Recording and Analysis of Circadian Rhythms in Running-wheel Activity in Rodents
Summary
Circadian rhythms in voluntary wheel-running activity in mammals are tightly coupled to the molecular oscillations of a master clock in the brain. As such, these daily rhythms in behavior can be used to study the influence of genetic, pharmacological, and environmental factors on the functioning of this circadian clock.
Abstract
When rodents have free access to a running wheel in their home cage, voluntary use of this wheel will depend on the time of day1-5. Nocturnal rodents, including rats, hamsters, and mice, are active during the night and relatively inactive during the day. Many other behavioral and physiological measures also exhibit daily rhythms, but in rodents, running-wheel activity serves as a particularly reliable and convenient measure of the output of the master circadian clock, the suprachiasmatic nucleus (SCN) of the hypothalamus. In general, through a process called entrainment, the daily pattern of running-wheel activity will naturally align with the environmental light-dark cycle (LD cycle; e.g. 12 hr-light:12 hr-dark). However circadian rhythms are endogenously generated patterns in behavior that exhibit a ~24 hr period, and persist in constant darkness. Thus, in the absence of an LD cycle, the recording and analysis of running-wheel activity can be used to determine the subjective time-of-day. Because these rhythms are directed by the circadian clock the subjective time-of-day is referred to as the circadian time (CT). In contrast, when an LD cycle is present, the time-of-day that is determined by the environmental LD cycle is called the zeitgeber time (ZT).
Although circadian rhythms in running-wheel activity are typically linked to the SCN clock6-8, circadian oscillators in many other regions of the brain and body9-14 could also be involved in the regulation of daily activity rhythms. For instance, daily rhythms in food-anticipatory activity do not require the SCN15,16 and instead, are correlated with changes in the activity of extra-SCN oscillators17-20. Thus, running-wheel activity recordings can provide important behavioral information not only about the output of the master SCN clock, but also on the activity of extra-SCN oscillators. Below we describe the equipment and methods used to record, analyze and display circadian locomotor activity rhythms in laboratory rodents.
Protocol
1. Animal Housing
- Cage: In order to record the running-wheel activity of an individual rodent, each cage should house a single rodent and running-wheel. Because running wheels can be considered a form of enrichment, all rodents in any study should have similar access to a running wheel.
- Bedding changes: Animal handling as well as changes in cages or bedding can all have non-photic effects on circadian rhythms21-23, so, cages with mesh-flooring are ideal because they minimize contact with the animal. Notwithstanding the availability of such a tray-system, bedding changes should be avoided during critical phases of an experiment. Alternatives include using longer-lasting bedding, which would allow for more infrequent cage changes, or changing cages on a pseudo-random schedule.
- Isolation Boxes: Cages should be kept in isolation boxes that are sound-attenuated, light-controlled, and well-ventilated. Depending on the size and configuration of the isolation boxes, the number of cages within each box will typically range from 1-8. When housing multiple cages in a single isolation box, one should be aware that various odors and sounds coming from other animals can have confounding effects on the circadian behavior of individual animals. To avoid these problems, one should attempt to house one cage per isolation box.
- Ventilation: Adequate air flow is imperative to making the boxes a comfortable home environment for rodents. The fan in each box should be hooded, so as to prevent light from outside the box from reaching the inside. Also, fans will typically remove air from the box and blow it into the room. Small light-tight vents allow air to enter the isolation boxes from several points, and help to avoid uncomfortable breezes. In order to verify that there is adequate ventilation, the temperature inside the isolation box (when closed for several hours, with the lights on) should be virtually identical to the temperature in the room where it is housed.
- Lighting: Environmental light intensity should be the same in all cages. Arrange a single light at a similar location above each cage, and always use the same brand/type of bulb. Use moderate intensity illumination (100-300 lux) at cage level. Avoid excessively high illumination levels, which are more likely to produce direct changes on behavior attributable to the light rather than the circadian system, per se (e.g. masking).
- Darkness/Dim red lighting: If it is necessary to handle or treat animals in the dark (e.g. in constant darkness or nighttime), night vision goggles should be used. Alternatively, because the circadian system is relatively insensitive to red-wavelengths, dim red lighting can be used. The specific red light you use should be tested to ensure it does not alter running-wheel activity (e.g. masking) or adjust the circadian clock (e.g. produce a phase shift).
2. Data Collection (See Figure 1 – Vitalview Hardware Configuration)
- Running wheels: The diameter and ergonomics of the running wheel will change the amount of use24. Thus, use smaller and lighter wheels for mice, and larger heavier wheels for rats. When washing and re-installing wheels, ensure that the wheels are able to spin unobstructed, do not “wobble”, and that the recording micro-switches are activated by each turn of the wheel.
- Micro-switches: Each revolution of the running wheel should activate a magnetic or mechanical micro-switch. Information from the micro-switch is transmitted via a single channel and recorded by a computer which can bin the data over time (e.g. every 2, 5, 6, or 10 min).
- Computer hardware: Our running-wheel recordings are made with Vitalview, a hardware and software platform developed by Mini Mitter (http://www.minimitter.com/vitalview_software.cfm). However, there are other recording platforms such as ClockLab, developed by Actimetrics (http://www.actimetrics.com/ClockLab/). Both platforms bring together data from many single-channel sources (e.g. a single micro-switch activated by a single running wheel) into a single computer file. Data from individual channels can then be graphed and analyzed separately at a later date.
3. Data Recordings
- Files: The above mentioned software platforms can be used to separate out single channels so that individual files are created for each running-wheel record. Such data are best visualized and graphed with specially designed programs such as Actiview (Minimitter, Bend, OR), Circadia, or Clocklab (Actimetrics, Wilmette, IL) which can all produce periodograms and actograms. However, single-channel files can also be opened and analyzed using general spreadsheet programs such as Excel (Microsoft, Redmond, WA).
- Calculating Circadian Time (CT): CT 12 is, by definition, the onset of running-wheel activity in nocturnal rodents. In parallel with the 24 hr day, by convention, one circadian day is broken into 24 circadian hr. Accordingly, if the circadian free-running period is 24 hr 30 min as measured by the wall clock, CT 0 will occur approximately 12 hr 15 min after CT12.
Representative Results
- Computer programs: Specialized computer programs are typically used in the generation of actograms and the calculation of circadian period. These programs include, but are not limited to, Actiview (Minimitter, Bend, OR) and Circadia.
- Actograms: Actograms provide a graphic illustration of the daily patterns of running-wheel activity. There are single-plotted (x-axis = 24 hr) and double-plotted (x-axis = 48 hr) actograms. Both methods plot sequential days from top to bottom, but double-plotted actograms plot two days on each horizontal line. Specifically, double-plotted actograms show the “second day” on the far right of each line, as well as at the start of the second horizontal line, and so on. Double-plotting is especially helpful to visualize non-24 hr rhythms.
- Periodogram: A periodogram is constructed from a spectral analysis of the running wheel activity over time. Periodograms show the relative power for a range of pre-set periods, and are commonly used to determine the free-running period.
- Results: In the laboratory, rodents are usually housed under a 24 hr LD cycle. Under these conditions the rhythm of activity is entrained, such that the daily pattern of running-wheel activity is aligned with the precise 24 hr LD cycle. In Figure 2A, the double-plotted actogram on the left shows the running-wheel activity of a rat that became active at the same time each day, soon after the environmental lights were turned off. The periodogram on the right shows a strong peak at 24 hr, consistent with entrainment to a precise 24 hr LD cycle. Figure 2B illustrates the running wheel use of a rat that was housed in constant darkness. In this case, the daily onset of running-wheel activity occurred slightly later each day, creating a rightward “drift”. This rightward “drift” indicates that the endogenous circadian period is greater than 24 hr, but it is the peak in the periodogram that quantifies this period. According to the periodogram, the maximum power is observed at 24.33 hr. In contrast to the automated periodogram analysis, Figure 3 illustrates a method to manually calculate the free-running period using the time of onset of running-wheel activity. It is important to realize that calculating the period by hand and calculating it with a periodogram may yield slightly different results.
The daily patterns of running-wheel activity can be disrupted in several ways. Figure 4 illustrates an arrhythmic pattern of running-wheel activity, produced by an electrolytic lesion of the SCN. This type of experiment provided some of the first evidence that the SCN contained the “master” circadian clock7,8. The periodogram on the right confirms this arrhythmic pattern of activity by showing equivalently low power for all periods in the circadian range (20-30 hr). The circadian pattern of running-wheel use can also be disrupted by housing rats in constant light. Figure 5 shows an actogram from a rat exposed sequentially to several of the lighting conditions already described. First, the rat was housed in constant darkness and exhibited a running wheel activity rhythm of approximately 24.33 hr. Second, the environmental light was kept on and the rat was housed in a constant light environment. Constant light is known to disrupt the SCN-based clock and produce arrhythmic patterns of running-wheel activity, similar to an SCN lesion. This disruption by light, however, occurs gradually over the course of 2-3 weeks. Therefore, when the running-wheel record is analyzed after the initial 3 weeks in constant light, the periodogram does not yield a peak. Finally, in the third phase the rat was put back on a 12 hr:12 hr LD cycle and the running wheel activity rhythms recover nearly immediately.
The amount of running, and the time of day it occurs, can also be manipulated by environmental factors. For instance, if rodents are fasted and given a temporally-restricted meal each day, this restricted feeding schedule will induce a daily bout of food-anticipatory activity. It is termed “anticipatory” because it occurs prior to the arrival of the daily meal, and is especially obvious when the meal is given in the middle of the day, a time when nocturnal rodents are relatively inactive. For instance, if an experiment provides a single 2 hr meal each day, food can be added to the cage at ZT 4 (4 hr after lights turned on) and removed at ZT 6 (2 hr later). Moreover, wire mesh flooring in the cages is also advantageous for this type of experiment because it makes it impossible for the rats to hide food and store it for later, thus ensuring that the rat is actually consuming all the food within the prescribed mealtime. Finally, one of the main advantages of an accurate running-wheel activity recording is that it allows for correlations to be made between running-wheel activity and daily oscillations in the expression of circadian clock gene expression throughout the brain and body. - Common pitfalls:
- Many software platforms automatically adjust for daylight saving time changes. When conducting an experiment at the time of yearly time changes, be sure this option is turned off in the recording software as well as the computer operating system software. This safeguard should help avoid discrepancies between the recording and the external light cycle.
- To be able to check on discrepancies in the data or unexpected changes in behavior, keep a text file with the precise time and date of every box opening, feeding and watering, bedding changes, experimental manipulations, and any other disruption that might occur. The precise start and end times of the data recording should also be noted in this file.
- It is important to check regularly that the lights are turning on and off at the expected times. Many problems can occur, including power outages and burnt out light bulbs. Some running-wheel platforms are equipped with light-sensors, but others do not verify lighting conditions.
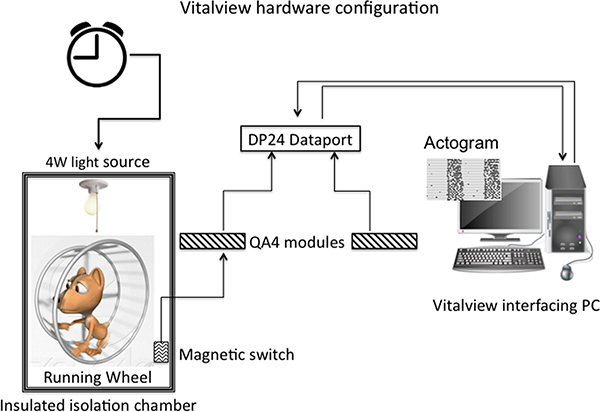
Figure 1. The Vitalview hardware configuration begins with a rodent running wheel, which is designed to activate a micro-switch with each revolution. This information then travels to the QA4 modules and is relayed to the DP24 dataport and finally is recorded by the Vitalview-equipped computer. The computer sums running-wheel revolutions from each channel every 10 min; these data can be viewed later as an actogram or periodogram. Depending on how the lights are set up, they can be controlled remotely either by the same Vitalview-equipped computer or by wall timers purchased from any electronics store.
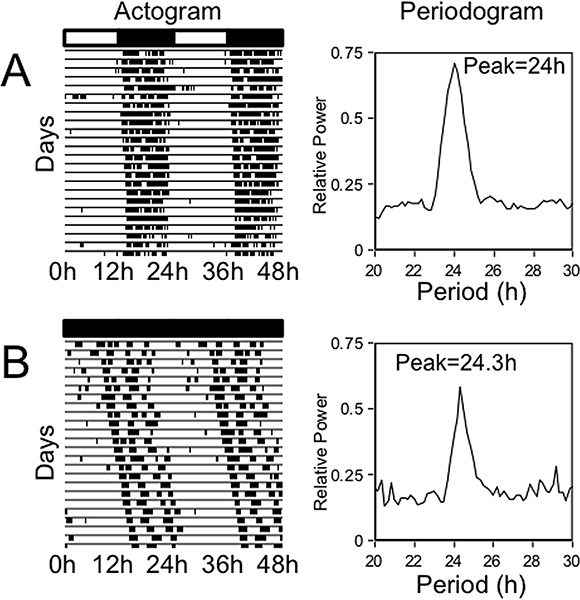
Figure 2. Representative actograms and periodograms for male Wistar rats housed in a 12 hr:12 hr LD cycle (A) and in constant darkness (B). Double-plotted actograms (left column) illustrate the lighting conditions along the top, 48 hr of running-wheel activity along the X-axis, and plot sequential days from top to bottom. Periodograms (right column) perform a spectral analysis on the running wheel data illustrated in the actograms. Figure 1A illustrates the behavior of a rat that was housed under a 24 hr LD cycle. Under these conditions the rat becomes active at the same time each day, showing an exact 24 hr peak in the periodogram. Figure 1B illustrates the behavior of a rat that was housed in constant darkness. Under these conditions the rat was active slightly later each day, hence the rightward drift in the actogram and 24.33 hr peak in the periodogram.
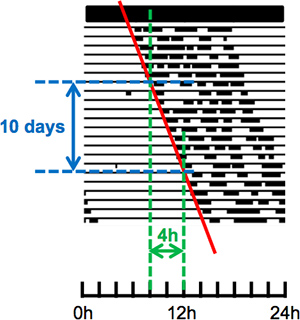
Figure 3. Period can also be extrapolated by hand. First draw a line of best-fit based on the daily onset of activity (red line). Next calculate the slope in h/day, remember that if the rhythm is <24 hr the slope will be a negative value, and finally add 24 hr. This procedure will provide an estimate of the circadian period for that animal. In this case, 4 hr/10 days suggests that the animal is becoming active approximately 0.4 hr later each day (slope 0.4 hr/day). Therefore, the circadian period is approximately 24.4 hr (or about 24 hr and 24 min). Any manipulation that is scheduled according to CT requires the free-running period to make an accurate prediction of CT.
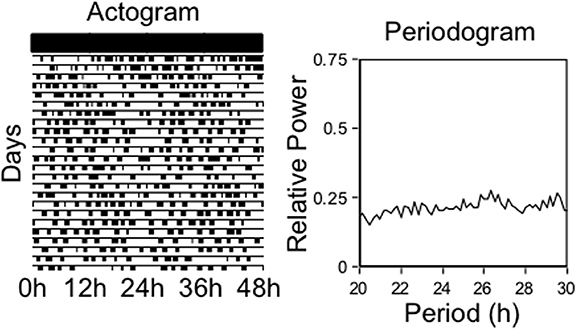
Figure 4. Electrolytic lesions of the SCN will produce arrhythmic patterns of running-wheel activity. In this case the rat is housed under constant darkness and, because the “master” circadian clock has been lesioned, the rat fails to show an endogenous circadian rhythm in running-wheel activity. The periodogram on the right confirms that there is no significant rhythm in the circadian range.
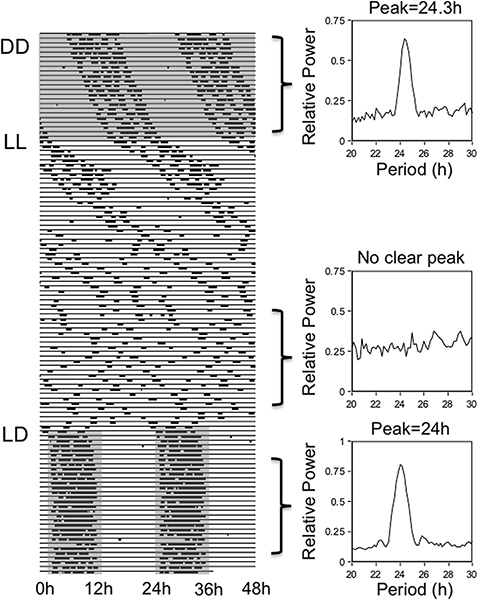
Figure 5. Lighting conditions have strong effects on the patterns of running wheel activity. In this record, the rat is initially housed under constant darkness (DD), as denoted by the shaded portion of the record. Under this condition, the circadian clock drives a circadian rhythm in running-wheel activity with a period of 24.33 hr, shown by the periodogram (top right). Next, the rat is housed under constant light (LL), as denoted by the white portion of the actogram. Under this condition the endogenous circadian clock is disrupted gradually over 2-3 weeks, and as shown by the periodogram (middle right) the rat becomes arrhythmic. Finally, the normal 12 hr:12 hr LD cycle was reinstated and the running-wheel activity rhythm was restored with a precise 24 hr rhythm as shown by the periodogram (bottom right).
Discussion
Monitoring daily activity rhythms using running wheels is the most commonly used and reliable method for assessing the output of the master circadian clock in nocturnal rodents. Wheel-running activity, however, is only one of many aspects of behavior and physiology that can be monitored continuously. Although the vast majority of running-wheel activity occurs during the night, over 30% of the total wakefulness occurs during the daytime25,26. Other endpoints can be used to assess circadian rhythms, including general activity, food-bin approaches, drinking, sleep, and body temperature. Thus, depending on the nature of the study, researchers may record several rhythms simultaneously.
Declarações
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge salary awards, equipment grants, and operating funds from the Fonds de la recherche en santé Québec (FRSQ), Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Concordia University Research Chairs Program (CRUC), as well as the thoughtful feedback on this manuscript from Dr. Jane Stewart.
Materials
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| Vitalview Card & Software | Mini Mitter | #855-0030-00 | (Bend, OR, USA) |
| DP24 Dataport | Mini Mitter | #840-0024-00 | (Bend, OR, USA) |
| QA4-Module | Mini Mitter | #130-0050-00 | (Bend, OR, USA) |
| Magnetic Switch | Mini Mitter | #130-0015-00 | (Bend, OR, USA) |
| C-50 Cable assembly | Mini Mitter | #060-0045-10 | (Bend, OR, USA) |
| Rat running wheel assembly | Mini Mitter | #640-0700-00 | (Bend, OR, USA) |
| Cage and tray support | Mini Mitter | #640-0400-00 | (Bend, OR, USA) |
| Useable cut away cage | Mini Mitter | #664-2154-00 | (Bend, OR, USA) |
| Grid floor for cage | Mini Mitter | #676-2154-00 | (Bend, OR, USA) |
| Waste tray | Mini Mitter | #684-2154-00 | (Bend, OR, USA) |
| Lamp housing | Microlites Scientific | #R-101 | (Toronto, ON, Canada) |
| 4W Fluorescent lamps | Microlites Scientific | #F4T5/CW | (Toronto, ON, Canada) |
| Isolation chambers | Custom built | 28″H x 20″W x 28″D ½” Black Melamine. |
Referências
- Pittendrigh, C. S., Daan, S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents. V. Pacemaker Structure: A Clock for All Seasons. J. Comp. Physiol. 106, 333-355 (1976).
- Pittendrigh, C. S., Daan, S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents. IV. Entrainment: Pacemaker as Clock. J. Comp. Physiol. 106, 291-331 (1976).
- Pittendrigh, C. S., Daan, S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents. III. Heavy Water and Constant Light: Homeostasis of Frequency?. J. Comp. Physiol. 106, 267-290 (1976).
- Pittendrigh, C. S., Daan, S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents. II. The Variability of Phase Response Curves. J. Comp. Physiol. 106, 253-266 (1976).
- Pittendrigh, C. S., Daan, S. A Functional Analysis of Circadian Pacemakers in Nocturnal Rodents. I. The Stability and Lability of Spontaneous Frequency. J. Comp. Physiol. 106, 223-252 (1976).
- Ralph, M. R., Foster, R. G., Davis, F. C., Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 247, 975-978 (1990).
- Moore, R. Y., Eichler, V. B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 42, 201-206 (1972).
- Stephan, F. K., Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. U.S.A. 69, 1583-1586 (1972).
- Abe, M., et al. Circadian rhythms in isolated brain regions. J. Neurosci. 22, 350-356 (2002).
- Yamazaki, S., et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 288, 682-685 (2000).
- Lamont, E. W., Robinson, B., Stewart, J., Amir, S. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc. Natl. Acad. Sci. U.S.A. 102, 4180-4184 (2005).
- Amir, S., Lamont, E. W., Robinson, B., Stewart, J. A circadian rhythm in the expression of PERIOD2 protein reveals a novel SCN-controlled oscillator in the oval nucleus of the bed nucleus of the stria terminalis. J. Neurosci. 24, 781-790 (2004).
- Yoo, S. H., et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U.S.A. 101, 5339-5346 (2004).
- Guilding, C., Piggins, H. D. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain?. Eur. J. Neurosci. 25, 3195-3216 (2007).
- Boulos, Z., Terman, M. Food availability and daily biological rhythms. Neurosci. Biobehav. Rev. 4, 119-131 (1980).
- Boulos, Z., Rosenwasser, A. M., Terman, M. Feeding schedules and the circadian organization of behavior in the rat. Behav. Brain Res. 1, 39-65 (1980).
- Verwey, M., Amir, S. Food-entrainable circadian oscillators in the brain. Eur. J. Neurosci. 30, 1650-1657 (2009).
- Davidson, A. J., Poole, A. S., Yamazaki, S., Menaker, M. Is the food-entrainable circadian oscillator in the digestive system?. Genes Brain Behav. 2, 32-39 (2003).
- Hara, R., et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 6, 269-278 (2001).
- Damiola, F., et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950-2961 (2000).
- Mrosovsky, N. Phase response curves for social entrainment. J. Comp. Physiol. A. 162, 35-46 (1988).
- Cain, S. W., et al. Reward and aversive stimuli produce similar nonphotic phase shifts. Behav. Neurosci. 118, 131-137 (2004).
- Antle, M. C., Mistlberger, R. E. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neurosci. 20, 9326-9332 (2000).
- Banjanin, S., Mrosovsky, N. Preferences of mice, Mus musculus, for different types of running wheel. Lab Anim. 34, 313-318 (2000).
- Verwey, M., Lam, G. Y., Amir, S. Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats. Eur. J. Neurosci. 29, 2217-2222 (2009).
- Gooley, J. J., Schomer, A., Saper, C. B. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 9, 398-407 (2006).

