Lectin-based Isolation and Culture of Mouse Embryonic Motoneurons
Summary
An alternative way of isolating mouse embryonic motoneurons from the spinal cord is described. The method takes into account the fact that lectin can bind to the low affinity nerve growth factor receptor p75NTR. This lectin-based preplating allows a purification similar to that with a specific antibody against the p75NTR.
Abstract
Spinal motoneurons develop towards postmitotic stages through early embryonic nervous system development and subsequently grow out dendrites and axons. Neuroepithelial cells of the neural tube that express Nkx6.1 are the unique precursor cells for spinal motoneurons1. Though postmitotic motoneurons move towards their final position and organize themselves into columns along the spinal tract2,3. More than 90% of all these differentiated and positioned motoneurons express the transcription factors Islet 1/2. They innervate the muscles of the limbs as well as those of the body and the inner organs. Among others, motoneurons typically express the high affinity receptors for brain derived neurotrophic factor (BDNF) and Neurotrophin-3 (NT-3), the tropomyosin-related kinase B and C (TrkB, TrkC). They do not express the tropomyosin-related kinase A (TrkA)4. Beside the two high affinity receptors, motoneurons do express the low affinity neurotrophin receptor p75NTR. The p75NTR can bind all neurotrophins with similar but lower affinity to all neurotrophins than the high affinity receptors would bind the mature neurotrophins. Within the embryonic spinal cord, the p75NTR is exclusively expressed by the spinal motoneurons5. This has been used to develop motoneuron isolation techniques to purify the cells from the vast majority of surrounding cells6. Isolating motoneurons with the help of specific antibodies (panning) against the extracellular domains of p75NTR has turned out to be an expensive method as the amount of antibody used for a single experiment is high due to the size of the plate used for panning. A much more economical alternative is the use of lectin. Lectin has been shown to specifically bind to p75NTR as well7. The following method describes an alternative technique using wheat germ agglutinin for a preplating procedure instead of the p75NTR antibody. The lectin is an extremely inexpensive alternative to the p75NTR antibody and the purification grades using lectin are comparable to that of the p75NTR antibody. Motoneurons from the embryonic spinal cord can be isolated by this method, survive and grow out neurites.
Protocol
For special reagents: see Tab. 1. All other reagents listed are in cell culture grade quality obtained from Sigma Aldrich.
1. PORN-H/laminin coating of dishes/coverslips
- Prepare working solution of 0.5 mg/ml Poly-DL-ornithine hydrobromide (PORN-H) in 150 mM borate buffer pH 8.35.
- A stock solution of 50 mg PORN/1 ml borate buffer can be prepared in advance and stored at -20°C.
- Sterilize glass coverslips by flaming in 100% ethanol and let them air-dry.
- Cover the surface with sufficient PORN-H solution overnight at 4°C.
- On the next day wash three times with sterilized water and air-dry coverslips.
- Prepare working solution of 2.5 μg/ml laminin in HBSS
- Aliquots can be stored at -20°C. Thaw at 4°C immediately before use.
- Cover the surface of the PORN-H-coated coverslips with the laminin solution and let them incubate for at least 2 h at room temperature until use.
2. Lectin coating of the purification plate
- Prepare a solution of 10 mM Tris in sterilized water, pH 9.5.
- Add lectin (resolve the lectin (Sigma L9640) powder in HBSS) to a final concentration of 10 μg/ml.
- Coat the surface of a 10 cm cell culture dish with 8 ml of the lectin solution and let it incubate for at least 30 min at room temperature.
- Wash the plate three times with HBSS and store in HBSS until use.
- Prepare a 30mM KCl, 0.8% (w/v) NaCl depolarisation-solution. Sterilize by filtration and store at room temperature (RT).
3. Motoneuron culture medium
- Thaw horse serum overnight at 4°C and inactivate at 55°C for 30 min, Aliquot into 5 ml and store at -20°C. Thaw aliquot directly before use at RT.
- Store B27-supplement in 1 ml aliqouts at -20°C. Thaw B27 supplement at RT immediately before use. Avoid freeze and thaw cycles.
- Aliquot glutamax into 0.5 ml aliquots and store at -20°C and use it at 1:100.
- Prepare a stock solution of 10 μg/ml CNTF in sterilized water with 0.1% BSA. Store at -20°C.
- Mix 46 ml neurobasal medium with 2.5 ml horse serum, 1 ml B27-supplement and 0.5 ml glutamax directly before use.
- Add CNTF to a final concentration of 10 ng/ml medium and pre-warm medium to 37°C.
4. Spinal cord dissection
- Sacrifice an E12.5 pregnant mouse and remove the embryos carefully. Add enough RT HBSS to completely cover the embryos.
- Remove the head and tail and place the embryo back side up with straddled limbs.
- Fix the embryo with one forceps and remove the outer skin with the other forceps.
- Remove the spinal cord by piercing the forceps under it and lift it up with saw-like movements on both sides of the spinal cord.
- Transfer the isolated spinal cord to a new dish with HBSS and open the central channel of the spinal cord on the dorsal side.
- Remove the dorsal root ganglia by removing the enclosing meninges of the spinal cord.
- Collect the lumbar parts of the spinal cords of up to 6 embryos per lectin plate in an eppendorf reaction tube filled with 1 ml HBSS and store on ice until the preparation is done.
5. Enrichment of motoneurons by lectin-based purification
Note: Before starting the next steps, latest here prepare and prewarm the medium (see step 3.)
- Prepare a trypsin solution by solving 1 g Trypsin in 100 ml HBSS, store at -20°C and thaw at 4°C.
- Mix 1g trypsin-inhibitor with 98 ml HBSS and 2 ml of 1 M HEPES pH 7.4, 1 ml aliquots should be stored at 4°C.
- Transfer the tube with the spinal cords to a cell culture hood and carefully remove 700 μl of the HBSS.
- Add 7.5 μl trypsin solution and mix by carefully inverting the tube.
- Perform trypsination for 8 min at 37°C.
- Stop the reaction by adding 30 μl trypsin inhibitor and pipette up and down (triturate) carefully 10-15 times with a 1000 μl pipette tip until no more cell aggregates are visible. Repeat the trituration with a 20-200 μl pipette and the corresponding tip.
- Pipette the cell solution into the HBSS-filled lectin plate and disperse the cells by gently rotating the plate.
- Keep the lectin plate for 60 min at RT on a vibration-free surface. Cover it to avoid contamination.
- Remove the HBSS very gently and wash the plate carefully 4 times with pre-warmed HBSS to remove cell fragments and the not attached/unattached cells.
- Immediately after the last washing step, add 500 μl depolarisation solution to the plate and let it incubate for 1min.
- Facilitate the detachment of the lectin bound cells by shaking and tapping the plate.
- Fill up the plate with 2 ml pre-warmed culture medium and transfer to a 15 ml falcon tube.
- Count cell number with a neubauer counting chamber.
- Plate the cell on the laminin coated coverslips or plates in an appropriate number depending on the application.
- Perform a medium exchange on day 1 and subsequently every second day by careful replacement of 50% of the old medium. Always use pre-warmed, freshly prepared new culture medium.
6. Representative results:
Embryonic motoneurons can be obtained from mouse embryos at E12 to E14. 2-3% of the total cell population of the lumbar spinal cord consists of motoneurons and as the dorsal root ganglionic neurons are p75NTR-positive as well, it is important to get rid of these cells before starting the lectin-based preplating procedure (For overview see Figure 1 and Figure 2). Secondly, the meninges include strongly dividing cells that should be excluded as well, as they cannot be properly removed by the lectin-based preplating procedure (Figure 2 and Figure 3). In worse cases, these cells can overgrow the culture plates. The representative pictures in Figure 3B and 3D also give an impression about the lower cell density after washing procedures have been performed (see step 5.9 of the protocol). Cultures of embryonic mouse motoneurons are usually maintained for up to five or seven days. During this time period the cells establish their neurites up to a maximum length (Figure 4). Neurite lengths strongly depend on the substrate on the plates8. The typical substrate is laminin which is coated on PORN-H covered culture dishes. Motoneurons can also grow out on other or less defined substrates, like extracellular matrices generated by water lysis8. In cases of suboptimal coating, neurite length and growth cone area are decreased and cell death usually increases. Trophic support plays a crucial role for survival of embryonic mouse motoneurons in culture. Survival of initially plated cells on day seven drop to 10% to 20% without trophic support6. Brain derived neurotrophic factor (BDNF) and Ciliary neurotrophic factor (CNTF) are the most commonly used factors but others can promote survival as well [Leukemia inhibitory factor (LIF), Cardiotrophin-1 (CT-1), basic fibroblast growth factor (bFGF), Insulin-like growth factor 1 (IGF-1)9].
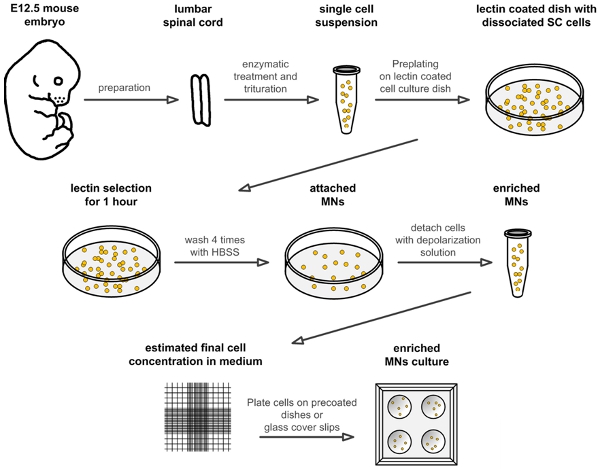
Figure 1. Flow scheme for the preparation of embryonic mouse motoneurons. The schematic drawing illustrates the different steps for isolation and culture of mouse embryonic motoneurons. Abbreviations: SC: spinal cord; HBSS: Hanks balanced salt solution; MN: motoneuron.
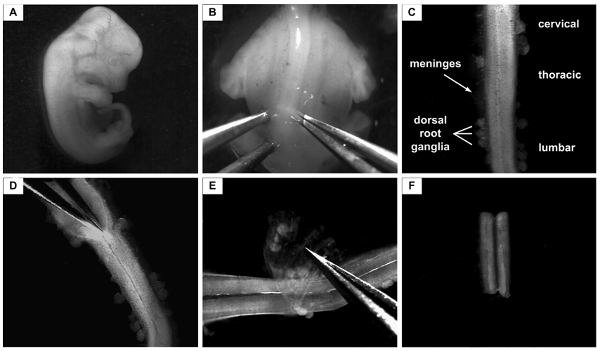
Figure 2. Isolation of the lumbar part of the spinal cord from E12.5 mouse. A: E12.5 mouse embryo. For the next step, the head and the tail are removed and the body is placed in a dorsal-up position B: Embryo in a dorsal-up position. Forceps at the left and right of the spinal cord are fixing the embryo body in its position. One of the forceps is subsequently used to cut the skin and to cut underneath the spinal cord the other is used to fix the embryo in its position. C: Isolated spinal cord from E12.5 mouse embryo. The parts of the spinal cord (cervical, thoracic, lumbar) are indicated. The spinal cord is surrounded by the meninges and part of the dorsal root ganglia still attach to them. D: The spinal cord is longitudinally cut on the dorsal side to open it towards the central canal. E: The meninges on the ventral side are now taken off from the flattened spinal cord. Note: While taken off, the lumbar part is marked by a small cut. F: Only the lumbar part of the spinal cord is then taken for further procedures.
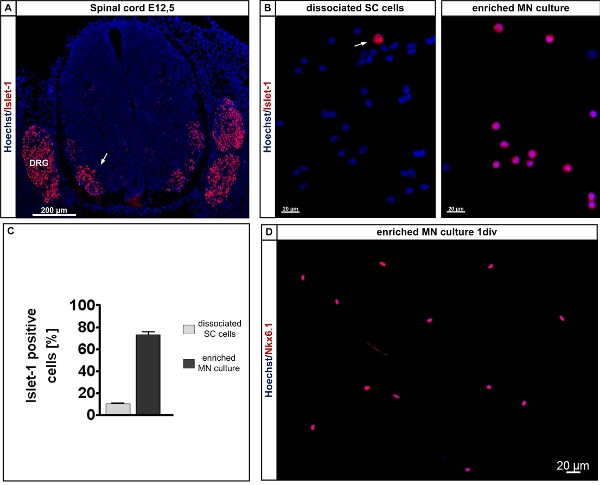
Figure 3. Determination and isolation of Islet-positive cells from the lumbar spinal cord of E12.5 mouse embryos. A: The Islet-1/2 antibody labels motoneuron columns within the embryonic lumbar spinal cord (red, arrow) and Hoechst labels all nuclei within the section. Cross section of an E12.5 lumar spinal cord. E12.5 mouse embryos were immersion-fixed in 4% paraformaldehyde for 6 h, washed 3 times with phosphate-buffered saline and treated with sucrose according to standard procedures. Cryosections of 20 μm were taken with a cryostat and the sections were handled for immunhistochemical staining according to standard procedures11. The sections ware subsequently labeled with Hoechst for localization of the cell nuclei. Note that the dorsal root ganglionic neurons are Islet-1/2-positive as well and that the preparation procedure excludes this tissue parts from the isolation procedure. B: Islet-1/2 labeled (red, arrow) dissociated lumbar spinal cord cells from E12.5 embryos, left – before and right – after lectin-based preplating. The cells were counterstained with Hoechst to visualize all cell nuclei. C: Quantification of Islet-1/2-positive cells before and after lectin-based preplating. D: Nkx6.1 labeled motoneurons from E12.5 embryos after one day in culture. The cells were counterstained with Hoechst to cisualieze all nuclei. Note that 90% of all cells are Nkx6.1 positive. Abbreviations: SC: spinal cord; MN: motoneuron; DRG: dorsal root ganglion.
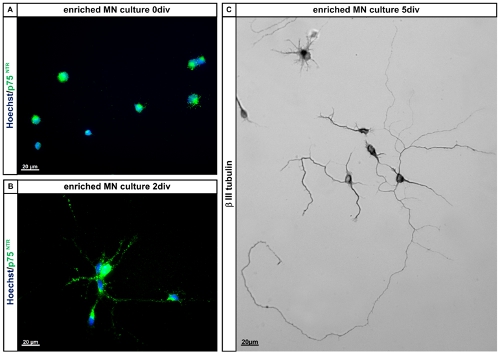
Figure 4. Characterisation of E12.5 mouse motoneurons. A and B: Enriched isolated E12.5 mouse lumbar spinal motoneurons express p75NTR at 0 days in vitro (0div) and on day 2 in vitro (2div). Cells were fixed with 4% paraformaldehyde and subsequently stained for p75NTR according to standard procedures. Cells were counterstained using Hoechst to visualize all nuclei. C: Enriched motoneurons after 5 days in vitro (5div) on PORN-H and laminin as culture substrates and in the presence of CNTF stained for β-III-tubulin. Note that the cells have grown out long neurites and display the typical motoneuron morphology with one longer (branched) process and one or more shorter processes (axons and dendrites). Abbreviations: MN: motoneuron; div: days in vitro.
| After dissociation without Lectin-based preplating | |||
| Total number of cells/SC | Trypan blue-positive cells [%] | Islet-1/2- positive cells [%] | |
| 1 | 995.0 | 13,1 | 9,5 |
| 2 | 889.4 | 8,0 | 10,0 |
| 3 | 1.180.0 | 11,0 | 10,8 |
| Mean ± SD | 1.021.0 ± 147.1 | 10,7 ± 2,6 | 10,1 ± 0,7 |
| With lectin-based preplating | |||
| Number of cells after preplating/SC | Trypan blue-positive cells [%] | Islet-1/2- positive cells [%] | |
| 1 | 189.6 | 9,9 | 70,5 |
| 2 | 168.8 | 3,7 | 76,1 |
| 3 | 125.0 | 0,0 | 72,5 |
| Mean ± SD | 161.1 ± 33.0 | 4,5 ± 5,0 | 73,0 ± 2,8 |
Table 1. Summary of representative isolation procedures for mouse embryonic motoneurons from stage E12.5. Results from 3 different isolation procedures especially for this purpose are given as total numbers or percentage numbers ± SD. Abbreviation: SC: spinal cord; SD: standard deviation.
| Islet-1/2 positive cells with p75 panning [%] | Islet-1/2 positive cells with Lectin-based panning [%] |
| 92,0 ± 3,5 1 | 73,0 ± 2,8 |
Table 2. Comparison of Lectin-based and p75-based1 panning motoneuron cell numbers. Results are given as percentage numbers ± SD.
Discussion
The advantage of this lectin-based preplating technique is that it is less expensive than the p75NTR-based panning procedure, and the lectin is more stable than antibodies. The enrichment listed in Fig. 2 and Tab. 1 shows that the procedure allows to the purification of similar numbers of cells and that a majority of these cells express the motoneuron marker Islet-1/2. The most critical step is the isolation procedure for the lumbar spinal cord. Removing the meninges and the DRGs (Fig. 2) is essential for the following purification procedure by lectin-based preplating. If this has been managed correctly, almost all cells express the p75NTR (for representative picture see Fig. 3a). The reason for the difference between expression of Islet-1/2 and p75NTR is most probably because lumbar motoneurons differentially express higher and lower levels of Islet-1/210. In case of low levels of Islet-1/2 expression this might have escaped our attention in the immuncytochemical staining and subsequent counting as we were stringent with respect to positive versus negative cells (Tab. 1). Additionally, Table 1 clearly shows that the isolated cells survive the procedure in a healthy condition, as there are only few trypan-blue positive cells that indicate that the cells are irreversibly damaged. This alterative procedure also allows isolation of motoneurons from single embryos and therefore of mixed genotype litters from embryonic mice. In conclusion, this alternative to the panning procedure with the p75NTR antibody6 has similar if not identical capacities in terms of possible application ranges and provides a cheap and efficient alternative purification method for mouse embryonic motoneurons.
Declarações
The authors have nothing to disclose.
Acknowledgements
We thank Sandra Bargen for excellent technical support. This work was supported by the Protein research department (PRD, TP A1.2 (R.C. and T.S.), and the RUB Research Fond, Rektoratsprogramme – wissenschaftlicher Nachwuchs (A.K.). The monoclonal antibodies 39.4D5 and F55A10 were obtained from the Developmental Studies Hybridoma Bank (DSHB, Iowa city, IA).
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| Poly-DL-ornithine hydrobromide | Sigma | P8638 | |
| Laminin | Invitrogen | 23017-015 | |
| HBSS | Gibco | 14170 | |
| Glass coverslips | Thermo | Ø 10 or 14mm | |
| Lectin | Sigma | L5142 | |
| Cell culture dish | Nunc | 150350 | Nunclon delta surface |
| Horse serum | Linaris | SHD3250ZK | Each batch has to be tested for MN culture |
| B27 Supplement | Gibco | 17504-044 | |
| Glutamax | Gibco | 35050-038 | |
| CNTF | Sigma | N0513 | |
| BSA | Applichem | A1391 | |
| Neurobasal | Gibco | 21103-041 | |
| Forceps | Stainless steel, size 4 or 5 | ||
| Trypsin | Worthington | LS003707 | |
| Trypsin-Inhibitor | Sigma | T6522 | |
| Anti-Islet-1 | DSHB | 39.4D5 | Cell culture supernatant |
| Anti-Nkx6.1 | DSHB | F55A10 | Cell culture supernatant |
| Anti-p75NTR | Abcam | Ab8874 |
Table 3. Table of specific reagents and equipment.
Referências
- Vallstedt, A. Different levels of repressor activity assign redundant and specific roles to Nkx6 genes in motor neuron and interneuron specification. Neuron. 31, 743-755 (2001).
- Tsuchida, T. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 79, 957-970 (1994).
- Lin, J. H. Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell. 95, 393-407 (1998).
- Wiese, S., Beck, M., Karch, C., Sendtner, M. Signalling mechanisms for survival of lesioned motoneurons. Acta Neurochir. , 21-35 (2004).
- Gavins, F. N., Chatterjee, B. E. Intravital microscopy for the study of mouse microcirculation in anti-inflammatory drug research: focus on the mesentery and cremaster preparations. J. Pharmacol. Toxicol. Methods. 49, 1-14 (2004).
- Bullen, A. Microscopic imaging techniques for drug discovery. Nat. Rev. Drug Discov. 7, 54-67 (2008).
- Hazelwood, K. L. Entering the Portal: Understanding the Digital Image Recorded Through a Microscope. Imaging Cellular and Molecular Biological Functions. , 3-43 (2007).
- Hickey, M. J. L-selectin facilitates emigration and extravascular locomotion of leukocytes during acute inflammatory responses in vivo. J. Immunol. 165, 7164-7170 (2000).
- Cara, D. C., Kubes, P. Intravital microscopy as a tool for studying recruitment and chemotaxis. Methods Mol. Biol. 239, 123-132 (2004).
- Liu, L. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J. Exp. Med. 201, 409-418 (2005).
- Heit, B. PI3K accelerates, but is not required for, neutrophil chemotaxis to fMLP. J. Cell Sci. 121, 205-214 (2008).

