Screening Assay for Oxidative Stress in a Feline Astrocyte Cell Line, G355-5
Summary
A screening method to detect oxidative cellular environments is to measure the oxidation of CM-H2DCFDA. Once oxidized within a cell, CM-H2DCFDA changes from non-fluorescent into a fluorescent compound. This change in fluorescence is measured by flow cytometry and indicates the number of cells in an oxidative environment.
Abstract
An often-suggested mechanism of virus induced neuronal damage is oxidative stress. Astrocytes have an important role in controlling oxidative stress of the Central Nervous System (CNS). Astrocytes help maintain a homeostatic environment for neurons as well as protecting neurons from Reactive Oxygen Species (ROS). CM-H2DCFDA is a cell-permeable indicator for the presence of ROS. CM-H2DCFDA enters the cell as a non-fluorescent compound, and becomes fluorescent after cellular esterases remove the acetate groups, and the compound is oxidized. The number of cells, measured by flow cytometry, that are found to be green fluorescing is an indication of the number of cells that are in an oxidative state. CM-H2DCFDA is susceptible to oxidation by a large number of different ROS. This lack of specificity, regarding which ROS can oxidize CM-H2DCFDA, makes this compound a valuable regent for use in the early stages of a pathogenesis investigation, as this assay can be used to screen for an oxidative cellular environment regardless of which oxygen radical or combination of ROS are responsible for the cellular conditions. Once it has been established that ROS are present by oxidation of CM-H2DCFDA, then additional experiments can be performed to determine which ROS or combination of ROSs are involved in the particular pathogenesis process. The results of this study demonstrate that with the addition of hydrogen peroxide an increase in CM-H2DCFDA fluoresce was detected relative to the saline controls, indicating that this assay is a valuable test for detecting an oxidative environment within G355-5 cells, a feline astrocyte cell line.
Protocol
1. Cell culture of feline astrocytes and treatment with H2O2
- Culture cell line G355-5 in a 75 cm2 flask with DMEM media plus nutrients at 37°C and 5% CO2 until 60% confluent (˜ 5 days). Replace the media every 1-2 days or as needed to maintain a healthy culture.
- Remove media and add 2 ml of 0.25% trypsin. Lift the cells by pipetting for 45 s – 1 min at room temperature (RT).
- Quickly transfer the suspension to a 15 ml conical tube containing 10 ml of media. Mix gently.
- Centrifuge at 300 x g, 23°C for 3 min to obtain a pellet. Discard the supernatant without disturbing the pellet.
- Add 6-7 ml of fresh media and resuspend the cells.
- Add ˜ 1 ml of cells to each well of a 6-well plate. Add 2-3 ml of media to each well and incubate until 80-90% confluent.
- Prepare a 100μM solution of peroxide (H2O2) in media.
- Remove media from each well and replace with either 2 ml fresh media (control), 2 ml of H2O2 (treatment) or 2ml of DPBS. Incubate at 37°C, 5% CO2 for 3 h.
- Remove solutions and add 1 ml of trypsin to lift the cells. Transfer cells to a 1.7 ml Eppendorf tube containing 0.5 ml DPBS. This step should be performed on each well individually in order to prevent cell death from prolonged trypsin incubation.
- Spin at 300 x g for 3 min, discard the supernatant and resuspend in 1 ml of DPBS.
2. Staining for ROS
- All steps for staining should be carried out with minimal light exposure to prevent bleaching.
- Add 1 μl of 50 mM CCCP to the positive control. Incubate at 37°C, 5% CO2 for 5 min.
- Add 5 μl of a 10 mM CM-H2DCFDA solution to the H2O2 group. Incubate at 37°C, 5% CO2 for 15-30 min. No stain is added to the saline sample.
- Wash samples with 2 ml of DPBS to remove residual stain.
- Spin at 300 x g for 3 min to obtain a pellet.
- Resuspend cells in 0.5 ml of DPBS. Add 1 μl PI to each sample, except the saline.
- Transfer samples to a 5ml flow cytometer tube.
- Run on the flow cytometer.
3. Flow cytometry
- Run samples on a Beckman Coulter FC500 (or equivalent) equipped with a 488 nm argon laser and the following band passes: 525, 775 and 620 nm, all ± 20 nm.
- Prepare the appropriate controls: unstained cells, CM-H2DCFDA stained only, PI only, double stained (negative control).
- Make a protocol that has the following histoplots: FS vs. SS, PI vs. CM-H2DCFDA. It should also have the following histograms: cell number vs. FL1, FL2 and FL3, which corresponds to the stains.
- Before running the samples, check equipment, fluids and waste container. Warm up the equipment for at least 20 min.
- Clean the equipment and perform a calibration with the appropriate beads according to the manufacturers standards.
- Select your protocol and run the samples.
- Clean the equipment as done previously.
- Clean the vacuum line according to the manufacturers standards.
- Turn off the flow and clean the head/vacuum line.
- Turn off the software and the computer.
4. Representative Results:
Flow cytometry results were analyzed using FlowJo 7.6 and the stain controls were used to objectively set the gating. Based on this, healthy cells appear on the left of the histogram and ROS (oxidative stress) was detected as a shift of cells to the right. Figure 1 shows the results of the effect of hydrogen peroxide on healthy feline astrocytes. Data from FL1 was used to measure the intensity of CM-H2DCFDA, which indicates the presence of ROS. As expected, a higher amount of ROS was detected in the sample treated with H2O2. This is displayed on the histogram as a shift in fluorescent intensity from left to right. When comparing healthy cells treated with DMEM versus DPBS, there was no significant change in the amount of ROS (Fig 2).
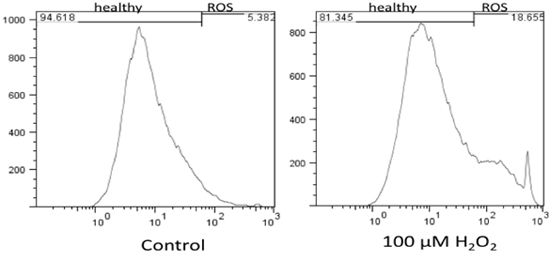
Figure 1. Flow cytometry results of levels of oxidative stress in healthy feline astrocytes and the effect of H2O2 on healthy cells. The results indicate that H2O2 increases levels of ROS in healthy cells as compared to cells with no treatment (DMEM).
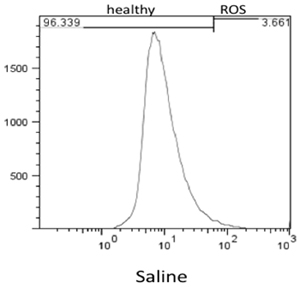
Figure 2. Flow Cytometry results of levels of ROS detected in healthy cells incubated for 3hrs with DPBS.
Discussion
An often suggested mechanism of virus induced neuronal damage is oxidative stress 1, 4, 7, 9, 13, 15, 16, 18-22, 27, 31. Basically, it is proposed that through viral exposure the glia (astrocytes and microglia) release reactive oxygen radicals such as, hydroxide, superoxide anion, nitric oxide, and/or hydrogen peroxide, which are toxic to neurons. Similarly, oxidative stress has also been suggested as a major pathologic mechanism in methamphetamine-induced neurotoxicity 2, 6, 17. Since our group is interested in the effects of both virus and drugs of abuse on the CNS, we elected to use a quick screening test to determine the present of excess ROS in astrocytes. The majority of neuronal glutathione is produced by the astrocytes and then delivered to the neurons 5, 8, 10-12, 24, 26, 29, 30. Thus, the astrocytes role in oxidative stress is important in maintaining a homeostatic environment for neurons as well as protecting neurons from ROS, and is the reason that astrocyte cell cultures were chosen for this study.
CM-H2DCFDA is also known as 2′,7′-dichlorofluorescein and H2DCF. CM-H2DCFDA is a cell-permeable indicator for presence of reactive oxygen species. CM-H2DCFDA enters the cell as a non-fluorescent compound, which becomes fluorescent after cellular esterases remove the acetate groups, and the compound becomes oxidized. Once inside the cell and the acetate groups are removed, the CM-H2DCFDA can be oxidized by a number of ROS species, including nitric oxide, peroxynitrite anions, hydrogen peroxide, and organic hydroperoxides 3, 14, 25, resulting in a green fluorescent product 23, 28. This lack of specify in the ROS, which can oxidize CM-H2DCFDA, makes it a valuable reagent in the early stages of a pathogenesis investigation, in which an oxidative stress mechanism is believed to be playing a role, but it is unknown which oxygen radical might be involved, rather than test for each ROS separately. Once it has been established that ROS are present by oxidation of CM-H2DCFDA, then additional experiments can be performed to determine, which ROS or combination of ROSs are involved in the particular pathogenesis process. The results of this present study demonstrate that with the addition of hydrogen peroxide an increase in CM-H2DCFDA fluoresce was detected when compared to the saline control, indicating that that this assay is valuable test for detecting an oxidative environment within the G355-5 cells, a feline astrocyte cell line.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank ReadiSorb Products for their generous support of these studies.
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| DMEM | Cellgro | 15-013-CV | |
| MEM Vitamins | Cellgro | 25-020-cl | 1% (v/v) |
| L-glutamine | Hyclone | 17-605E | 1% (v/v) |
| Non Essential Amino Acids | Hyclone | 25-025-cl | 1% (v/v) |
| FBS | Hyclone | SH30071.03 | 20% (v/v) |
| Essential Amino Acids | Cellgro | 25-030-cl | 0.2% (v/v) |
| 500ml vacuum filtration system | VWR | 87006-076 | |
| 15 ml conical tubes | Falcon | 352097 | |
| 75 cm2 tissue culture flasks | Falcon | 353136 | |
| 6 well tissue culture plate | Falcon | 353224 | |
| CM-H2DCFDA | Invitrogen | C6827 | |
| Propidium Iodide (PI) | Sigma | P4170-10mg | |
| Trypsin | Lonza | 17-160F | |
| H2O2 | Sigma | H6520 | |
| HyQ Antibiotic | Hyclone | SV30079.01 | 0.1% (v/v) |
| G355-5 cells | ATCC | CRL-2033 | Normal feline brain |
Referências
- Bukrinsky, M. I., Nottet, H. S., Schmidtmayerova, H. L., Dubrovsky, H., Flanagan, C. R., Mullins, M. E., Lipton, S. A., Gendelman, H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 181, 735-745 (1995).
- Cadet, J. L., Ali, S., Epstein, C. Involvement of oxygen-based radicals in methamphetamine-induced neurotoxicity: evidence from the use of CuZnSOD transgenic mice. Ann N Y Acad Sci. 738, 388-3891 (1994).
- Cathcart, R., Schwiers, E., Ames, B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 134, 111-116 (1983).
- Chao, C. C., Hu, S., Peterson, P. K. Glia: the not so innocent bystanders. J Neurovirol. 2, 234-239 (1996).
- Cooper, A. J., Kristal, B. S. Multiple roles of glutathione in the central nervous system. Biol Chem. 378, 793-802 (1997).
- Cubells, J. F., Rayport, S., Rajendran, G., Sulzer, D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 14, 2260-2271 (1994).
- Dawson, V. L., Dawson, T. M., Uhl, G. R., Snyder, S. H. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci U S A. 90, 3256-329 (1993).
- Desagher, S., Glowinski, J., Premont, J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 16, 2553-2562 (1996).
- Dewhurst, S., Gelbard, H. A., Fine, S. M. Neuropathogenesis of AIDS. Mol Med Today. 2, 16-23 (1996).
- Dringen, R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 62, 649-671 (2000).
- Dringen, R., Hamprecht, B. Glutathione restoration as indicator for cellular metabolism of astroglial cells. Dev Neurosci. 20, 401-407 (1998).
- Dringen, R., Pfeiffer, B., Hamprecht, B. Synthesis of the Antioxidant Glutathione in Neurons: Supply by Astrocytes of CysGly as Precursor for Neuronal Glutathione. J Neurosci. 19, 562-569 (1999).
- Everall, I. P. H. u. d. s. o. n., L, R. W. K. e. r. w. i. n. Decreased absolute levels of ascorbic acid and unaltered vasoactive intestinal polypeptide receptor binding in the frontal cortex in acquired immunodeficiency syndrome. Neurosci Lett. 224, 119-1122 (1997).
- Gabriel, C., Camins, A., Sureda, F. X., Aquirre, L., Escubedo, E., Pallas, M., Camarasa, J. Determination of nitric oxide generation in mammalian neurons using dichlorofluorescin diacetate and flow cytometry. J Pharmacol Toxicol Methods. 38, 93-938 (1997).
- Gendelman, H. E., Genis, P., Jett, M., Zhai, Q. H., Nottet, H. S. An experimental model system for HIV-1-induced brain injury. Adv Neuroimmunol. 4, 189-1893 (1994).
- Hayman, M., Arbuthnott, G., Harkiss, G., Brace, H., Filippi, P., Philippon, V., Thomson, D., Vigne, R., Wright, A. Neurotoxicity of peptide analogues of the transactivating protein tat from Maedi-Visna virus and human immunodeficiency virus. Neurociência. 53, 1-6 (1993).
- Hirata, H., Ladenheim, B., Rothman, R. B., Epstein, C., Cadet, J. L. Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res. , 677-6345 (1995).
- Koka, P., He, K., Zack, J. A., Kitchen, S., Peacock, W., Fried, I., Tran, T., Yashar, S. S., Merrill, J. E. Human immunodeficiency virus 1 envelope proteins induce interleukin 1, tumor necrosis factor alpha, and nitric oxide in glial cultures derived from fetal, neonatal, and adult human brain. J Exp Med. 182, 941-951 (1995).
- Kong, L. Y., Wilson, B. C., McMillian, M. K., Bing, G., Hudson, P. M., Hong, J. S. The effects of the HIV-1 envelope protein gp120 on the production of nitric oxide and proinflammatory cytokines in mixed glial cell cultures. Cell Immunol. 172, 77-83 (1996).
- Koutsilieri, E., Gotz, M. E., Sopper, S., Sauer, U., Demuth, M., ter Meulen, V., Riederer, P. Regulation of glutathione and cell toxicity following exposure to neurotropic substances and human immunodeficiency virus-1 in vitro. J Neurovirol. 3, 342-349 (1997).
- Lipton, S. A. Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: potential treatment with NMDA open-channel blockers and nitric oxide-related species. Brain Pathol. 6, 507-517 (1996).
- Lipton, S. A., Yeh, M., Dreyer, E. B. Update on current models of HIV-related neuronal injury: platelet-activating factor, arachidonic acid and nitric oxide. Adv Neuroimmunol. 4, 181-188 (1994).
- Mills, E. M., Takeda, K., Yu, Z. X., Ferrans, V., Katagiri, Y., Jiang, H., Lavigne, M. C., Leto, T. L., Guroff, G. Nerve growth factor treatment prevents the increase in superoxide produced by epidermal growth factor in PC12 cells. J Biol Chem. 273, 22165-22168 (1998).
- Peuchen, S., Duchen, M. R., Clark, J. B. Modulation of the glutathione redox state in adult astrocytes. Biochem Soc Trans. 24, 449S-449S (1996).
- Possel, H., Noack, H., Augustin, W., Keilhoff, G., Wolf, G. 2,7-Dihydrodichlorofluorescein diacetate as a fluorescent marker for peroxynitrite formation. FEBS Lett. 416, 175-178 (1997).
- Sagara, J., Makino, N., Bannai, S. Glutathione efflux from cultured astrocytes. J Neurochem. 66, 1876-1881 (1996).
- Stefano, G. B., Smith, E. M., Paemen, L. R., Hughes, T. K. HIV gp120 associated neurological deficits: a potential role for nitric oxide and other signal molecules. Advances in Neuroimmunology. 3, 47-57 (1993).
- Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K., Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 270, 296-299 (1995).
- Wang, X. F., Cynader, M. S. Astrocytes provide cysteine to neurons by releasing glutathione. J Neurochem. 74, 1434-1442 (2000).
- Wilson, J. X. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 75, 1149-1163 (1997).
- Zenger, E., Collisson, E. W., Barhoumi, R., Burghardt, R. C., Danave, I. R., Tiffany-Castiglioni, E. Laser cytometric analysis of FIV-induced injury in astroglia. Glia. 13, 92-100 (1995).

