Patient-derived Tumor Xenografting: A Technique to Generate Experimental Mouse Model for Evaluating Urothelial Cell Carcinoma
Abstract
Source: Moret, R. et al. Patient-derived Orthotopic Xenograft Models for Human Urothelial Cell Carcinoma and Colorectal Cancer Tumor Growth and Spontaneous Metastasis. J. Vis. Exp. (2019)
This video describes the procedure to generate an experimental xenograft murine model, which can be used for studying urothelial cell carcinoma progression.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. UCC Mouse Model
- Preparation of mice for procedure
- Obtain 6- to 8-week old female NOD/SCID mouse. Shave the lower backs of the mouse using hair removal cream. Anesthetize the mouse in an induction chamber with isoflurane (2.5% in 100% oxygen, 1 L/min).
- Once sedated, place the mouse in the supine position with its snout in an isoflurane nose cone and bareback firmly grounded on a dispersive electrode.
NOTE: Mouse is completely sedated if unresponsive to toe pinch.
- Take the harvested UCC tumor cells and resuspend them in 50 µL RPMI media. Instill the UCC cells to the bladder using an angiocatheter (Figure 1Aa, Ab).
- Set up a monopolar electrocautery machine and set it to a power of 4 W. Lubricate a 24 G sterile angiocatheter with lubricating jelly and insert it through the urethra of the female mouse.
NOTE: Slight resistance may be felt. Gently push forward or remove the angiocatheter and repeat. Do not force. If the catheter bends on entry, insert a sterile guide wire halfway into the catheter to provide stability. - Fully insert the 0.025" fixed core straight guide wire 1 mm past the end of the angiocatheter.
NOTE: The wire is marked with tape prior to the procedure to indicate the 1 mm stopping point and ensure consistency. - Hold the monopolar pin to the guidewire for 1 s, allowing for electrical irritation of the bladder mucosa.
- Attach a fresh sterile angiocatheter to 1 cc Luer-Lok syringe and draw up 200 µL of collected cells.
NOTE: At least 100 µL is lost from the angiocatheter to the syringe. Compensate for loss volume when calculating the volume of cell suspension needed. - Remove guide wire and angiocatheter from mouse urethra. Insert angiocatheter with syringe of cells attached into urethra.
NOTE: Advancement should be easier than before. - Instill 50 µL of cells into the mouse bladder. Wait a few seconds before removing the angiocatheter to allow for cells to adhere to the bladder wall.
NOTE: Cells remain in the bladder and develop into a primary tumor.
- Set up a monopolar electrocautery machine and set it to a power of 4 W. Lubricate a 24 G sterile angiocatheter with lubricating jelly and insert it through the urethra of the female mouse.
Representative Results
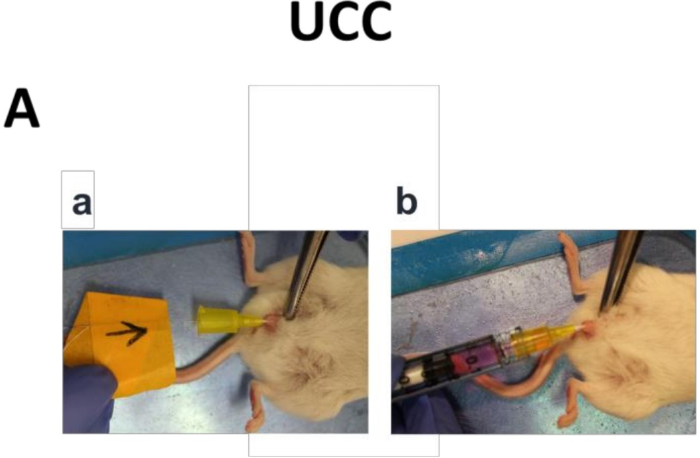
Figure 1: Orthotopic UCC mouse models. (Aa) An angiocatheter was inserted into the bladder of a female NOD/SCID mouse and an electrocautery shock was applied to the bladder wall via a guidewire. (Ab) Luciferase tagged UCC tumor cells, BlCaPt15 (2 x 104 cells), or BlCaPt37 (5 x 105 cells) with the addition of 3 x 105 LN stromal HK cells, were instilled into the NOD/SCID mouse bladder through the angiocatheter.
Declarações
The authors have nothing to disclose.
Materials
| Hair removal cream | Church & Dwight Co., Inc | 1(800)248-8820 | |
| Isoflurane | Henry Schein Animal Health | 108333 | |
| 6-8 week old NOD/SCID mice (female) | Jackson Lab | 1303 | |
| Forceps | Symmetry Surgical Inc | 06-0011 | |
| Electrosurgical generator | ValleyLab | FORCE1C20 | |
| Isoflurane induction chamber | Perkin Elmer | 119038 |

