An Assay To Evaluate the Anticancer Effects of Lactobacillus Cell-Free Supernatant
Abstract
Source: Lee, J., et al. Evaluating Cell Death Using Cell-Free Supernatant of Probiotics in Three-Dimensional Spheroid Cultures of Colorectal Cancer Cells. J. Vis. Exp. (2020).
This video demonstrates an in vitro assay to study the anticancer effects of Lactobacillus cell-free supernatant (LCFS). Upon exposing colorectal cancer spheroids to varying doses of LCFS, the interaction with the bacterial metabolites in LCFS induces apoptosis in the cancer cells, disrupting the spheroid structure and leading to cell death in a dose-dependent manner.
Protocol
1. Bacterial cell cultures and preparation of Lactobacillus cell-free supernatant (LCFS)
NOTE: Steps 1.2 – 1.9 are conducted in an anaerobic chamber.
- Prepare a De Man–Rogosa–Sharpe (MRS) agar plate and broth containing L-cysteine and sterilize by autoclaving.
- Pre-incubate the MRS agar plate in H2 anaerobic chamber maintained at 37 ˚C with 20 ppm oxygen.
- Thaw Lactobacillus bacterial stock and inoculate the agar plate with the bacterial culture (Figure 1A (i)).
- Incubate bacteria for 2 – 3 days in H2 anaerobic chamber at 37 ˚C and 20 ppm oxygen until single bacterial colonies are obtained.
- Wash and dry the Hungate type anerobic culture tube. Autoclave the culture tube at 121 ˚C for 15 min.
- Then incubate the tube in H2 anaerobic chamber at 37 ˚C and 20 ppm oxygen to remove oxygen.
- Place 2 – 3 mL of MRS broth into the tube. Seal the tube with a butyl rubber stopper and screw the cap.
- Obtain a single colony with a loop and place it into the 1.5 mL culture tube with 500 µL of 1x PBS. (Figure 1A (ii)).
- Suspend the colony using a 1 mL syringe (Figure 1A (iii)). Do this by, inserting the needle of the 1 mL syringe in the center of the tube lid, aspirating the suspended colony and then resuspending it back into the MRS broth media. (Figure 1A (iv)).
- Incubate the MRS broth media in a shaker incubator for 2 days (37 °C, 5% CO2, 200 rpm).
- Measure the optical density (OD) using a spectrophotometer to monitor bacterial growth curves until the absorbance at OD620 reaches to 2.0.
- Separate the bacterial pellets and the conditioned media by centrifuging at 1,000 x g for 15 min. Wash the collected bacterial pellets with 1x PBS and resuspend in 4 mL of RPMI 1640 supplemented with 10% fetal bovine serum. Do not include any antibiotics in the medium.
- Maintain the bacterial pellets in RPMI and incubate in a shaker incubator for 4 h at 37 °C with 5% CO2 at a speed of 100 rpm.
- For the preparation of the probiotic supernatant, remove the bacterial pellet via centrifugation at 1000 x g, for 15 min at 4 °C. Sterile-filter the recovered supernatant using a 0.22 μm filter and store at −80 °C until use.
2. Generation of spheroids
- Preparing colorectal cancer cell lines
- Grow DLD-1, HT-29, and WiDr cell lines as monolayers until 70-80% confluency and incubate the plate at 37 °C in a 5% CO2 incubator (Growth medium: RPMI containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin).
- For cells grown in 100 mm Petri dish, wash the plate twice with 4 mL of 1x PBS. Add 1 mL of 0.25% trypsin-EDTA and incubate the Petri dish for 2 min at 37 °C in a 5% CO2 incubator to dissociate the cells.
- After incubation, check for the cell dissociation under a microscope and neutralize trypsin-EDTA with 5 mL of growth medium.
- Transfer the dissociated cells to a 15 mL conical tube and centrifuge for 3 min at 300 x g.
- Discard the supernatant and resuspend gently with 3 mL of growth media.
- Count the cells with trypan blue to determine viable cells using a hemocytometer. (Figure 1B (i))
- Spheroid formation
- In a 15 mL conical tube, dilute the cells from 2.1.5 to obtain 1 – 2 x 105 cells/mL (Figure 1B (ii))
- Add final concentration of 0.6% methylcellulose to the cell suspension and transfer the diluted cells to a sterile reservoir.
NOTE: For each cell line, the amount of methylcellulose needed should be titrated and determined accordingly. - Use a multichannel pipette to dispense 200 µL of cells to each well of an ultra-low attachment 96-well round bottom microplate. (Figure 1B (iii))
- Incubate the plate at 37 °C in a 5% CO2 incubator for 24 – 36 h.
- After 24 – 36 h, observe the plate under a light microscope to ensure spheroid formation.
3. Treating 3D colorectal cancer cells with LCFS
- Generate spheroids as described in steps 2 and 3.
- Before performing the LCFS treatment, thaw the frozen LCFS at room temperature (RT) for 10 – 20 min.
- Inoculate the LCFS stock solution into a growth medium. Serially dilute to 25%, 12.5%, and 6% in the growth medium (i.e., 25% LCFS = 150 µL of growth medium + 50 µL of LCFS).
- Take out the cell culture plate containing spheroids from the incubator and remove as much of the growth medium as possible from each well using a 200 μL pipette.
- Add the growth media with LCFS on the cells and incubate at 37 °C in a 5% CO2 incubator for 24 – 48 h.
NOTE: The volume to be used will depend on the plate size as follows: 2 mL for 6-well cell culture plates; 200 μL for 96-well cell culture plates.
Representative Results
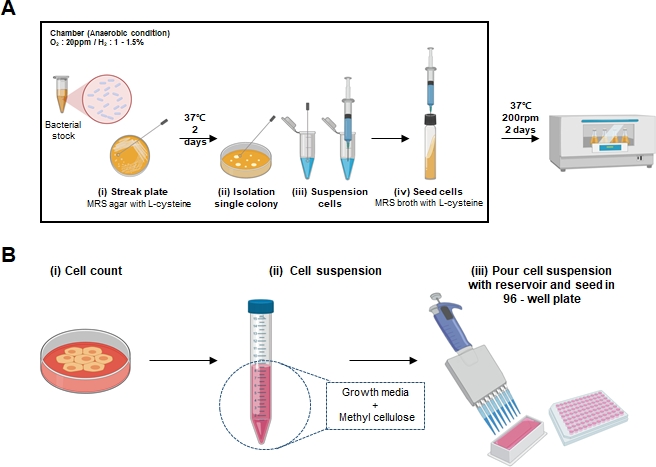
Figure 1: Schematic representation of spheroid formation and LCFS preparation.
(A) Schematic representation images of the LCFS generation protocol are marked by (i-iv) (B) Schematics of the methylcellulose-mediated spheroid formation are marked by (i-iii).
Declarações
The authors have nothing to disclose.
Materials
| Applied Biosystems MicroAmp Optical Adhesive Film | Thermo Fisher Scientific | 4311971 | 100 covers |
| Axygen 2.0 mL MaxyClear Snaplock Microcentrifuge Tube, Polypropylene, Clear, Nonsterile, 500 Tubes/Pack, 10 Packs/Case | Corning | SCT-200-C | 500 Tubes/Pack, 10 Packs/Case |
| BD Difco Bacto Agar | BD | 214010 | 500 g |
| BD Difco Lactobacilli MRS Broth | BD | DF0881-17-5 | 500 g |
| Corning Phosphate-Buffered Saline, 1X without calcium and magnesium, PH 7.4 ± 0.1 | Corning | 21-040-CV | 500 mL |
| Falcon 5 mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap | Corning | 352235 | 25/Pack, 500/Case |
| Fetal Bovine Serum, certified, US origin | Thermo Fisher Scientific | 16000044 | 500 mL |
| Lactobacillus fermentum | Korean Collection for Type Cultures | KCTC 3112 | |
| L-Cysteine hydrochloride monohydrate | Sigma-Aldrich | C6852-25G | 25 g |
| Methyl Cellulose (3500-5600mPa·s, 2% in Water at 20°C) | TCI | M0185 | 500 g |
| MicroAmp Fast Optical 96-Well Reaction Plate with Barcode, 0.1 mL | Applied Biosystems | 4346906 | 20 plates |
| Millex-GS Syringe Filter Unit, 0.22 µm, mixed cellulose esters, 33 mm, ethylene oxide sterilized | Millipore | SLGS033SB | 250 |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | 15140122 | 100 mL |
| RPMI-1640 | Gibco | 11875-119 | 500 mL |
| Trypsin-EDTA (0.25%), phenol red | Thermo Fisher Scientific | 25200056 | 100 mL |
| Materials/Equipment/Software | |||
| CO2 incubator | Thermo fisher | HERAcell 150i | |
| Conical tube 15 ml | SPL | 50015 | |
| Conical tube 50 ml | SPL | 50050 | |
| Corning Costar Ultra-Low Attachment Multiple Well Plate | Sigma-Aldrich | CLS7007 | |
| Corning Costar Ultra-Low Attachment Multiple Well Plate | Sigma-Aldrich | CLS3471 | |
| Costar 50 mL Reagent Reservoirs, 5/Bag, Sterile | Costar | 4870 | |
| Countess Cell Counting Chamber Slides | Thermofisher | C10228 | |
| Countess II FL Automated Cell Counter | invitrogen | AMQAF1000 | |
| EnSpire Multimode Reader | Perkin Elmer | Enspire 2300 | |
| Eppendorf Research Plus Multi Channel Pipette, 8-channel | Eppendorf | 3122000051 | |
| Incubated shaker | Lab companion | SIF-6000R | |
| Multi Gauge Ver. 3.0, | Fujifilm | Tokyo, Japan | |
| Optical density (OD)LAMBDA UV/Vis Spectrophotometers | Perkin Elmer | Waltham, MA, USA | |
| Phase-contrast microscope | Olympus | Tokyo, Japan | |
| SPL microcentrifuge tube 1.5mL | SPL | 60015 | |
| SPL Multi Channel Reservoirs, 12-Chs, PS, Sterile | SPL | 21012 | |
| Vibra-Cell Ultrasonic Liquid Processors | SONICS-vibra cell | VC 505 | 500 Watt ultrasonic processor |
| Vinyl Anaerobic Chamber | COY LAB PRODUCTS |
Tags

.
