In vivo Liver Endocytosis Followed by Purification of Liver Cells by Liver Perfusion
Summary
The study of liver sinusoidal endothelial cells (SECs) must be performed with primary cells obtained from the animal as no cell lines exist. This method relies on liver digestion and differential centrifugation for SEC purification for subsequent culturing and experimentation.
Abstract
The liver is the metabolic center of the mammalian body and serves as a filter for the blood. The basic architecture of the liver is illustrated in figure 1 in which more than 85% of the liver mass is composed of hepatocytes and the remaining 15% of the cellular mass is composed of Kupffer cells (KCs), stellate cells (HSCs), and sinusoidal endothelial cells (SECs). SECs form the blood vessel walls within the liver and contain specialized morphology called fenestrae within in the cytoplasm. Fenestration of the cytoplasm is the appearance of holes (˜100 μm) within the cells so that the SECs act as a sieve in which most chylomicrons, chylomicron remnants and macromolecules, but not cells, pass through to the hepatocytes and HSCs 1 (Fig. 1). Due to the lack of a basement membrane, the gap between the SECs and hepatocytes form the Space of Disse. HSCs occupy this space and play a prominent role in regulation and response to injury, storage of retinoic acid and immunoregulation of the liver 2.
SECs are among the most endocytically active cells of the body displaying an array of scavenger receptors on their cell surface 3. These include SR-A, Stabilin-1 and Stabilin-2. Generally, small colloidal particles less than 230 nm and macromolecules in buffer phase are taken up by SECs, whereas, large particles and cellular debris is endocytosed (phagocytosed) by KCs 4. Thus, the bulk clearance of extracellular material such as the glycosaminoglycans from blood is largely dependent on the health and endocytic functions of SECs 5,6. For example, an increase in blood hyaluronan levels is indicative of liver disease ranging from mild to more severe forms 7.
With the exception of one report 8, there are no immortalized SEC cell lines in existence. Even this immortalized cell line is de-differentiated in that it does not express scavenger receptors that are present on primary SECs (our data, not shown). All cell biological studies must be performed on primary cells obtained freshly from the animal. Unfortunately, SECs dedifferentiate under standard culture conditions and must be used within 1 or 2 days upon isolation from the animal. Differentiation of SECs is marked by the expression of Stabilin-2 or HARE receptor 9 , CD31, and the presence of cytoplasmic fenestration 1. Differentiation of SECs can be extended by the addition of VEGF in culture media or by culturing cells in hepatocyte conditioned medium 10,11.
In this report, we will demonstrate the endocytic activity of SECs in the intact organ using radio-labeled heparin for hyaluronan for the SEC-specific Stabilin-2 receptor. We will then purify hepatocytes and SECs from the perfused liver to measure endocytosis.
Protocol
1. Excision of the liver (adopted from P.O. Seglen12 and R. Blomhoff13 with modifications 14,15)
- Add 10 mL 30% isoflurane in polyethylene glycol to a petri dish in a closed 5.5 L chamber. It is optimal to wait 10-15 min after addition of isoflurane to create a stabilized atmosphere within the chamber.
- Place a rat in the sealed chamber and allow it to become anesthetized. Full effects of the anesthesia are apparent when the animal becomes limp, unresponsive, and exhibits deep breathing.
- Place 2 mL of isoflurane in polyethylene glycol in some cotton balls at the bottom of a 30 mL syringe tube.
- Place the rat on its back on a diaper-covered tray and place the syringe tube over the snout.
- Immediately confirm deep anesthesia by wetting the abdomen with 70% ethanol. Do not let the animal die by isoflurane overdose.
- Using bandage scissors and forceps, expose the entire abdominal cavity.
- Locate the vena porta and, using forceps, draw two strands of surgical silk or polyester thread (ligature) beneath the vena porta immediately above the mesenteric branch.
- Withdraw the forceps and tie a loose overhand knot.
- Using forceps underneath the vena porta and gently pulling back to straighten out the vein, cannulate the vein with an Insyte Autoguard catheter (18 GA, 1.3 x 300 mm, BD Biosciences) or similar catheter. The catheter should go several mm beyond the loop formed by the thread.
- Retract and remove the needle by releasing the spring-loaded trigger on the catheter. Blood should start seeping up into the catheter indicating proper ligature placement.
- Tighten down the overhand knot and secure it with another overhand knot.
- Sever either the inferior vena cava or descending aorta (aorta abdominalis) to allow blood to drain from the circulatory system.
- Begin flushing the liver with oxygenated TBS at 37°C to remove the blood (blanching) at a flow rate of 20 mL/min. The liver should turn from a reddish purple to a loam color.
- While the liver is flushing, excise the liver by cutting away the GI tract and connective tissue. It is important not to lacerate the liver or puncture the Glisson’s capsule.
- Place the liver on a plastic net over a funnel that allows buffers to be collected and recirculated. Buffers at this point should not be recirculated, but flow into the waste beaker. Flushing should not last more than 10 min. (Fig. 2A). Prepare the endocytosis solution required in step 2.1.
2. Internalization of 125I-SA-b-Hep (or other suitable labeled ligand)
- To 50 mL RPMI media in a small beaker, add to a final concentration 0.05% BSA and 0.01 mCi 125I-SA-b-Hep or other appropriately labeled ligand for endocytosis.
- Attach this beaker to the apparatus to allow for oxygenation.
- Turn off the pump, switch the inlet tubing, and then turn on the pump at 20 mL/min.
- As the labeled RPMI approaches the liver, turn off the pump and allow excess fluid in the funnel and tubing drain.
- Place the drain tubing into the media beaker to allow recirculation of labeled media and then restart the pump.
- Allow fluids to recirculate through the liver for no more than 1 hour.
- During this internalization step, be sure the temperature in the liver is stable by covering it and prepare the collagenase for digestion.
3. Digestion of the liver by collagenase digestion
- Freshly dissolved and filtered (0.45 μm) collagenase (100 mg/kg rat weight) should be added to Buffer 2 in a total volume not to exceed 60 mL at 37°C. The volume is dependent on the length/internal volume of the tubing and should be adjusted accordingly.
- Stop the pump and exchange the inlet tubing from the media beaker to the TBS.
- Flush the liver for 2 min at 50 mL/min which allows for loosening of the desmosomal cell junctions which are calcium dependent.
- While the liver is flushing, exchange the media beaker with the beaker containing collagenase on the apparatus.
- Immediately after flushing, begin the digestion with collagenase in a circulatory loop at a flow rate of 20 mL/min for up to 15 min. Since collagenase batches vary by lot number, variations in the amount and time of digestion need to be optimized for every new lot of collagenase. Collagenase is also calcium dependent. Turn off the pump and switch the buffer and gas lines from Flask A to Flask B. Transfer the effluent line from the waste container to Flask B (Fig. 2B).
- Stop the pump, remove the catheter and transfer the liver to a dish containing 20-30 mL Buffer 1.
- Peel back the Glisson’s capsule of the liver and shake the cells out in the liquid.
- As the liquid becomes opaque with cellular material, transfer the cells through a 100 μm mesh followed by filtration through a 30 μm mesh and then into a 50 mL conical on ice.
- Add more Buffer 1 to the liver and continue shaking cells from the liver matrix, transferring the liquid to the mesh filters and conical.
- Repeat steps 3.7 to 3.9 until no more cells can be dislodged with reasonable shaking of the liver.
4. Hepatocyte and SEC purification
- Centrifuge the 50 mL conicals containing cells at 150 x g for 3 min. to pellet the hepatocytes.
- Transfer the liquid fraction containing non-parenchymal cells to fresh 50 mL conicals on ice.
- Wash the hepatocytes be resuspending the pellet in Buffer 3 and repeating steps 4.1 and 4.2 three more times.
- At the end of the washes, the pellets are ≥97% pure hepatocytes. To obtain the SECs, centrifuge all of the tubes containing pooled supernatant (containing HSCs, SECs, and KCs and some small hepatocytes) at 200 x g for 10 min. at 4°C.
- Aspirate the buffer and resuspend all the pellets in 5 mL RPMI/BSA at 4°C.
- Pool the cells together into 2 tubes and add RPMI/BSA up to a volume of 35 mL.
- Centrifuge the conicals at 100 x g for 3 min.
- Collect the top 25 mL of liquid and transfer to a clean 50 mL conical.
- Resuspend the pellet in the remaining 10 mL media and add back 25 mL of cold RPMI/BSA and centrifuge at 100 x g for 3 min.
- Repeat steps 3.8 and 3.9 once more, discard the cell pellet and lower 10 mL media.
- Centrifuge the supernatents to pellet SECs, KCs, and HSCs at 200 x g for 10 min. at 4°C.
- Prepare three 50 mL tubes containing 20 mL 25% Percoll in PBS on ice. Underlay with 15 mL 50% Percoll in each tube.
- Resuspend the cell pellets in a total volume of 30 mL RPMI/BSA.
- Carefully overlay each gradient with 10 mL of cells and centrifuge at 900 x g for 20 min at 4°C.
- SECs and KCs have very close buoyant densities and are at the 25/50% interface. HSCs are typically at the 25%/media interface due to their higher buoyancy as lipid storing cells. Any remaining hepatocytes and blood cells have the lower buoyancies and are pelleted. Aspirate from the top down near the 25/50% interface and discard this material.
- Collect the cells in the 25/50% interface and transfer to a fresh conical with cold RPMI. The final volume should be ~40 mL to dilute out the Percoll.
- Centrifuge the conical at 350 x g for 10 min at 4°C to pellet the cells.
- Resuspend the cells in ~10 mL pre-warmed RPMI containing 100 U/mL Penicillin/100 g/mL Streptomycin and place cells in an acid-washed glass Petri dish or crystallizing dish.
- Incubate the cells for 15 min. at 37°C in a tissue culture incubator.
- Rock or swirl the plate a little and then collect the SECs in the supernatant. The KCs adhere to the glass much more rapidly than SECs. Alternatively, SECs may be separated from KCs by immunopurification using anti-CD31 or anti-Stabilin2/HARE antibodies conjugated to magnetic beads.
- Viability and cell numbers may be assessed by trypan blue exclusion using a hemacytometer or with using an automated cell counter.
- Culture the SECs on fibronectin-coated plastic dishes at 37°C, 5% CO2, RPMI/0.25% BSA with 40 ng/mL VEGF, 100 U/mL Penicillin, 100 μg/mL Streptomycin or with hepatocyte conditioned media.
- Hepatocytes, KCs, and SECs may be assess for internalization of labeled material by the use of a gamma counter and normalizing to total cellular protein or by microscopy (if fluorescently labeled) using these culturing methods.
5. Representative results:
Hepatocyte purification is ≥97% and SEC purification is typically ≥95% using this method. Excision of the abdominal cavity, cannulation of the portal vein, and blanching of the liver all should occur within a minute for best results. HARE/Stabilin-2 is a specific receptor on liver SECs and is not expressed in other liver cell types. In this sample, cell lysates of both hepatocytes and SECs were separated by 5% SDS-PAGE and probed with a monoclonal antibody against both isoforms of Stabilin-2 (Fig. 3).
Unfractionated heparin injected into the blood stream is cleared by the liver16. Clearance is primarily performed by liver SECs in contrast to hepatocytes and KCs17. The Stabilin-2/HARE receptor is the main clearance receptor that binds and internalizes heparin in SECs18. In our example here, we labeled heparin with a biotin tag on the carboxylate group of GlcNAc/NS. The biotin was conjugated with iodinated streptavidin for detection of internalized heparin proteoglycan. Injection of the labeled heparin followed by exsanguination 30 minutes post-injection allows us to monitor which organs internalize this form of heparin. In this short time interval, the liver is the primary clearance organ (Fig. 4).
To more clearly understand which cell type is responsible for clearance, we added 125I-SA-b-Hep to RPMI media supplemented with 0.05% BSA and allowed it to circulate through the liver for 20 min. Following collagenase digestion and cellular purification, the vast majority of labeled heparin is internalized by SECs in contrast to hepatocytes (Fig. 5).
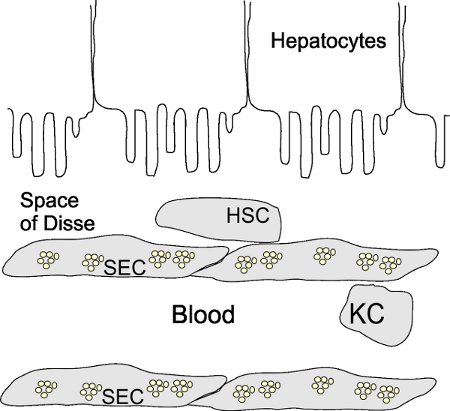
Figure 1. Basic liver architecture. SECs form the walls of the sinusoids and are normally fenestrated (small clusters of circles). Stellate cells (HSCs) are found in the Space of Disse in contrast to Kupffer cells (KCs) which are normally present in the microvasculature of the sinusoids. Hepatocytes occupy the other side of the Space of Disse.
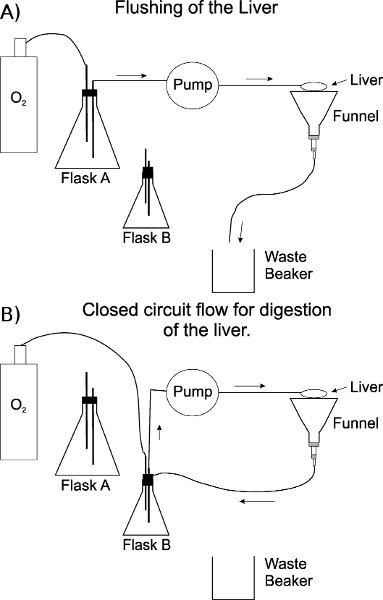
Figure 2. Schematic of the perfusion apparatus. A) Set-up of apparatus during the flushing/washing of the sinusoids of the liver. TBS is in a 1 L flask. B) A 125 mL flask containing ~60 mL Buffer 2 with collagenase is used for digestion of the liver in a closed circuit. The effluent line is also changed from the waste container to flask B through a notch cut in the rubber stopper. The oxygen line is not submerged into the buffer containing collagenase (flask B) to avoid foaming. The stoppers on each flask are notched to allow efficient transfer of the effluent line and to prevent increased pressure from the oxygen gas.
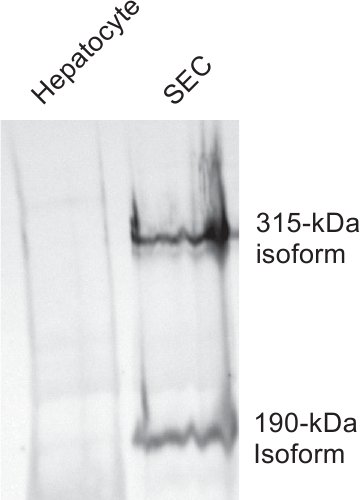
Figure 3. Hepatocyte preparations are not contaminated with SECs. Equal amounts of cell lysates from purified batches of hepatocytes (lane 1) and SECs (lane 2) were separated by 5% SDS-PAGE and blotted to nitrocellulose. Monoclonal antibody #30 which is specific against both isoforms (315 kDa and 190 kDa) of HARE/Stabilin-2 was used to probe the lysates.
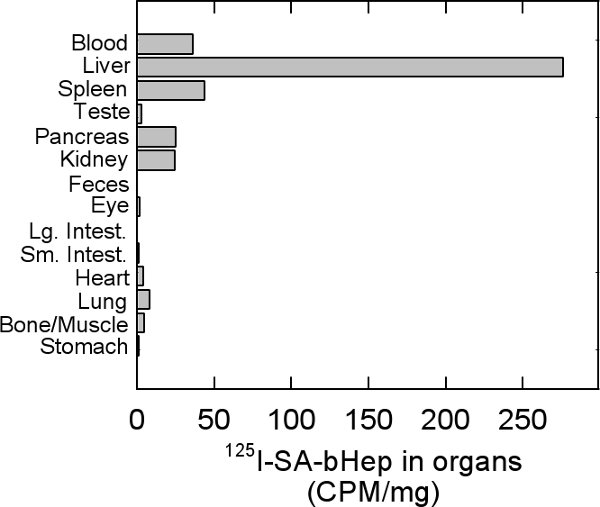
Figure 4. Distribution of labeled heparin in organs. Rats were injected via the lateral tail vein with 125I-SA-b-hep wait for 30 minutes and then exsanguinated. Blood was collected so as to not to give any organs artificially high readings. Counts per minute (CPM) of each organ were normalized against the weight of the organ in grams.
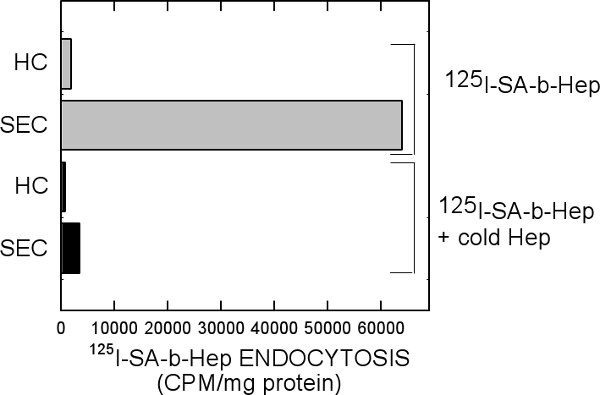
Figure 5. Amount of labeled heparin in hepatocytes and SECs. RPMI media containing 125I-SA-b-Hep was allowed to circulate through an intact liver for 20 min followed by collagenase digestion and cellular purification. Equal amounts of hepatocyte and SEC cell protein lysates were quantified with the Bradford Assay and counted by a gamma counter.
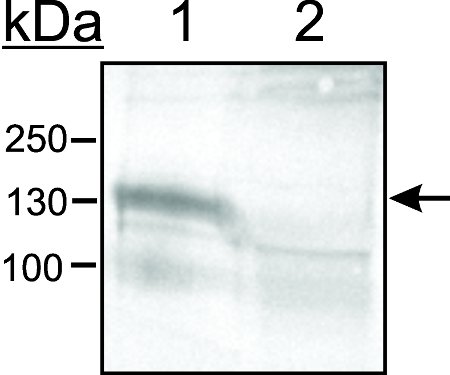
Figure 6. Kupffer cells are separated from SECs by adhesion on glass. Cells were placed in an acid-washed glass crystallizing dish and allowed to adhere for 15 min at 37°C, 5% CO2. The supernatent containing non-adhered cells was gently swirled and placed in a centrifuge tube. Lysates from both adhered cells on glass (lane 1) and from non-adhered cells (lane 2) were separated by 8% SDS-PAGE, blotted, and probed with an antibody against CD163 which is specific for Kupffer cells.
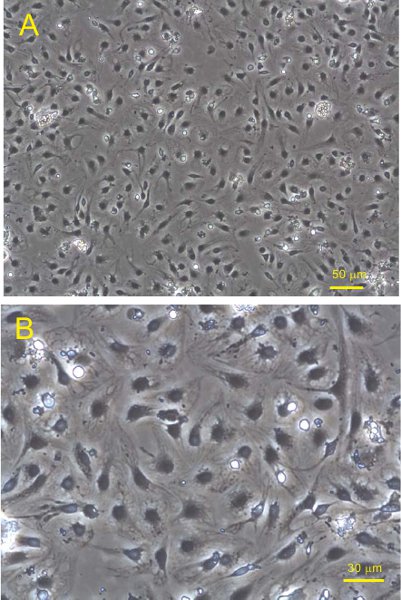
Figure 7. SECs plated on fibronectin coated plastic 6-well dishes were incubated overnight in RPMI supplemented with 0.1% BSA. Phase contrast images were taken at (A) 100x and (B) 200x magnification.
Discussion
Anesthesia of the rat by the hanging drop method in which isoflurane vapor induces unconsciousness is the preferred method for our studies. A vaporizer may also be used instead of a syringe with cotton to maintain the animal outside the chamber, but the surgical procedures are so quick, it is not required. Polyethylene glycol is added to the isoflurane to decrease the vapor pressure and evaporation rate. Too much isoflurane vapor will induce death too quickly. If the animal dies before the liver is cannulated and blanched with TBS, pooling blood may clot in the liver and prevent efficient washing of the sinusoids and digestion with collagenase. The addition of heparin may be useful as an anticoagulant but is not used in these studies since we use labeled heparin as our probe.
Gey’s buffered saline solution (GBSS) is often substituted by other laboratories for the purification of SECs. While it is useful for SEC purification, hepatocytes do not tolerate GBSS as well as Buffers 1-3 and viabilities are not nearly as high. When measuring endocytosis, it is good practice to measure background levels in the organ and in purified hepatocytes.
This method is one of two for efficient purification of SECs following the liver perfusion with collagenase. The other method separates the non-parenchymal cells by elutriation centrifugation 19 instead of Percoll gradient. The advantages for using elutriation over Percoll gradients are slightly higher cell viabilities and numbers. The downside is that elutriation centrifugation requires a specialized rotor, dedicated centrifuge, and peristaltic pump together which costs tens of thousands of dollars. This method only requires the use of a refrigerated table top centrifuge and peristaltic pump which is standard in many laboratories.
Separation of SECs and KCs by selective adhesion is a standard method20-22. While it is true that SECs will adhere to the glass and plastic, the key point is to use acid-washed clean glass with no more than a 15 minute incubation at 37°C at 5% CO2. KCs will always adhere faster than SECs and it is advisable to gently wash the KCs with media to extract any remaining SECs that have become loosely attached. Pronase and other proteases are also omitted from the collagenase digestion steps in this protocol to increase viabilities of the cells. Proteases digest extracellular receptors and may inhibit cellular adhesion in the final purification steps.
The method presented here has a wide variety of applications. Although we show the end result in which heparin is initially taken up for clearance, perfusion of the liver for obtaining primary hepatocytes, KC, SECs, and HSCs may be useful for a number of studies involving metabolic pathways, immunoregulation, scavenger activities, and other physiological studies. The most difficult part of this procedure is good cannulation, digestion of the liver, and handling of the liver without having the catheter slip from the portal vein.
Declarações
The authors have nothing to disclose.
Acknowledgements
We would like to thank Dr. Paul Weigel at the Univ. of Oklahoma for the use of monoclonal antibody 30 for detection of the HARE/Stabilin-2 receptor and Janet Weigel for her technical assistance. This research is funded by University of Nebraska Research Funds.
Materials
- Buffer 1: 142 mM NaCl 6.7 mM KCl, 10 mM HEPES, 1.5% BSA, pH 7.4
- Buffer 2 (perfusion buffer): 67.0 mM NaCl, 6.7 mM KCl, 4.8 mM CaCl2-H2O, 101.0 mM HEPES, BSA 1.5%, pH 7.4.
- Buffer 3: 137 mM NaCl, 4.7 mM KCl, 0.7 mM MgSO4, 1.2 mM CaCl2-2H2O, 10 mM HEPES, 1.5% BSA, pH 7.4
- RPMI/BSA: 15g/L BSA in RPMI
- TRIS-buffered Saline (TBS): 154 mM NaCl, 10 mM Trizma Base salt.
- Collagenase: Collagenase A (#11088785103) from Roche, Collagenase Type I (#LS004691) from Worthington Biochemical or Collagenase I (#C0130) or IA (#C9891) from Sigma-Aldrich.
All salts are from Sigma-Aldrich
| Name | Company | Model/catalog # | Comment |
| 20L water bath | ThermoFisher | 2231 | Water is about 45°C to compensate for cooling within the tubing. |
| Peristaltic Pump | Cole-Parmer | 7553-70 | Masterflex series |
| Refrigerated Centrifuge | Sorvall | Legend XTR | |
| Catheter | BD Biosciences | 381444 | |
| Dessicator chamber | Fisher | 08595E | Use internal plate |
| Percoll | Sigma | P4937 | |
| Bovine Serum Albumin | SeraCare | AP-4510-01 | |
| 100 μm mesh | Spectrum labs | 146488 | |
| 30 μm mesh | Spectrum labs | 146506 | |
| RPMI 1640 | Invitrogen | 21870 | |
| Polyethylene glycol | Spectrum labs | PO107 |
Referências
- Elvevold, K., Smedsrod, B., Martinez, I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am. J. Physiol. Gastrointest. Liver. Physiol. 294, G391-G400 (2008).
- Friedman, S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125-172 (2008).
- Smedsrod, B., Pertoft, H., Gustafson, S., Laurent, T. C. Scavenger functions of the liver endothelial cell. Biochem. J. 266, 313-327 (1990).
- Shiratori, Y. Quantification of sinusoidal cell function in vivo. Semin. Liver. Dis. 13, 39-49 (1993).
- Smedsrod, B., Pertoft, H., Eriksson, S., Fraser, J. R., Laurent, T. C. Studies in vitro on the uptake and degradation of sodium hyaluronate in rat liver endothelial cells. Biochem. J. 223, 617-626 (1984).
- Harris, E. N., Kyosseva, S. V., Weigel, J. A., Weigel, P. H. Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa hyaluronic acid receptor for endocytosis (HARE). J. Biol. Chem. 282, 2785-2797 (2007).
- Alkhouri, N. A Combination of the Pediatric NAFLD Fibrosis Index and Enhanced Liver Fibrosis Test Identifies Children with Fibrosis. Clin. Gastroenterol. Hepatol. , (2010).
- Huebert, R. C. Immortalized liver endothelial cells: a cell culture model for studies of motility and angiogenesis. Lab. Invest. 90, 1770-1781 (2010).
- Zhou, B., Weigel, J. A., Fauss, L., Weigel, P. H. Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 275, [pii]- (2000).
- Krause, P. Hepatocyte-supported serum-free culture of rat liver sinusoidal endothelial cells. J. Hepatol. 32, 718-726 (2000).
- Esser, S. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell. Biol. 140, 947-959 (1998).
- Seglen, P. O. Preparation of isolated rat liver cells. Methods. Cell. Biol. 13, 29-83 (1976).
- Blomhoff, R., Berg, T. Isolation and cultivation of rat liver stellate cells. Methods. Enzymol. 190, 58-71 (1990).
- Harris, E. N., Baggenstoss, B. A., Weigel, P. H. Rat and human HARE/stabilin-2 are clearance receptors for high- and low-molecular-weight heparins. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1191-G1199 (2009).
- Karaa, A., Kamoun, W. S., Clemens, M. G. Oxidative stress disrupts nitric oxide synthase activation in liver endothelial cells. Free. Radic. Biol. Med. 39, 1320-1331 (2005).
- Gustafson, S., Bjorkman, T. Circulating hyaluronan, chondroitin sulphate and dextran sulphate bind to a liver receptor that does not recognize heparin. Glycoconj. J. 14, 561-568 (1997).
- Oie, C. I., Olsen, R., Smedsrod, B., Hansen, J. B. Liver sinusoidal endothelial cells are the principal site for elimination of unfractionated heparin from the circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 294, 520-528 (2008).
- Harris, E. N., Weigel, J. A., Weigel, P. H. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 283, 17341-17350 (2008).
- Knook, D. L., Sleyster, E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp. Cell. Res. 99, 444-449 (1976).
- Graupera, M. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am. J. Physiol. Gastrointest. Liver Physiol. 288, 763-770 (2005).
- Yannariello-Brown, J., Zhou, B., Ritchie, D., Oka, J. A., Weigel, P. H. A novel ligand blot assay detects different hyaluronan-binding proteins in rat liver hepatocytes and sinusoidal endothelial cells. Biochem. Biophys. Res. Commun. 218, 314-319 (1996).
- Duryee, M. J. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin. Exp. Res. 28, 1931-1938 (2004).

