Abstract
Source: Wang, J., et al. A Mouse Model of Vascularized Heterotopic Spleen Transplantation for Studying Spleen Cell Biology and Transplant Immunity. J. Vis. Exp. (2019).
The video demonstrates a surgical method for generating a mouse model of vascularized heterotopic spleen transplantation. The abdominal cavity of an anesthetized donor mouse is surgically opened to remove the spleen. The inferior vena cava and infrarenal aorta are identified, their lumbar branches are ligated, and openings are made for the heterotopic transplantation of a spleen from a congenic donor mouse. Upon completing vascular anastomosis and restoring blood flow to the implanted spleen, the incision is closed, and the mouse is monitored for recovery.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Surgical Preparation, Anesthetization, and Analgesia Regimen
- Place a sterile disposable drape (45.7 cm x 66 cm) on the surgical platform. Gently grab the mouse, inject ketamine (50 mg/kg) and xylazine (10 mg/kg) intraperitoneally (i.p) for anesthesia, and inject 0.05 mg/kg buprenorphine subcutaneously for analgesia.
- Ensure the depth of anesthesia by toe-pinch, shave the hair in the whole abdomen area with a razor and place the mouse on the sterile surgical platform under the operating microscope at 6-10x magnification.
2. Donor Spleen Harvest
- Sterilize the abdomen with an alcohol prep pad, secure the limbs with surgical tape, and make a 3-4 cm midline vertical skin incision from the pubis to the xiphoid process with scissors.
- Retract the abdominal wall with sterile retractors made from paperclips. Move the intestines to the right flank of the abdomen (surgeon's left) side with a sterile cotton swab to expose the spleen. Cauterize the short gastric vein attached to the spleen with a sterile low temperature cautery (Figure 1A). Place a piece of sterile gauze soaked with 37 °C saline over the spleen to keep it moist (Figure 1B).
- Separate and mobilize the portal vein from the pancreatic tissue (Figure 1C) by ligating the portal vein branches (superior pancreaticoduodenal vein and right gastric vein); place a suture around the portal vein distal from the splenic vein (Figure 1D).
- Flip the spleen to the right side to expose the aorta and celiac trunk with the splenic artery (Figure 1E). Dissect and mobilize aortic-celiac-splenic artery by ligating the hepatic artery and gastric artery; place a suture around the aorta proximal to the celiac artery (Figure 1F-G).
- Inject 100 international units (IU) heparin into the inferior vena cava (IVC) to heparinize the whole body and wait 3 min to ensure the heparin take effects. Ligate the aorta proximal to celiac artery, transect the portal vein, and then perfuse the whole body using 10 mL of heparinized cold (4 °C) saline (10 mL/20 s) from the abdominal aorta distal to celiac trunk (Figure 1H).
- Collect the spleen graft en bloc with the associated aortic-celiac-splenic segment and the portal vein along with a segment of splenic vein and a small portion of pancreatic tissue. Preserve the graft in 5 mL of 4 °C saline before transplant. Euthanize the mouse by cervical dislocation.
3. Recipient Splenectomy and Spleen Graft Implantation
- Place a heating pad on the surgical platform and adjust the temperature to 37 °C. Place a sterile drape (45.7 cm x 66 cm) on top of the heating pad to create a sterile surgical platform. Repeat steps 2.1 and 2.2 for surgical preparation and anesthetization. Make a 3-4 cm midline incision and retract the abdominal wall as described in step 2.1 and step 2.2.
- Carefully move the intestine to right side of the mouse with a sterile cotton swab to expose the recipient's spleen. Ligate the splenic vein and artery and remove the spleen.
- Carefully move the intestine to left side of the mouse and cover the intestines with wet gauze (soaked with sterile 37 °C saline). Dissect and ligate the lumbar branches of the infrarenal aorta and IVC; cross-clamp the infrarenal aorta and IVC by using two 4 mm microvascular clamps.
- Place an 11-0 nylon suture through the infrarenal aorta (a full thickness) and retract to create an elliptical aortotomy by a single cut with microscissors (the length should match the diameter of the donor aorta, Figure 2A). Pierce the IVC using a 30 G needle to create an elliptical venotomy and extend the opening to donor portal vein-matched length using microscissors (Figure 2A).
- Clear the intraluminal blood or blood clot (in the aorta and IVC) with 500 μL of heparinized saline (10 units/mL).
- Place the spleen graft in the right flank of the recipient mouse abdomen; carefully identify the donor's aortic cuff and the donor's portal vein. After making sure that the vessels are not twisted, cover the spleen graft with gauze soaked with cold (4 °C) saline.
- Connect the donor's aortic cuff to the proximal and distal apex of the recipient's aortaotomy with two stay sutures (11-0 nylon suture, same as below) (Figure 2B,C). Make an anastomosis with 2-3 bites of continuous 11-0 nylon sutures between the donor's aortic cuff and the recipient's aortaotomy (anterior wall) (Figure 2D). Turn the spleen graft over to the left side of the recipient; make the anastomosis between the donor's aortic cuff and the recipient's aortotomy (posterior wall) (Figure 2E).
- Perform an anastomosis to connect the donor's portal vein to the posterior wall of the recipient's IVC, using 4 to 5 bites of continuous sutures on the inside of the IVC and then close the suture on the outside of the IVC (Figure 2F,G).
- Release the vessel clamps and use sterile cotton swab to tamponade bleeding until the spleen color is recovered (Figure 2H).
- Close the abdomen with a 5-0 synthetic absorbable vicryl suture in a continuous pattern. Close the skin layer with a 5-0 nylon suture in an interrupted pattern.
NOTE: For the steps 3.7-3.8, alternatively, make an anastomosis between the donor's portal vein and the recipient's IVC first (step 3.8); then make an anastomosis between the donor's aortic cuff and the posterior wall of the recipient's aortotomy, using 2 to 3 continuous sutures in the inside of the aorta and close the suture on the outside of the aorta.
4. Animal Recovery
- Inject 1 mL of warm saline subcutaneously via 4 separate locations (0.25 mL/location) after closing the abdomen.
- Keep the mouse in a temperature-controlled incubator (30 °C) for the first few hours post-operation, monitor the mouse until it has regained sufficient consciousness, and then transfer the mouse to a new clean cage with regular food and water, with a heating pad (30 °C) underneath the cage. Keep the mouse post-surgery in a separate cage.
5. Post-surgical Pain Management
- Inject 0.05 mg/kg buprenorphine subcutaneously 24 h and 48 h post-surgery to maintain analgesia regimen.
Representative Results
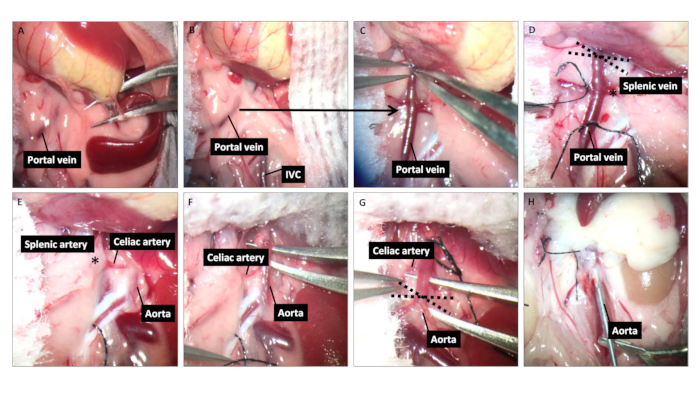
Figure 1: Donor spleen harvest. (A) Cauterize the short gastric vein attached to the spleen. (B) Place a small piece of sterile warm wet gauze over the spleen to keep it moist. (C) Dissect and isolate the portal vein behind the pancreas. (D) Ligate the side branches of the portal vein and place a suture round the portal vein distal to the splenic vein. The dashed lines represent the location transecting later for the anastomosis with the recipient IVC. (E) Flip the spleen over to the right side of the abdomen (surgeon's left) to expose the aorta and celiac trunk with its branches including splenic artery. (F) Dissect and mobilize aortic -celiac -splenic artery by ligating the two other branches. (G) Place a suture around the aorta proximal to the celiac artery. The dashed lines represent the location transecting later used for the anastomosis with recipient abdominal aorta. (H) After ligating the aorta and transecting the portal vein, perfuse the spleen graft with 10 mL of heparinized saline through the aorta.
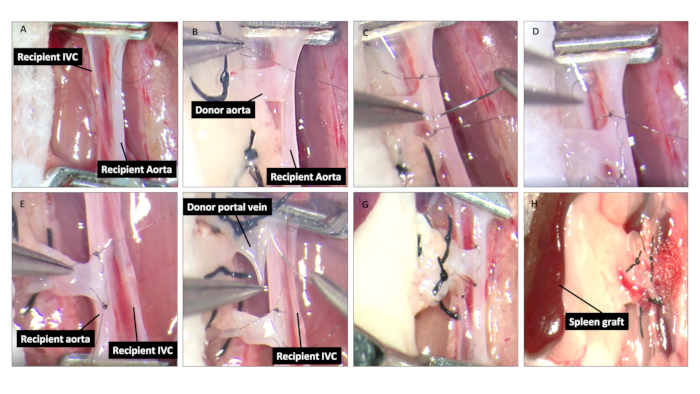
Figure 2: Spleen graft implantation. (A) After isolation and cross clamping the aorta and IVC, make a longitudinal aortotomy and venotomy in the aorta and IVC, respectively. (B-D) Place the spleen graft on the right side of the abdomen (surgeon's left side). Make the end-to-side anastomosis between donor aortic cuff and the anterior wall of the recipient's aortotomy, using 2-3 bites of continuing suture. (E) Turn the spleen graft over to the left flank of the recipient; repeat the previous procedure between the donor aortic cuff and the posterior wall of the recipient's aortotomy and close the suture on the outside of aorta. (F-G) Make an end-to-side anastomosis between the donor portal vein and the posterior wall of the recipient's IVC, using 4 to 5 bites of continuous 11-0 nylon suture in the inside of the IVC and then close the suture on the outside of the IVC. (H) After completing the anastomosis, release the vessel clamps and place some cotton buds to help stop the bleeding.
Declarações
The authors have nothing to disclose.
Materials
| Ketamine | Wyeth | 206205-01 | |
| Xylazine | Lloyd Laboratories | 139-236 | |
| Heparin solution | Abraxis Pharmaceutical Products | 504031 | |
| Injection grade normal saline | Hospira Inc. | NDC 0409-4888-20 | |
| 70% Ethanol | Pharmco Products Inc. | 111000140 | |
| ThermoCare Small Animal ICU System | Thermocare, Inc. | ||
| Adson Forceps | Roboz Surgical Instruments | RS-5230 | |
| Derf Needle Holder | Roboz Surgical Instruments | RS-7822 | |
| Extra Fine Micro Dissecting Scissors | Roboz Surgical Instruments | RS-5881 | |
| Micro-clip | Roboz Surgical Instruments | RS-5420 | |
| 7-0 silk | Braintree Scientific | SUT-S 103 | |
| 11-0 nylon on 4-mm (3/8) needle | Sharpoint DR4 | AK-2119 | |
| Ms CD45.2 antibody | BD Bioscience | 553772 | |
| Ms CD45.1 antibody | BD Bioscience | 553776 | |
| Ms CD11b antibody | BD Bioscience | 557657 | |
| Ms B220 antibody | BD Bioscience | 553089 | |
| Ms Ly6C antibody | eBioscience | 48-5932-80 | |
| Ms Ly6G antibody | BD Bioscience | 561236 | |
| Ms F4/80 antibody | BD Bioscience | 565614 | |
| Ms CD11c antibody | BD Bioscience | 558079 | |
| Ms CD3 antibody | eBioscience | 48-0032-82 | |
| Ms CD4 antibody | BD Bioscience | 552051 | |
| Ms CD8 antibody | BD Bioscience | 563786 | |
| LIVE/DEAD™ Fixable Violet Dead Cell Stain Kit | Thermo Fisher | L34955 |
Tags

.
