Differentiation of Human-Induced Pluripotent Stem Cells into Macrophages
Abstract
Source: Lopez-Yrigoyen, M., et al. Production and Characterization of Human Macrophages from Pluripotent Stem Cells. J. Vis. Exp. (2020).
This video describes an in vitro method for generating macrophages from human induced pluripotent stem cells (iPSCs). Macrophage generation from iPSCs occurs in three stages: (i) aggregation of iPSCs in suspension to form three-dimensional embryoid bodies (EBs) that differentiate into mesodermal cells, (ii) formation of macrophage precursor cells from the differentiated EBs, and (iii) differentiation of the macrophage precursor cells into mature, functional macrophages.
Protocol
1. Human iPSC Differentiation to Macrophages
NOTE: A schematic summary of the macrophage differentiation protocol is depicted in Figure 1.
- Preparing cell differentiation growth factors and other reagents
- Prepare human embryonic stem cell-serum-free media (hESC-SFM; see Table of Materials) by supplementing Dulbecco's modified Eagle medium-F12 (DMEM/F12) with hESC supplement, 1.8% w/v bovine serum albumin (BSA), and 0.1 mM 2-mercaptoethanol.
- Prepare 0.1% w/v solution of porcine gelatin by dissolving the gelatin into sterile water. Gelatin solution can be stored at 4 °C for up to 2 years.
- Prepare human bone morphogenetic protein (BMP4) stock solution (25 μg/mL) by dissolving BMP4 into a 4 mM hydrogen chloride (HCl)-0.2% BSA PBS solution. Distribute the stock solution as 50 μL aliquots in cryotubes. Stock solutions can be stored at -20 °C for up to 1 year. Once thawed, stock BMP4 can be stored at 4 °C for 5 days.
- Prepare human vascular endothelial growth factor (VEGF) stock solution (100 μg/mL) by dissolving VEGF into a 0.2% BSA PBS solution. Distribute the stock solution as 10 μL aliquots in cryotubes. Stock solutions can be stored at -20 °C for up to 1 year. Once thawed, stock VEGF can be stored at 4 °C for 7 days.
- Prepare human stem cell factor (SCF) stock solution (100 μg/mL) by dissolving SCF into a 0.2% BSA PBS solution. Distribute the stock solution as 5 μL aliquots in cryotubes. Stock solutions can be stored at -20 °C for up to 1 year. Once thawed, stock SCF can be stored at 4 °C for 10 days.
- Prepare human interleukin-3 (IL3) stock solution (10 μg/mL) by dissolving IL3 into a 0.2% BSA PBS solution. Distribute the stock solution as 500 μL aliquots in cryotubes. Stock solutions can be stored at -20 °C for up to 2 years. Once thawed, stock IL3 can be stored at 4 °C for 15 days.
- Prepare human macrophage colony-stimulating factor (CSF1) stock solution (10 μg/mL) by dissolving CSF1 into a 0.2% BSA PBS solution. Distribute the stock solution as 1 mL aliquots in cryotubes. Stock solutions can be stored at -20 °C for up to 2 years. Once thawed, stock CSF1 can be stored at 4 °C for 15 days.
- Stage 1: Generation of embryoid bodies (day 0–day 3)
- On day 0, add 2.25 mL of Stage 1 media (hESC-SFM supplemented with 50 ng/mL BMP4, 50 ng/mL VEGF, and 20 ng/mL SCF) into two wells of an ultralow attachment 6-well plates.
- Replace maintenance media of one 80% confluent well of iPSCs in a 6-well plate with 1.5 mL of Stage 1 media.
- Cut colonies using the cell passaging tool and transfer cut colonies with a pipette into the two wells of an ultralow attachment 6-well plate (see Table of Materials).
- On day 2, bring cytokines to a final concentration of 50 ng/mL BMP4, 50 ng/mL VEGF, and 20 ng/mL SCF using 0.5 mL of hESC-SFM media.
NOTE: IPSC colonies will become EBs (Figure 2A).
- Stage 2: Emergence of hematopoietic cells in suspension
- On day 4, coat 4 wells of a 6-well tissue culture plate with 0.1% w/v gelatin and incubate for at least 10 min.
- Remove gelatin and add 2.5 mL of Stage 2 media (X-VIVO15 supplemented with 100 ng/mL CSF1, 25 ng/mL IL-3, 2 mM glutamax, 1% penicillin-streptomycin, and 0.055 mM 2-mercaptoethanol).
- Collect formed EBs into a 50 mL centrifuge tube and allow them to settle at the bottom of the tube by gravity. Carefully aspirate the media.
- Resuspend EBs in 2 mL of Stage 2 media.
- Transfer 10–15 EBs (no more than 15) to a gelatin-coated well containing 2.5 mL of Stage 2 media.
- Incubate EBs at 37 °C and 5% CO2 air.
- Change media on plated EBs every 3–4 days for 2–3 weeks.
- After 2–3 weeks, the EBs start releasing nonadherent hematopoietic cells into suspension.
NOTE: This period of suspension cell release can vary and is cell line dependent. Cells in this suspension can be harvested and matured into macrophages (see Stage 3) (Figure 2A).
- Stage 3: Terminal macrophage maturation
- Collect suspension hematopoietic cells and replenish media (Stage 2 media) on the EB plate.
- Centrifuge suspension cells at 200 x g for 3 min.
- Resuspend suspension cells in Stage 3 media (X-VIVO15 supplemented with 100 ng/mL CSF1, 2 mM glutamax, and 1% penicillin-streptomycin).
- Plate collected and spun cells onto untreated plastic 10 cm bacteriological-grade plates (10 mL) or uncoated 6-well tissue culture plates (3 mL) at a 0.2 x 106 cells/mL density.
- Keep cells in Stage 3 media for 9–11 days, changing media every 5 days.
NOTE: Steps 1.4.1–1.4.5 from Stage 3 can be repeated every 3–4 days and suspension cells can be harvested from the original EB plate for up to 3 months.
Representative Results
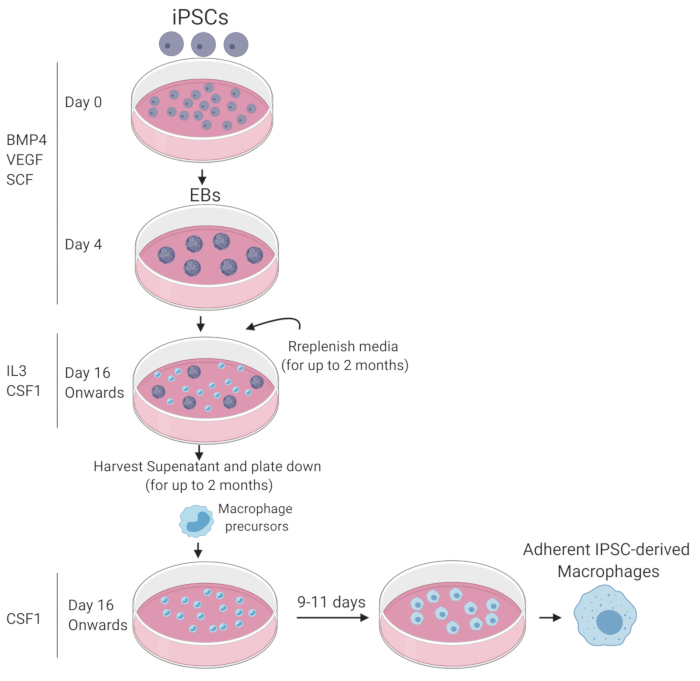
Figure 1: Graphic summary of iPSC differentiation to mature functional macrophages. Diagram drawn with Biorender.
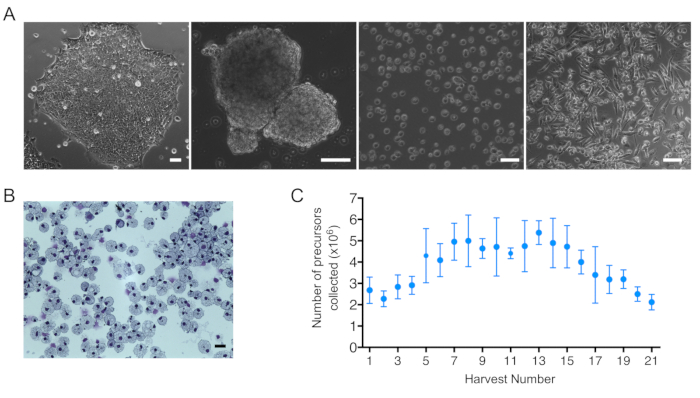
Figure 2: iPSC differentiation towards macrophages and iPSC-DM number and morphology. (A) Bright Field images obtained from (left to right): an IPSC colony, embryoid bodies (EBs), harvested suspension cells, and mature macrophages. Scale bar = 100 μm. (B) Image of macrophage cytospins stained with Kwik-diff kit. Scale bar = 25 μm. (C) Number of suspension cells collected per harvest per one 6-well plate of EBs. Plot shows mean + SEM; (n = 6 biologically independent experiments).
Declarações
The authors have nothing to disclose.
Materials
| CellMask Deep Red Plasma Membrane Stain | Thermofisher | C10046 | Cryopreservation media |
| Cryostor CS10 | Sigma | C2874 | |
| CTS CELLstart Substrate | Invitrogen | A1014201 | Stem cell substrate |
| Porcine Skin Gelatin | Sigma | G9136 | |
| Recombinant Human BMP4 Protein | R&D | 314-BP-010 | |
| Recombinant Human IL3 | Preprotech | 0200-03-10 | |
| Recombinant Human MCSF (carrier-free) 100ug | Biolegend | 574806 | |
| Recombinant Human VEGF Protein | R&D | 293-VE-010 | |
| SCF (C-Kit Ligand) Recombinant Human Protein | Thermofisher | PHC2111 | |
| StemPro hESC SFM | Thermofisher | A1000701 | |
| StemPro EZPassage Disposable Stem Cell Passaging Tool | Thermofisher | 23181010 | |
| Ultralow attachment plates: Cell culture multi-well plate, 6 well, cell star cell repellent surface | Greiner | 657970 | |
| 2-Mercaptoethanol (50 mM) | Invitrogen | 31350010 | |
| DPBS, calcium, magnesium (500ml) | Thermofisher | 14040091 | |
| GlutaMAX Supplement | Thermofisher | 35050061 |

