An Assay to Study the Impact of Extracellular Matrix Stiffness on Bacterial Infection
Abstract
Source: Bastounis, E. E., et al. A Multi-well Format Polyacrylamide-based Assay for Studying the Effect of Extracellular Matrix Stiffness on the Bacterial Infection of Adherent Cells. J. Vis. Exp. (2018).
This video demonstrates an assay to study the effect of extracellular matrix (ECM) stiffness on bacterial infection of adherent cells. Polyacrylamide hydrogels of varying stiffness, overlaid with collagen, are layered in a multi-well plate to mimic the stiffness of physiological ECM. Upon overlaying human endothelial cells on the hydrogels and infecting the cells with Listeria monocytogenes bacteria engineered to express a fluorescent protein once intracellular, flow cytometry is used to compare internalized bacteria in cells residing on stiffer hydrogels versus softer hydrogels.
Protocol
1. Manufacturing Thin Two-layered Polyacrylamide (PA) Hydrogels on Multi-Well Plates
- Polyacrylamide hydrogel fabrication
NOTE: See Figure 1.- Prepare aqueous solutions that contain 3 – 10% of 40% stock acrylamide solution (see Table of Materials) and 0.06 – 0.6% of 2% stock bis-acrylamide solution (see Table of Materials) to manufacture hydrogels of tunable stiffness ranging from 0.6 kPa to 70 kPa. See Table 1.
- For 0.6 kPa hydrogels, mix 3% acrylamide with 0.045% bis-acrylamide. For 3 kPa hydrogels, mix 5% acrylamide with 0.074% bis-acrylamide. For 10 kPa hydrogels, mix 10% acrylamide with 0.075% bis-acrylamide. For 20 kPa hydrogels, mix 8% acrylamide with 0.195% bis-acrylamide. For 70 kPa hydrogels, mix 10% acrylamide with 0.45% bis-acrylamide.
NOTE: Further details on achieving the desirable PA hydrogel stiffness can be found elsewhere.
- For 0.6 kPa hydrogels, mix 3% acrylamide with 0.045% bis-acrylamide. For 3 kPa hydrogels, mix 5% acrylamide with 0.074% bis-acrylamide. For 10 kPa hydrogels, mix 10% acrylamide with 0.075% bis-acrylamide. For 20 kPa hydrogels, mix 8% acrylamide with 0.195% bis-acrylamide. For 70 kPa hydrogels, mix 10% acrylamide with 0.45% bis-acrylamide.
- Prepare two aqueous solutions for each desirable hydrogel stiffness. Prepare Solution 1 to be bead-free and Solution 2 to contain 0.03% 0.1-µm diameter fluorescent micro-beads (see Table of Materials).
- Degas Solutions 1 and 2 by vacuum for 15 min to eliminate oxygen that is known to inhibit polymerization of the solutions.
- Add 0.6% of the 10 g/mL stock APS solution and 0.43% tetramethylethylenediamine (TEMED) to Solution 1 to enable a polymerization initiation. Act fast.
- Add 3.6 µL of Solution 1 to the center of each well of the 24-well dish.
- Immediately cover the wells with 12-mm untreated circular coverslips and let Solution 1 sit for 20 min so that it fully polymerizes.
- Gently tap a syringe needle to a surface to create a small hook at its tip to facilitate the removal of the coverslips. Lift the coverslips using the syringe needle.
- Add 0.6% of the 10 g/mL stock APS solution and 0.43% TEMED to Solution 2. Deposit 2.4 µL of the mixture on top of the first layer in each well of the 24-well dish.
- Cover Solution 2 with 12-mm circular glass coverslips, gently pressing downwards using a pair of forceps to ensure the thickness of the second layer is minimal. Let Solution 2 polymerize for 20 min.
- Add 500 µL of 50 mM HEPES pH 7.5 to each of the wells and then remove the glass coverslips with the syringe needle and forceps.
- Prepare aqueous solutions that contain 3 – 10% of 40% stock acrylamide solution (see Table of Materials) and 0.06 – 0.6% of 2% stock bis-acrylamide solution (see Table of Materials) to manufacture hydrogels of tunable stiffness ranging from 0.6 kPa to 70 kPa. See Table 1.
- Sterilization, collagen-coating, and equilibration of polyacrylamide hydrogels
- UV-expose the hydrogels for 1 h in the tissue culture hood to allow sterilization.
- Prepare a mixture of 0.5% weight/volume sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate (Sulfo-SANPAH, see Table of Materials) in 1% DMSO and 50 mM HEPES pH = 7.5.
- Add 200 µL of this solution to the upper surface of the hydrogels. Acting fast, expose them to UV (302 nm) for 10 min to activate them.
- Wash the hydrogels twice with 1 mL of 50 mM HEPES pH = 7.5. Repeat if needed to ensure that any excess crosslinker is removed.
- Protein-coat the hydrogels with 200 µL of 0.25 mg/mL rat tail Collagen I (see Table of Materials) in 50 mM of HEPES. Incubate the hydrogels, with the collagen solution on top, overnight at room temperature.
NOTE: To prevent dehydration/evaporation, place the multi-well plates in a secondary containment and add laboratory cleaning tissues soaked in water in the inner periphery of the containment. - Use an epifluorescence or confocal microscope with a 40X objective to measure the thickness of the hydrogels. Do so by locating the z positions of the bottom (where the glass surface is) and top planes of the hydrogel (where the fluorescent beads' intensity is maximum). Then subtract the z positions to determine the height.
NOTE: We used an inverted epifluorescence microscope and a 40X objective with numerical aperture 0.65 to measure the thickness of the hydrogels. Atomic Force Microscopy measurements (AFM) can also be performed at this point to confirm the exact stiffness of the hydrogels (see Figure 2). - Before seeding the cells of interest on the hydrogels, add 1 mL of media on the hydrogels and incubate them at 37 ˚C for 30 min to 1 h to ensure equilibration.
NOTE: We added MCDB-131 full media because that is the media where the model host cells (HMEC-1) were cultured in (see step 2.1 for details).
2. Human Microvascular Endothelial Cell Culture and Seeding on Hydrogels
- Culture HMEC-1 (human, microvascular endothelial) cells in MCDB-131 full media containing MCDB-131 media supplemented with 10% fetal bovine serum, 10 ng/mL of epidermal growth factor, 1 µg/mL of hydrocortisone, and 2 mM of L-glutamine (see Table of Materials).
- Split the confluent cultures 1:6 every 3 – 4 days and keep the cells until passage 40.
- One day prior to the experiment, detach the cells from their culture vessel using 0.25% trypsin/EDTA. First wash the cells and their culture vessel 1x with sterile phosphate buffered saline (PBS), and then add the appropriate amount of 0.25% trypsin/EDTA (2 mL per 100-mm dish or 75-cm flask, 1 mL per 60-mm dish or 25-cm flask), incubating the flask at 37 °C for 5 – 10 min to allow the detachment of the cells from their substrate.
- Neutralize the trypsin by adding the desired volume of MCDB-131 full media, pipet gently to break up the clumps of cells, and then place the solution into a conical centrifuge tube.
- Gently swirl the solution of cells to ensure that the cells are evenly distributed and then take out 20 µL of the solution and very gently fill out the two chambers underneath the coverslip of a glass hemocytometer.
- Pellet down the solution of cells contained in the conical centrifuge tube using centrifugation for 10 min at 500 x g.
- During the 10 min waiting period, count the cells using a hemocytometer. Use a microscope, focus on the grid lines of the hemocytometer with a 10X objective, and then use a hand tally counter to count the number of cells in one 1 mm x 1 mm square.
- Move the hemocytometer to another 1 mm x 1 mm square, count the cells there and then repeat the process two more times. Calculate the average of the four measurements and then multiply the average by 104. The final value is the number of viable cells/mL in the cell suspension that is being centrifuged.
- Remove the liquid out of the conical centrifuge tube while ensuring that the cell pellet is not disrupted. Resuspend the cells in the MCDB-131 full media at a concentration of 4 x 105 cells/mL.
- Seed the cells in suspension on the hydrogels by first removing the media with which the hydrogels were incubated and then adding 1 mL of cell suspension on each hydrogel.
3. Infection of Human Microvascular Endothelial Cells with L. monocytogenes
- Three days before the infection, streak out the Lm strain to be used from a glycerol stock (stored at -80 °C) onto a BHI-agar plate that contains 7.5 µg/mL of chloramphenicol and 200 µg/mL of streptomycin, if appropriate.
NOTE: The strain to be streaked out can be a wild-type or mutant, constitutively expressing fluorescence (for immunostaining JAT1045 was used) or expressing fluorescence under the ActA promoter (for flow cytometry or traction force microscopy JAT983 or JAT985 were used). - Incubate plates at 37 °C until discrete colonies are formed (1 – 2 days).
- The day before the infection, grow the desired strain overnight, shaking it at 150 rpm at 30 °C in BHI media with 7.5 µg/mL of chloramphenicol (if appropriate).
- Place 5 mL of BHI media in a 15-mL conical centrifuge tube, add 7.5 µg/mL of chloramphenicol (if appropriate), and then inoculate a single colony from the agar plate using a sterile 10 µL tip.
- The next day, just prior to infection, measure the optical density of the bacteria solution at 600 nm (OD600) by diluting the sample 1:5; use a cuvette containing BHI alone to serve as a blank.
- Dilute the overnight culture to an OD600 of 0.1 and incubate it, shaking for 2 h at 30 °C, in BHI media with 7.5 µg/mL of chloramphenicol (if appropriate) to allow the bacteria to reach log-phase growth.
- Measure the OD600 of the bacterial solution, which is expected to be around 0.2 – 0.3. If the OD600 is higher, dilute it to 0.2 – 0.3 with BHI alone.
- Take 1 mL of bacterial solution into a microcentrifuge tube. Spin it down for 4 min at 2,000 x g using centrifugation at room temperature. Remove the supernatant and resuspend the bacterial pellet in 1 mL of tissue culture-grade PBS, in the tissue culture hood. Wash the bacteria twice more by spinning them down for 4 min at 2,000 x g at room temperature. Remove the supernatant and resuspend the bacteria in 1 mL of PBS.
- Prepare the infection mix by mixing 10 or 50 µL of the bacteria resuspended in PBS with 1 mL of MCDB-131 full media for a multiplicity of infection (MOI; i.e., the number of bacteria per host cell) of approximately 50 bacteria per host cell or 10 bacteria per host cell.
- Remove the media from the wells of the 24-well plates, careful not to disrupt the hydrogels or the cells. Wash the cells 1x with 1 mL of MCDB-131 full media and then add 1 mL of the bacteria to each well.
- Cover the plate with its lid and wrap the plates with polyethylene food wrap to avoid leakage. Place the plates in the centrifuge and spin the samples for 10 min at 200 x g to synchronize the invasion. Move the plates into the tissue culture incubator and incubate them for 30 min at 37 °C.
- Wash the samples 4x with MCDB-131 full media and move them into the tissue culture incubator. After an additional 30 min, replace the media with media supplemented with 20 µg/mL of gentamicin.
4. Flow Cytometry to Quantify Extracellular-matrix-stiffness Dependent Susceptibility of Host Cells to Infection
- Weigh 5 mg of collagenase and place it in a 15-mL conical centrifuge tube. Add 10 mL of 0.25% trypsin-EDTA and mix them well.
- 8 h post-infection, remove the media from the wells of the 24-well plate and wash the wells 1x with tissue culture PBS. Remove the PBS from the wells and add 200 µL of the trypsin-EDTA/collagenase mix to each well. Place this in the tissue culture incubator for 10 min to allow full detachment of the cells.
- Use a fresh pipette for each well and pipet the mix up and down 8x. Be gentle so as to not damage the cells. Add 200 µL of full media to each well to neutralize the trypsin.
- Transfer the 400-µL cell solution of each well into a 5-mL polystyrene tube with a 35-µm cell strainer cap (see Table of Materials).
- Analyze 10,000 – 20,000 cells per sample by flow cytometry. Perform data acquisition through flow cytometry and determine the percentage of infected cells per well using relevant commercially available software. Use the forward scatter versus side scatter plots to gate the bulk of the distribution of the cell counts.
NOTE:: This will ensure the analysis of single cells and eliminate debris or cell doublets or triplets.- Measure the fluorescence signal from control-uninfected cells and gate the population of infected cells excluding autofluorescence.
Table 1. Composition of polyacrylamide (PA) hydrogels of varying stiffness. In this table, the percentage of stock 40% acrylamide solution and the percentage of stock 2% bis-acrylamide solution to achieve a given stiffness (Young's modulus, E) are indicated in different columns.
| Young's modulus (E, kPa) | Acrylamide % (from 40% stock) | Bisacrylamide % (from 2% stock) |
| 0.6 | 3 | 0.045 |
| 3 | 5 | 0.075 |
| 10 | 10 | 0.075 |
| 20 | 8 | 0.195 |
| 70 | 10 | 0.45 |
Representative Results
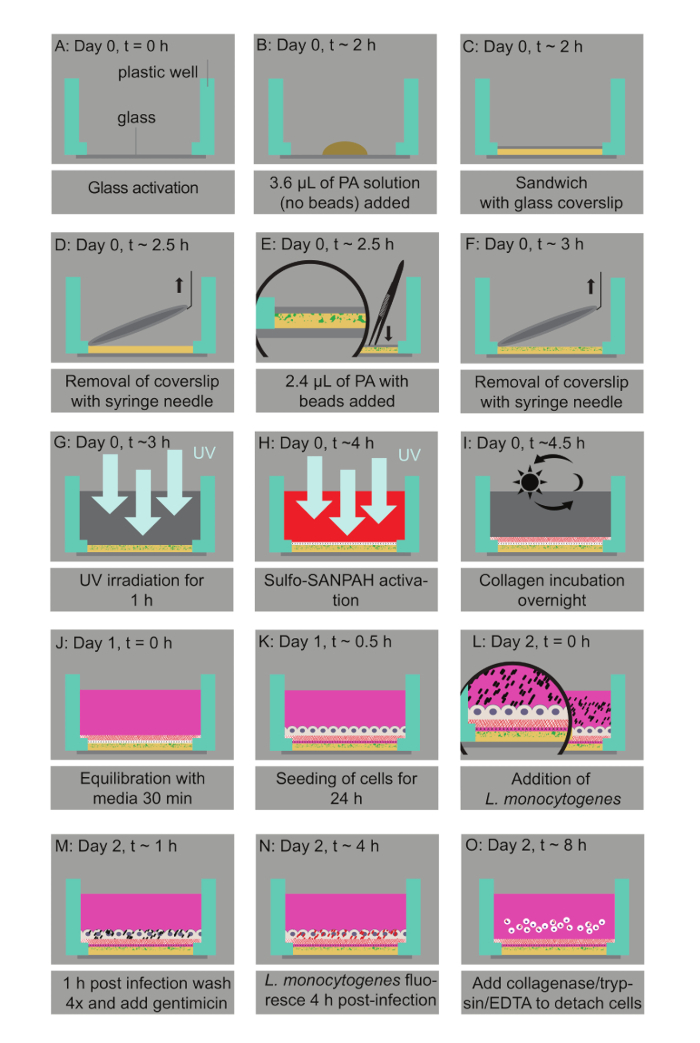
Figure 1: Bacterial infection assay of host cells residing on thin two-layered fluorescent bead-embedded polyacrylamide (PA) hydrogels of varying stiffness. A. Glass coverslips are chemically modified to enable hydrogel attachment. B. 3.6 µL of PA mixtures are deposited on the glass bottoms. C. The mixture is covered with a 12-mm circular glass coverslip to enable polymerization. D. The coverslip is removed with a needle syringe. E. 2.4 µL of a PA solution with microbeads is added on top of the bottom layer and capped with a circular glass coverslip. F. A buffer is added in the well and the coverslip is removed. G. UV irradiation for 1 h ensures sterilization. H. A Sulfo-SANPAH-containing solution is added on the gels, which are then placed under UV for 10 min. I. The hydrogels are washed with a buffer and then incubated overnight with collagen I. J. The hydrogel is equilibrated with cell media. K. The host cells are seeded. L. Lm bacteria are added to the solution and the infection is synchronized via centrifugation. M. 1 h post-infection bacteria in the solution are washed away and media supplemented with an antibiotic is added. N. At 4 h post-infection, Lm (JAT985) starts fluorescing. O. HMEC-1 cells are detached from their matrix and the solutions are transferred to tubes to perform flow cytometry measurements. Note that days and approximate times for each step of the assay are also indicated. This figure has been modified from Bastounis and Theriot.
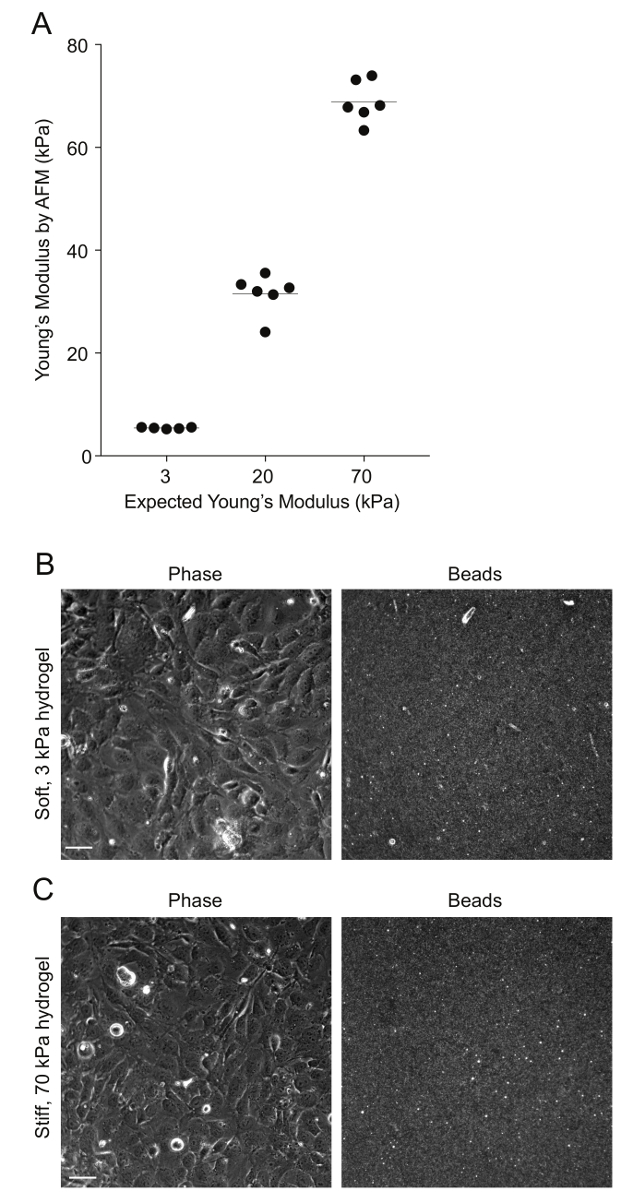
Figure 2: AFM measurements of PA hydrogel stiffness and beads' distribution. A. Data show the expected Young's modulus (measure of stiffness) of the PA hydrogels, given the amount of acrylamide and bis-acrylamide used versus the Young's modulus measured through AFM (N = 5 – 6). The horizontal bars depict the mean. The stiffness of the 0.6 kPa hydrogels could not be measured because the hydrogels were very soft and adhered to the AFM tip. B. This is a phase image of confluent HMEC-1 cells and the corresponding image of the beads embedded on the uppermost surface of a soft 3 kPa-PA hydrogel. The HMEC-1 were seeded for 24 h at a concentration of 4 x 105 cells per well. C. This image is the same as Figure 2B but for cells residing on a stiff 70-kPa PA hydrogel.
Declarações
The authors have nothing to disclose.
Materials
| Reagents | |||
| Sodium hydroxide pellets | Fisher | S318-500 | |
| (3-Aminopropyl)triethoxysilane | Sigma | A3648 | |
| 25% gluteraldehyde | Sigma | G6257-100ML | |
| 40% Acrylamide | Sigma | A4058-100ML | |
| Bis-acrylamide solution (2%w/v) | Fisher Scientific | BP1404-250 | |
| Fluorospheres carboxylate-modified microspheres, 0.1 μm, yellow-green fluorescent (505/515) | Invitrogen | F8803 | |
| Ammonium Persulfate | Fisher | BP17925 | |
| TEMED | Sigma | T9281-25ML | |
| Sulfo-SANPAH | Proteochem | c1111-100mg | |
| Collagen, Type I Solution from rat | Sigma | C3867-1VL | |
| Dimethyl sulfoxide (DMSO) | J.T. Baker | 9224-01 | |
| HEPES, Free acid | J.T. Baker | 4018-04 | |
| Leibovitz's L-15 medium, no phenol red | Thermofischer | 21083027 | |
| MCDB 131 Medium, no glutamine | Life technologies | 10372019 | |
| Foundation Fetal Bovine Serum, Lot: A37C48A | Gemini Bio-Prod | 900108 500ml | |
| Epidermal Growth Factor, EGF | Sigma | E9644 | |
| Hydrocortisone | Sigma | H0888 | |
| L-Glutamine 200mM | Fisher | SH3003401 | |
| DPBS 1X | Fisher | SH30028FS | |
| Gentamicin sulfate | MP biomedicals | 194530 | |
| Cloramphenicol | Sigma | C0378-5G | |
| DifcoTM Agar, Granulated | BD | 214530 | |
| BBL TM Brain-heart infusion | BD | 211059 | |
| Hoechst 33342, trihydrochloride, trihydrate – 10 mg/ul solution in water | Invitrogen | H3570 | |
| Formaldehyde 16% EM grade | Electron microscopy | 15710-S | |
| Anti-Listeria monocytogenes antibody | Abcam | ab35132 | |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor® 546 conjugate | Thermofischer | A-11035 | |
| 0.25% trypsin-EDTA , phenol red | Thermofischer | 25200056 | |
| COLLAGENASE FROM CLOSTRIDIUM HISTOLYTIC | Sigma | C8051 | |
| Streptomycin sulfate | Fisher Scientific | 3810-74-0 | |
| Sucrose | Calbiochem | 8510 | |
| Sodium dodecyl sulfate | Thermofischer | 28364 | |
| MES powder | Sigma | M3885 | |
| KCl | J.T. Baker | 3040-05 | |
| MgCl2 | J.T. Baker | 2444-1 | |
| EGTA | Acros | 40991 | |
| Disposable lab equipment | |||
| 12 mm circular glass coverslips | Fisherbrand | 12-545-81 | No. 1.5 Coverslip | 10 mm Glass Diameter | Uncoated |
| Glass bottom 24 well plates | Mattek | P24G-1.5-13-F | |
| 5 ml polystyrene tubes with a 35 μm cell strainer cap | Falcon | 352235 | |
| T-25 flasks | Falcon | 353118 | |
| 50 ml conical tubes | Falcon | 352070 | |
| 15 ml conicals tubes | Falcon | 352196 | |
| Disposable Serological Pipettes (1 ml, 2 ml, 5 ml, 10 ml, 25 ml) | Falcon | 357551 | |
| Pasteur Glass Pipettes | VWR | 14672-380 | |
| Pipette Tips (1-200 μl, 101-1000 μl) | Denville | P1122, P1126 | |
| Powder Free Examination Gloves | Microflex | XC-310 | |
| Cuvettes bacteria | Sarstedt | 67.746 | |
| Razors | VWR | 55411-050 | |
| Syringe needle | BD | 305167 | |
| 0.2um sterilizng bottles | Thermo Scientific | 566-0020 | |
| 20 ml syringes | BD | 302830 | |
| 0.2um filters | Thermo Scientific | 723-2520 | |
| wooden sticks | Grainger | 42181501 | |
| Saran wrap | Santa Cruz Biotechnologies | sc-3687 | |
| Plates bacteria | Falcon | 351029 | |
| Large/non-disposable lab equipment | |||
| Tissue Culture Hood | Baker | SG504 | |
| Hemacytometer | Sigma | Z359629 | |
| Bacteria incubator | Thermo Scientific | IGS180 | |
| Tissue culture Incubator | NuAire | NU-8700 | |
| Vacuum chamber/degasser | Belart | 42025 | 37 °C and 5% CO2 |
| Inverted Nikon Diaphot 200 epifluorescence microscope | Nikon | NIKON-DIAPHOT-200 | |
| Cage Incubator | Haison | Custom | |
| Scanford FACScan analyzer | Stanford and Cytek upgraded FACScan | Custom | |
| Pipette Aid | Drummond | 4-000-110 | |
| Pipettors (10 μl, 200 μl, 1000ul) | Gilson | F144802, F123601, F123602 | |
| pH meter | Mettler Toledo | 30019028 | |
| forceps | FST | 11000-12 | |
| 1 L flask | Fisherbrand | FB5011000 | |
| Autoclave machine | Amsco | 3021 | |
| Stir magnet plate | Bellco | 7760-06000 | |
| Magnet stirring bars | Bellco | 1975-00100 | |
| Spectrophotometer | Beckman | DU 640 | |
| Scanford FACScan analyzer | Cytek Biosciences | Custom Stanford and Cytek upgraded FACScan | |
| Software | |||
| Microscope Software (μManager) | Open Imaging | ||
| Matlab | Matlab Inc | ||
| Flowjo | FlowJo, LLC | ||
| Automated image analysis software, CellC | https://sites.google.com/site/cellcsoftware/ | The software is freely available. Eexecutable files and MATLAB source codes can be obtained at https://sites.google.com/site/cellcsoftware/ |

