cone, clean off any blood from the injection site with sterile gauze (if there is any present), and place the mouse in a cage on a cage warmer until awake.
3. Confirming the injection location
- Deeply anesthetize and perfuse the mouse with saline and 4% paraformaldehyde in phosphate-buffered saline (PBS) as previously described8. Store the brain in 30% sucrose in PBS at 4 °C until the brain sinks (typically 2 days).
- Cut 20-50 µm coronal sections on a cryostat as described previously9. While sectioning, observe the injection tract in the tissue block. Record whether the injection tract clearly intersects the lateral ventricle.
Representative Results
When performed successfully, this technique allows the rapid delivery of an experimental agent into the ventricular system. A luteinizing hormone (LH) pulse profile from an ovariectomized mouse that received an ICV injection of 3 µL of sterile isotonic saline, the vehicle for many pharmacological compounds, is shown in Figure 2A. This example demonstrates that brief exposure to gas anesthesia and the injection of 3 µL of fluid into the ventricular system alone did not alter the pulsatile LH secretion. At 3 h after the ICV injection, the animal was euthanized, and fresh-frozen neural tissue was collected and cut on a cryostat; the injection tract clearly intersected with the lateral ventricle. The injection tract was also visible in neural tissue from mice perfused with fixative (10 mL of heparinized saline followed by 20 mL of 4% paraformaldehyde in phosphate buffer). Examples of correctly placed injections are shown in Figure 2B. Two examples of incorrectly placed injections are shown in Figure 2C. Examples of correctly (left) and incorrectly placed injections of India ink are shown in Figure 2D. The injection of India ink is useful for practicing this technique as it allows for the clear visualization of the injection location. The flow of dye into both the lateral ventricles and the third ventricle can be seen in correctly placed injections (Figure 2D, left).
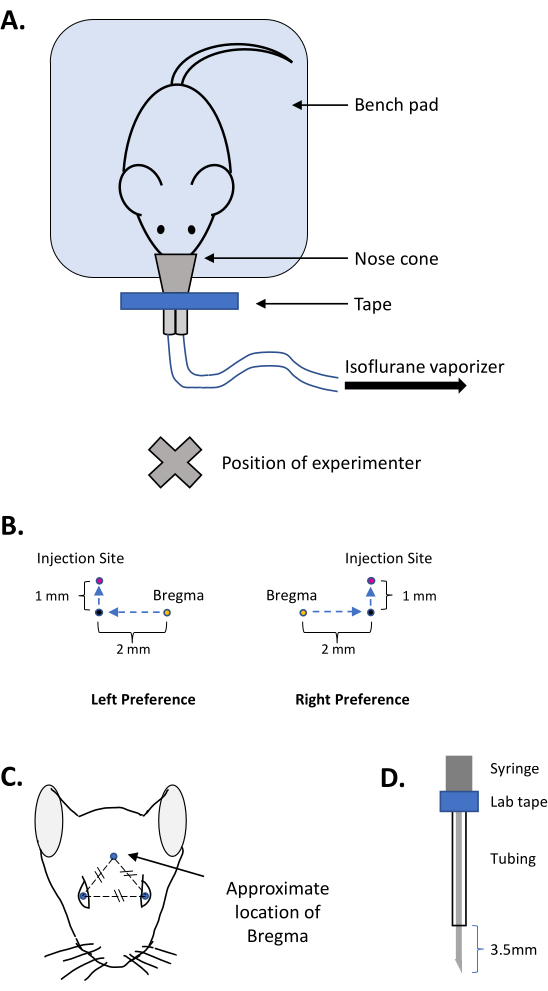
Figure 1: Images depicting the preparation of the equipment and the injection coordinates for free-hand ICV injections. (A) Configuration of the workstation for free-hand ICV injections. (B) Schema for practicing the movement necessary to place the ICV injection in 3-4 month-old female C57/BL6 mice. (C) Approximate location of bregma in adult mice. (D) Placement of the tubing to reveal 3.5 mm of the tip of the needle for the injection. Please click here to view a larger version of this figure.
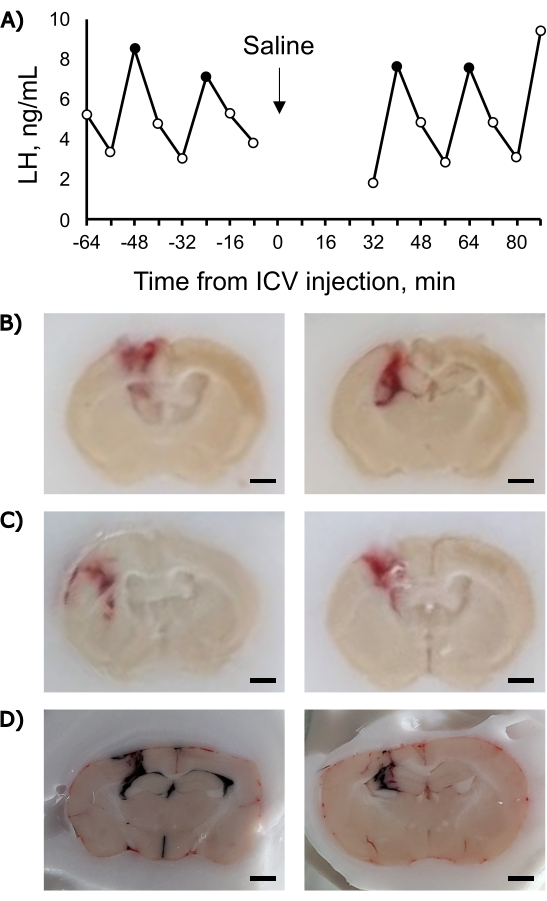
Figure 2: Representative luteinizing hormone and histology data related to free-hand ICV injections. (A) LH pulse profile before and after the ICV injection. The data points with solid black fill represent pulses as determined using a reformulation of PULSAR10 with the parameters suggested in that reference for an OVX mouse and a level of detection of 0.32 ng/mL. (B) Photographs of a coronal section of mouse brain with a correctly placed ICV injection. (C) Photographs of a coronal section of mouse brain with an incorrectly placed ICV injection: an injection tract lateral to the ventricle (left), and an injection tract dorsal to the ventricle (right). (D) Photographs of a coronal section of mouse brain with a correctly placed ICV injection of India ink (left) and an incorrectly placed ICV injection of India ink (right). Note: panels B and C depict paraformaldehyde-perfused brains collected 3 h after injection of saline; panel D depicts fresh brains collected on dry ice 2 min after the injection of India ink. Scale bar = 1 mm Please click here to view a larger version of this figure.
Discussion
Here, a simple and effective means for making ICV injections in mice is described. Since this technique does not require a stereotaxic frame, this approach for the central delivery of drugs and experimental agents is accessible to more researchers. Additionally, this approach is relatively high throughput since the preparation and injection procedure can be performed quickly.
Since this procedure requires the manipulation of needles and a glass syringe by hand using approximate distances, it is advised that the new practitioner dedicate some time to practicing the movements before working with live animals. Additionally, mouse cadavers can be useful for practicing identifying bregma with the needle. Once some confidence in the movement is gained, experience with dedicated live practice animals is helpful. During the practice sessions, the injection of 3 µL of India ink makes the visual assessment of the injection placement easier. Correctly placed injections will result in black staining in the ipsilateral lateral ventricle and often in the third ventricle and contralateral lateral ventricle. Injection of ink is a terminal procedure and mice should not be allowed to recover from anesthesia following ink injection. Even experienced practitioners can benefit from rehearsing the 2 mm lateral and 1 mm caudal movement a few times before making an injection. Additionally, it is important to hold the glass syringe vertical when placed into the brain. With practice, >75% of injections can be expected to be correctly placed. Holding the needle in place for ~1 min after the full volume has been injected can help prevent the backflow of the liquid out of the brain. During this time, it is essential to hold the needle in the correct position without movement. The successful performance of this procedure requires experience in reliably manipulating and holding the needle and glass syringe.
If some injections are not placed correctly, there are several troubleshooting options. First, check that the injection needle is straight (not bent) and that the needle is tightly fixed to the glass syringe. Next, if the missed injections are variable in their location, additional practice manipulating and holding the glass syringe is warranted. Having an additional person to spot whether the syringe is being held vertically during the injection can also be helpful. Finding bregma over intact skin with the needle also requires practice. Taking time to confidently identify this landmark before proceeding with the injection is highly recommended. If injections are consistently placed in an incorrect location, then altering the injection coordinates will be necessary. The coordinates described here (±2 mm lateral, 1 mm caudal to bregma) are effective in adult C57/BL6 mice, but these coordinates may have to be adjusted for other strains or age groups.
Though this technique can be highly effective in rapidly delivering agents centrally, there are some limitations. Since the plunger of the syringe is moved by hand, the rate of injection can be variable. It is advised to inject it relatively slowly to avoid potential off-target effects of pressure in the ventricular system. An injection volume of 3 µL is recommended in this protocol; however, a larger injection volume (5 µL) has been utilized in other laboratories11. Depending on the outcome measured, the injection volume and rate may need to be adjusted. For example, juvenile mice may only tolerate smaller injection volumes. Thus, preliminary experiments to test for vehicle effects are recommended. An additional limitation is that the confirmation of correct injection placement is limited to a somewhat subjective assessment of the injection tract after the tissue has been collected. The injection tract is visible in both fresh-frozen and paraformaldehyde-perfused tissue collected several hours after the ICV injection (see images in representative results). If multiple injections are made, it may not be clear from the tract marks if each injection was successfully placed.
This free-hand approach to making ICV injections requires anesthesia. With practice, the injections can be made rapidly, resulting in brief (3-5 min) exposure to the anesthetic. By shaving the head of the mouse and performing the craniotomy (piercing the skull with a sharp needle) prior to the experimental day, the time under anesthesia during the experiment can be reduced. Mice typically fully recover from this brief exposure to anesthesia within a few minutes. Collecting tail-tip blood samples immediately after anesthesia can be challenging, so sampling can be suspended for ~30 min after the injection to minimize stress in the mice, though this is not strictly necessary. Other laboratories have also successfully measured changes in LH secretion (one-off samples) 30 min following the administration of pharmacological agents using this free-hand ICV technique1,12. Changes in locomotor and ingestive behavior over a 24 h period have also been detected following ICV injections13, so long-term monitoring can be performed. Performing preliminary tests to determine the ease of sampling and the potential effects of anesthesia on outcomes of interest is recommended.
In summary, the free-hand ICV injection technique described here is a simple yet powerful method of delivering experimental agents into the brain. The specific advantages are that the approach is quick and technically simple and enables a high rate of successfully placed injections. Moreover, since this technique requires only brief exposure to anesthesia, it can be combined with a variety of other techniques such as frequent blood sampling, neural circuit manipulation, or in vivo recording to investigate neuroendocrine processes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Kellie Breen Church, Mr. Michael Kreisman, and Ms. Jessica Jang for their contributions to collecting the data shown in the representative results. This work was supported by the National Institutes of Health (NIH) R00 HD104994 (R.B.M.).
Materials
| 18-gauge blunt needles | SAI Infusion | B18-150 | |
| 18-gauge needles | BD Medical | 305195 | |
| Alcohol pads | Fisher Scientific | 22-363-750 | |
| Bench pad | Fisher Scientific | 14-206-62AC22 | |
| Betadine solution | Fisher Scientific | NC1696484 | |
| Buprenorphine | Patterson Vet Supply | 07-892-5235 | Controlled substance |
| Eyelube | Fisher Scientific | 50-218-8442 | |
| Glass syringe | Hamilton | 7634-01 | |
| Injection needle | Hamilton | 7803-01 | 27 gauge, Small Hub RN needle, point style: 4, Needle length: 10cm, Angle: 45 |
| Isoflurane | Patterson Vet Supply | 07-893-8441 | |
| Isoflurane vaporizer | Vet Equip | V-10 | |
| Laboratory Tape | VWR | 89098-128 | |
| Medical grade oxygen | Airgas | OX USPEA | |
| Paraformaldehyde | Millipore-Sigma | 8.18715.1000 | |
| Phosphate Buffered Saline | Fisher Scientific | J67802.K2 | |
| PulsaR Software | Open source, University of Otago | See ref 9 | |
| Ruler | Fisher Scientific | 12-00-152 | |
| Silastic tubing (0.040" I.D.) | DOW | 508-005 | |
| Silastic tubing (0.078" I.D.) | DOW | 508-009 | |
| Sterile saline | VWR | 101320-574 | |
| Sucrose | Fisher Scientific | S5-500 |
References
- Roseweir, A. K., et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. Journal of Neuroscience. 29 (12), 3920-3929 (2009).
- Kim, J. Y., Grunke, S. D., Levites, Y., Golde, T. E., Jankowsky, J. L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. Journal of Visualized Experiments. (91), e51863 (2014).
- Taylor, Z. V., Khand, B., Porgador, A., Monsonego, A., Eremenko, E. An optimized intracerebroventricular injection of CD4(+) T cells into mice. STAR Protocols. 2 (3), 100725 (2021).
- Russo, K. A., et al. Circadian control of the female reproductive axis through gated responsiveness of the RFRP-3 system to VIP signaling. Endocrinology. 156 (7), 2608-2618 (2015).
- Laursen, S. E., Belknap, J. K. Intracerebroventricular injections in mice. Some methodological refinements. Journal of Pharmacological Methods. 16 (4), 355-357 (1986).
- Haley, T. J., McCormick, W. G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. British Journal of Pharmacology and Chemotherapy. 12 (1), 12-15 (1957).
- McCosh, R. B., et al. Insulin-induced hypoglycaemia suppresses pulsatile luteinising hormone secretion and arcuate Kiss1 cell activation in female mice. Journal of Neuroendocrinology. 31 (12), e12813 (2019).
- Wu, J., et al. Transcardiac perfusion of the mouse for brain tissue dissection and fixation. Bio-Protocol. 11 (5), e3988 (2021).
- Comba, A., et al. Laser capture microdissection of glioma subregions for spatial and molecular characterization of intratumoral heterogeneity, oncostreams, and invasion. Journal of Visual Experiments. (158), e60939 (2020).
- Porteous, R., et al. Reformulation of PULSAR for analysis of pulsatile LH secretion and a revised model of estrogen-negative feedback in mice. Endocrinology. 162 (11), (2021).
- Hohmann, J. G., et al. Differential role of melanocortins in mediating leptin’s central effects on feeding and reproduction. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 278 (1), R50-R59 (2000).
- Gottsch, M. L., et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 145 (9), 4073-4077 (2004).
- Krasnow, S. M., et al. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology. 144 (3), 813-822 (2003).

