Modeling Cancerous Neural Invasion In Vitro: A Method to Co-culture Dorsal Root Ganglia and Cancer Cells
Published: April 30, 2023
Abstract
Source: Na'ara, S. et al. In Vitro Modeling of Cancerous Neural Invasion: The Dorsal Root Ganglion Model. J. Vis. Exp. (2016)
In this video, we demonstrate an in vitro method for co-culturing DRG and cancer cells to study neurotropism and neural invasion.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Isolating the Dorsal Root Ganglia (DRG)
- Use a stereomicroscope with 4X magnification.
- Position the spine on a non-adherent, non-absorbing platform (Telfa/nylon). Position the spine face up at the same craniocaudal orientation.
- Remove any spinal muscles and connective tissue. Use the ribs as a landmark for nerves as they leave the spine. Ribs also protect the nerves from surgical tools. The DRG are located at the cervical, thoracic, and lumbar sites of the vertebrae.
- Next, using scissors, cut the vertebral body in the midline and expose the spinal cord and DRG roots by gentle lateral retraction of the vertebral body.
- In the intercostal space, follow the peripheral nerve medially along the rib lateral to the DRG.
- Identify the DRG. It looks like the yolk of a 'fried egg' lying on the nerve.
- Cut the intercostal nerve distal to the DRG, leaving 2–3 mm of nerve distal to the ganglia to be used for retraction. This "tail" will be removed after harvesting.
- Grasp the nerve using forceps. Pinching the DRG body itself will cause neural cell damage and should be avoided.
- Approach the DRG proximally along its attachment to the spinal cord via its anterior and posterior roots.
- Apply gentle retraction to the DRG by pulling the efferent nerve (intercostal nerve stump) laterally and cut the anterior and posterior roots close to the DRG.
- Keep the DRG in a 35 mm Petri dish filled with fresh, ice-cold DMEM supplemented with 10% fetal calf serum (FCS), 1% penicillin-streptomycin, 1% non-essential amino acids, and 1% sodium pyruvate.
2. Implantation in the Petri Dish
NOTE: Perform the next steps on ice using pre-cooled pipette tips to prevent ECM solidification.
- Under a stereomicroscope at 2–4X magnification, place a 35 mm glass-bottom Petri dish on a paper grid.
IMPORTANT: Work on ice in order to keep the ECM in liquid state. Create a 1 mm diameter spot (approximately 20 µL) of growth factor-depleted ECM at the center of the grid. - Under direct visualization, place the DRG at the center of the ECM spot close to the bottom of the dish.
NOTE: The cancer cells used in this protocol are murine pancreatic cancer cells (KPC) established from freshly isolated tumor specimens from KPC mice, as described elsewhere. - Harvest 40,000 cancer cells from confluent cultures, wash them once with PBS, and resuspend them in 40 µL ECM on ice.
- Under direct visualization of the grid using a stereomicroscope, measure 500 µm at each direction from the DRG. At this point, slowly seed 10,000 cells/10 µL ECM. Use a 2–10 µL pre-cooled tip to inject the cells close to the dish bottom, avoiding their spread in the matrix or detachment of the matrix. (Figure 1).
- Leave the dish within a laminar flow hood for 10–15 min to solidify. Avoid ECM drying.
- Slowly add DMEM, prepared as previously mentioned, against the sidewall of the plate. Add enough medium to cover the ECM (about 2 mL).
- Put the dish back in the incubator at 37 °C.
- Replace the medium with a fresh medium on the following day.
- Replace the medium every two days.
Representative Results
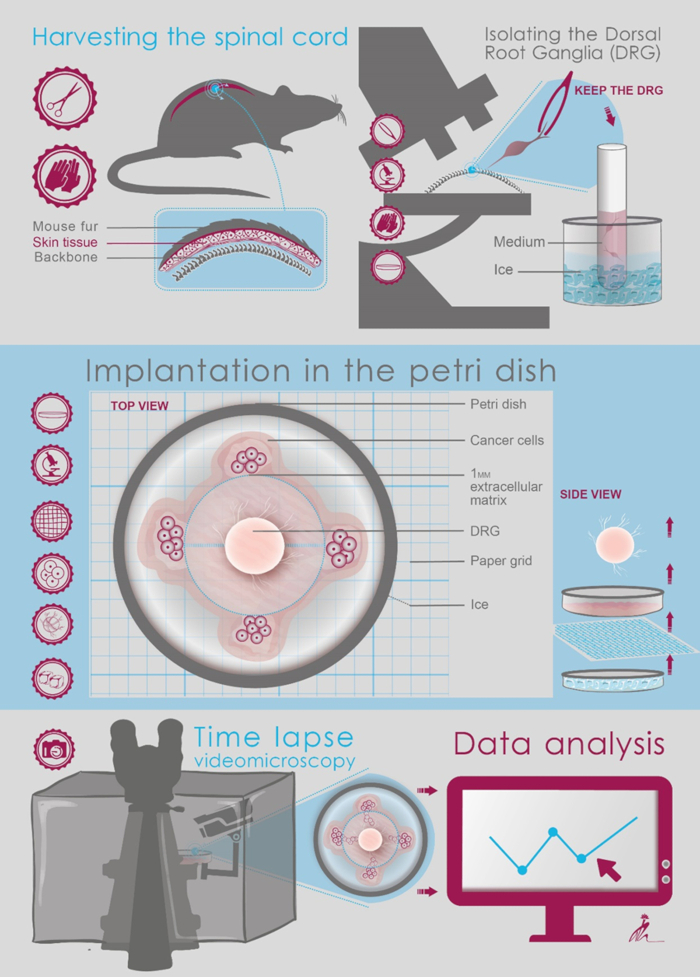
Figure 1: Schematic illustration of the protocol steps.
Declarações
The authors have nothing to disclose.
Materials
| Operating microscope | Leica | M205 | |
| Forceps | Sigma-Aldrich | F4142 | |
| Surgical blade | Sigma-Aldrich | Z309036 | |
| Scissors | Sigma-Aldrich | S3271 | |
| 35 mm Petri dishes glass bottom | de groot | 60-627860 | |
| 70% ethanol | Sigma | ||
| Cold PBS | Biological industries | 02-023-1A | |
| DMEM | Biological industries | 01-055-1A | |
| FCS | Rhenium | 10108165 | |
| Penicillin and streptomycin | Biological industries | 01-031-1B | |
| Sodium Pyruvate | Biological industries | 03-042-1B | |
| L-Glutamine | Biological industries | 03-020-1B | |
| Growth factor-depleted matrigel | Trevigen | 3433-005-01 |
Tags
Citar este artigo
Modeling Cancerous Neural Invasion In Vitro: A Method to Co-culture Dorsal Root Ganglia and Cancer Cells. J. Vis. Exp. (Pending Publication), e20490, doi: (2023).

